How Can Animal Models Inform the Understanding of Cognitive Inflexibility in Patients with Anorexia Nervosa?
Abstract
:1. Introduction
Cognitive Inflexibility: A Characteristic of AN
2. Cognitive Flexibility: An Essential Executive Function
2.1. Testing Cognitive Flexibility in Humans
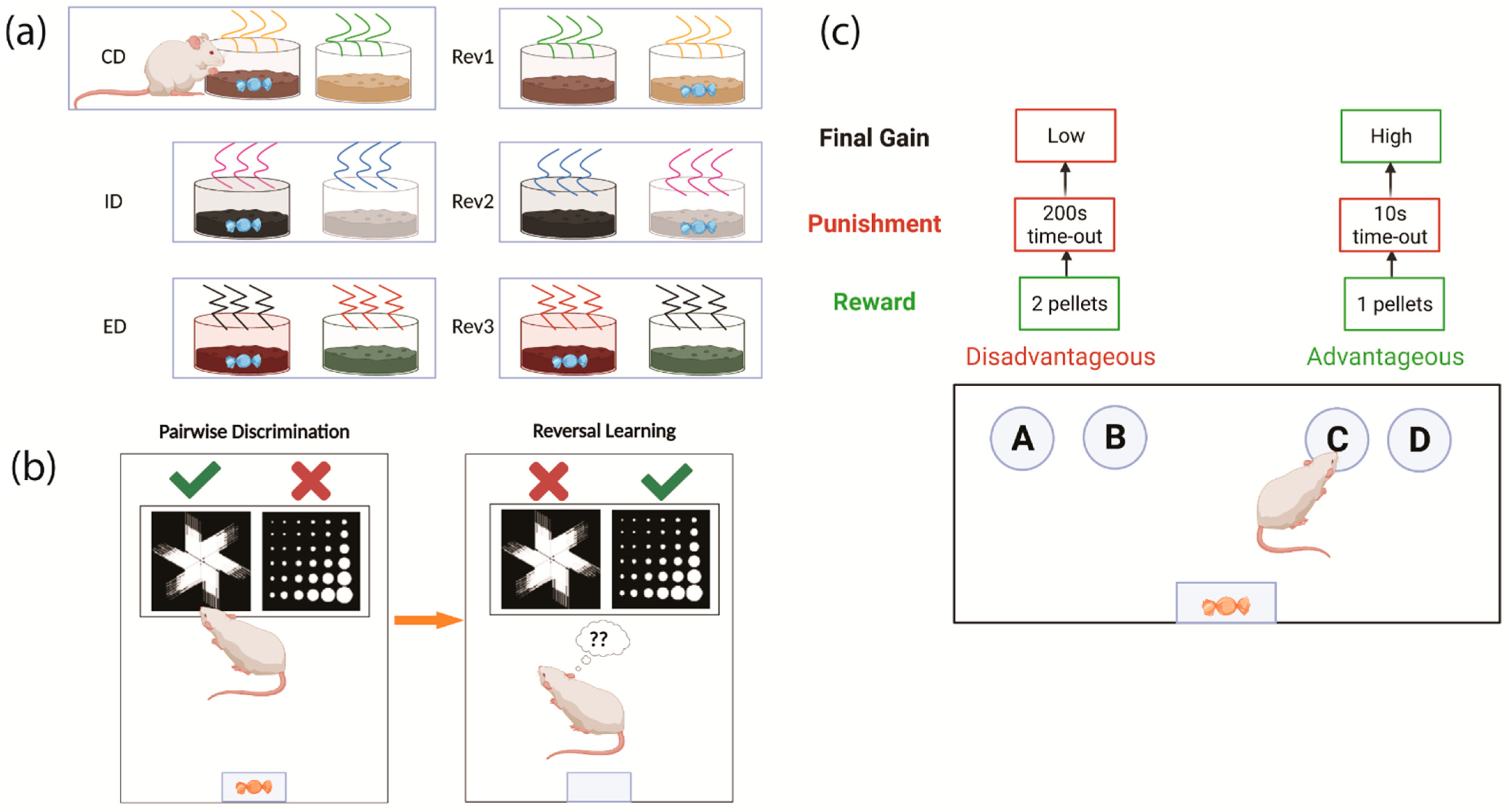
2.2. Testing Cognitive Flexibility in Rodents
2.3. Neuronal Control of Cognitive Flexibility
3. Common Neurological Drivers of AN and Cognitive Flexibility
3.1. Neurobiology Underlying AN
3.2. Cognitive Flexibility in AN
4. The Utility of the Activity-Based Anorexia (ABA) Model for Understanding the Neurobiology of AN
5. Investigating Cognitive Inflexibility in ABA
6. New Technologies to Improve the Assessment of Cognition and Behaviour in Animal Models of AN
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Steinhausen, H.C. Outcome of Eating Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality Rates in Patients with Anorexia Nervosa and Other Eating Disorders: A Meta-Analysis of 36 Studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berends, T.; Boonstra, N.; van Elburg, A. Relapse in Anorexia Nervosa: A Systematic Review and Meta-Analysis. Curr. Opin. Psychiatry 2018, 31, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Frank, G.K.W.; Shott, M.E. The Role of Psychotropic Medications in the Management of Anorexia Nervosa: Rationale, Evidence and Future Prospects. CNS Drugs 2016, 30, 419–442. [Google Scholar] [CrossRef] [Green Version]
- Lock, J. Updates on Treatments for Adolescent Anorexia Nervosa. Child Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Powers, P.S.; Bruty, H. Pharmacotherapy for Eating Disorders and Obesity. Child Adolesc. Psychiatr. Clin. N. Am. 2009, 18, 175–187. [Google Scholar] [CrossRef]
- Kaye, W.H.; Wierenga, C.E.; Bailer, U.F.; Simmons, A.N.; Bischoff-Grethe, A. Nothing Tastes as Good as Skinny Feels: The Neurobiology of Anorexia Nervosa. Trends Neurosci. 2013, 36, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-Wide Association Study Identifies Eight Risk Loci and Implicates Metabo-Psychiatric Origins for Anorexia Nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Friederich, H.-C.; Herzog, W. Cognitive-Behavioral Flexibility in Anorexia Nervosa. Behav. Neurobiol. Eat. Disord. 2011, 6, 111–123. [Google Scholar] [CrossRef]
- Sato, Y.; Saito, N.; Utsumi, A.; Aizawa, E.; Shoji, T.; Izumiyama, M.; Mushiake, H.; Hongo, M.; Fukudo, S. Neural Basis of Impaired Cognitive Flexibility in Patients with Anorexia Nervosa. PLoS ONE 2013, 8, e61108. [Google Scholar] [CrossRef] [Green Version]
- Dajani, D.R.; Uddin, L.Q. Demystifying Cognitive Flexibility: Implications for Clinical and Developmental Neuroscience. Trends Neurosci. 2015, 38, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinglass, J.E.; Walsh, B.T.; Stern, Y. Set Shifting Deficit in Anorexia Nervosa. J. Int. Neuropsychol. Soc. 2006, 12, 431–435. [Google Scholar] [CrossRef]
- Tenconi, E.; Santonastaso, P.; Degortes, D.; Bosello, R.; Titton, F.; Mapelli, D.; Favaro, A. Set-Shifting Abilities, Central Coherence, and Handedness in Anorexia Nervosa Patients, Their Unaffected Siblings and Healthy Controls: Exploring Putative Endophenotypes. World J. Biol. Psychiatry 2010, 11, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Tchanturia, K.; Harrison, A.; Davies, H.; Roberts, M.; Oldershaw, A.; Nakazato, M.; Stahl, D.; Morris, R.; Schmidt, U.; Treasure, J. Cognitive Flexibility and Clinical Severity in Eating Disorders. PLoS ONE 2011, 6, e20462. [Google Scholar] [CrossRef] [PubMed]
- Tchanturia, K.; Davies, H.; Roberts, M.; Harrison, A.; Nakazato, M.; Schmidt, U.; Treasure, J.; Morris, R. Poor Cognitive Flexibility in Eating Disorders: Examining the Evidence using the Wisconsin Card Sorting Task. PLoS ONE 2012, 7, e28331. [Google Scholar] [CrossRef]
- Perpiñá, C.; Segura, M.; Reales, S.S. Cognitive Flexibility and Decision-Making in Eating Disorders and Obesity. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2017, 22, 435–444. [Google Scholar] [CrossRef]
- Holliday, J.; Tchanturia, K.; Landau, S.; Collier, D.; Treasure, J. Is Impaired Set-Shifting an Endophenotype of Anorexia Nervosa? Am. J. Psychiatry 2005, 162, 2269–2275. [Google Scholar] [CrossRef]
- Lang, K.; Treasure, J.; Tchanturia, K. Is Inefficient Cognitive Processing in Anorexia Nervosa a Familial Trait? A Neuropsychological Pilot Study of Mothers of Offspring with a Diagnosis of Anorexia Nervosa. World J. Biol. Psychiatry 2016, 17, 258–265. [Google Scholar] [CrossRef]
- Duriez, P.; Lefèvre, H.K.; Di Lodovico, L.; Viltart, O.; Gorwood, P. Increased Cognitive Flexibility Mediates the Improvement of Eating Disorders Symptoms, Depressive Symptoms and Level of Daily Life Functioning in Patients with Anorexia Nervosa Treated in Specialised Centres. Eur. Eat. Disord. Rev. 2021, 29, 600–610. [Google Scholar] [CrossRef]
- Jansingh, A.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A. Developments in the Psychological Treatment of Anorexia Nervosa and Their Implications for Daily Practice. Curr. Opin. Psychiatry 2020, 33, 534–541. [Google Scholar] [CrossRef]
- Tchanturia, K.; Giombini, L.; Leppanen, J.; Kinnaird, E. Evidence for Cognitive Remediation Therapy in Young People with Anorexia Nervosa: Systematic Review and Meta-analysis of the Literature. Eur. Eat. Disord. Rev. 2017, 25, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errichiello, L.; Iodice, D.; Bruzzese, D.; Gherghi, M.; Senatore, I. Prognostic Factors and Outcome in Anorexia Nervosa: A Follow-up Study. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2015, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.J.; Laiks, L.S. Behavioral and Neural Mechanisms Underlying Habitual and Compulsive Drug Seeking. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Fröber, K.; Dreisbach, G. How Sequentially Changing Reward Prospect Modulates Meta-control: Increasing Reward Prospect Promotes Cognitive Flexibility. Cogn. Affect. Behav. Neurosci. 2021, 21, 534–548. [Google Scholar] [CrossRef]
- Stad, F.E.; Van Heijningen, C.J.M.; Wiedl, K.H.; Resing, W.C. Predicting School Achievement: Differential Effects of Dynamic Testing Measures and Cognitive Flexibility for Math Performance. Learn. Individ. Differ. 2018, 67, 117–125. [Google Scholar] [CrossRef]
- Magalhães, S.; Carneiro, L.; Limpo, T.; Filipe, M. Executive Functions Predict Literacy and Mathematics Achievements: The Unique Contribution of Cognitive Flexibility in Grades 2, 4, and 6. Child Neuropsychol. 2020, 26, 934–952. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, W.; Li, W.; Wei, D.; Li, H.; Lei, Q.; Zhang, Q.; Qiu, J. Association of Creative Achievement with Cognitive Flexibility by a Combined Voxel-Based Morphometry and Resting-State Functional Connectivity Study. NeuroImage 2014, 102, 474–483. [Google Scholar] [CrossRef]
- Davis, J.C.; Marra, C.A.; Najafzadeh, M.; Liu-Ambrose, T. The Independent Contribution of Executive Functions to Health Related Quality of Life in Older Women. BMC Geriatr. 2010, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Gruner, P.; Pittenger, C. Cognitive Inflexibility in Obsessive-Compulsive Disorder. Neuroscience 2016, 345, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Verdejo-Garcia, A.; Clark, L.; Verdejo-Román, J.; Albein-Urios, N.; Martinez-Gonzalez, J.M.; Gutiérrez, B.; Soriano-Mas, C. Neural Substrates of Cognitive Flexibility in Cocaine and Gambling Addictions. Br. J. Psychiatry 2015, 207, 158–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Kwok, N.T.-K.; Chan, T.C.-W.; Chan, G.H.-K.; So, S.H.-W. Inflexibility in Reasoning: Comparisons of Cognitive Flexibility, Explanatory Flexibility, and Belief Flexibility between Schizophrenia and Major Depressive Disorder. Front. Psychiatry 2021, 11, 609569. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Chica, M.; Rogers, B.; Damon, S.M.; Landman, B.A.; Woodward, N.D. Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol. Psychiatry 2018, 83, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, D.P.; Finger, E.C.; Brotman, M.A.; Rich, B.A.; Pine, D.S.; Blair, J.R.; Leibenluft, E. Impaired Probabilistic Reversal Learning in Youths with Mood and Anxiety Disorders. Psychol. Med. 2010, 40, 1089–1100. [Google Scholar] [CrossRef] [Green Version]
- Wildes, J.E.; Forbes, E.E.; Marcus, M.D. Advancing Research on Cognitive Flexibility in Eating Disorders: The Importance of Distinguishing Attentional Set-Shifting and Reversal Learning. Int. J. Eat. Disord. 2014, 47, 227–230. [Google Scholar] [CrossRef]
- Allen, P.J.; Jimerson, D.C.; Kanarek, R.B.; Kocsis, B. Impaired Reversal Learning in an Animal Model of Anorexia Nervosa. Physiol. Behav. 2017, 179, 313–318. [Google Scholar] [CrossRef]
- Kopp, B.; Lange, F.; Steinke, A. The Reliability of the Wisconsin Card Sorting Test in Clinical Practice. Assessment 2020, 28, 248–263. [Google Scholar] [CrossRef]
- Arbuthnott, K.; Frank, J. Trail Making Test, Part B as a Measure of Executive Control: Validation Using a Set-Switching Paradigm. J. Clin. Exp. Neuropsychol. 2010, 22, 518–528. [Google Scholar] [CrossRef]
- van den Berg, E.; Nys, G.M.; Brands, A.M.; Ruis, C.; van Zandvoort, M.J.; Kessels, R.P. The Brixton Spatial Anticipation Test as a Test for Executive Function: Validity in Patient Groups and Norms for Older Adults. J. Int. Neuropsychol. Soc. 2009, 15, 695–703. [Google Scholar] [CrossRef]
- Verharen, J.P.H.; Danner, U.N.; Schroder, S.; Aarts, E.; van Elburg, A.A.; Adan, R.A.H. Insensitivity to Losses: A Core Feature in Patients with Anorexia Nervosa? Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 995–1003. [Google Scholar] [CrossRef]
- van der Staay, F.J.; Arndt, S.S.; Nordquist, R.E. Evaluation of Animal Models of Neurobehavioral Disorders. Behav. Brain Funct. 2009, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffalo, E.A.; Movshon, J.A.; Wurtz, R.H. From Basic Brain Research to Treating Human Brain Disorders. Proc. Natl. Acad. Sci. USA 2019, 116, 26167–26172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. A Focus on Reward in Anorexia Nervosa through the Lens of the Activity-Based Anorexia Rodent Model. J. Neuroendocr. 2017, 29, e12479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, C.B.; Campbell, I.C.; Schmidt, U. A Reward-Centred Model of Anorexia Nervosa: A Focussed Narrative Review of the Neurological and Psychophysiological Literature. Neurosci. Biobehav. Rev. 2015, 52, 131–152. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent Models in Neuroscience Research: Is It a Rat Race? Dis. Model Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Janitzky, K.; Lippert, M.T.; Engelhorn, A.; Tegtmeier, J.; Goldschmidt, J.; Heinze, H.-J.; Ohl, F.W. Optogenetic Silencing of Locus Coeruleus Activity in Mice Impairs Cognitive Flexibility in an Attentional Set-Shifting Task. Front. Behav. Neurosci. 2015, 9, 286. [Google Scholar] [CrossRef] [Green Version]
- Heisler, J.M.; Morales, J.; Donegan, J.J.; Jett, J.D.; Redus, L.; O’Connor, J.C. The Attentional Set Shifting Task: A Measure of Cognitive Flexibility in Mice. J. Vis. Exp. 2015, 96, e51944. [Google Scholar] [CrossRef] [Green Version]
- Young, J.W.; Powell, S.B.; Geyer, M.A.; Jeste, D.V.; Risbrough, V.B. The Mouse Attentional-Set-Shifting Task: A Method for Assaying Successful Cognitive Aging? Cogn. Affect. Behav. Neurosci. 2010, 10, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Birrell, J.M.; Brown, V.J. Medial Frontal Cortex Mediates Perceptual Attentional Set Shifting in the Rat. J. Neurosci. 2000, 20, 4320–4324. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, A.; Brigman, J.L.; Radke, A.; Rudebeck, P.; Holmes, A. The Neural Basis of Reversal Learning: An Updated Perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Mar, A.C.; Horner, A.E.; Nilsson, S.R.; Alsio, J.; Kent, B.A.; Kim, C.H.; Holmes, A.; Saksida, L.M.; Bussey, T.J. The Touchscreen Operant Platform for Assessing Executive Function in Rats and Mice. Nat. Protoc. 2013, 8, 1985–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivalan, M.; Valton, V.; Seriès, P.; Marchand, A.R.; Dellu-Hagedorn, F. Elucidating Poor Decision-Making in a Rat Gambling Task. PLoS ONE 2013, 8, e82052. [Google Scholar] [CrossRef]
- Buckley, M.J.; Mansouri, F.A.; Hoda, H.; Mahboubi, M.; Browning, P.G.F.; Kwok, S.C.; Phillips, A.; Tanaka, K. Dissociable Components of Rule-Guided Behavior Depend on Distinct Medial and Prefrontal Regions. Science 2009, 325, 52–58. [Google Scholar] [CrossRef]
- Tsuchida, A.; Doll, B.B.; Fellows, L.K. Beyond Reversal: A Critical Role for Human Orbitofrontal Cortex in Flexible Learning from Probabilistic Feedback. J. Neurosci. 2010, 30, 16868–16875. [Google Scholar] [CrossRef]
- Stuss, D.; Levine, B.; Alexander, M.; Hong, J.; Palumbo, C.; Hamer, L.; Murphy, K.; Izukawa, D. Wisconsin Card Sorting Test Performance in Patients with Focal Frontal and Posterior Brain Damage: Effects of Lesion Location and Test Structure on Separable Cognitive Processes. Neuropsychologia 2000, 38, 388–402. [Google Scholar] [CrossRef]
- Mansouri, F.A.; Buckley, M.J.; Fehring, D.; Tanaka, K. The Role of Primate Prefrontal Cortex in Bias and Shift between Visual Dimensions. Cereb. Cortex 2020, 30, 85–99. [Google Scholar] [CrossRef] [Green Version]
- McAlonan, K.; Brown, V.J. Orbital Prefrontal Cortex Mediates Reversal Learning and Not Attentional Set Shifting in the Rat. Behav. Brain Res. 2003, 146, 97–103. [Google Scholar] [CrossRef]
- Cordova, C.A.; Jackson, D.; Langdon, K.D.; Hewlett, K.A.; Corbett, D. Impaired Executive Function Following Ischemic Stroke in the Rat Medial Prefrontal Cortex. Behav. Brain Res. 2014, 258, 106–111. [Google Scholar] [CrossRef]
- Carlén, M. What Constitutes the Prefrontal Cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef] [Green Version]
- Goyal, N.; Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A. Neuropsychology of Prefrontal Cortex. Indian J. Psychiatry 2008, 50, 202–208. [Google Scholar] [CrossRef]
- Seamans, J.K.; Lapish, C.C.; Durstewitz, D. Comparing the Prefrontal Cortex of Rats and Primates: Insights from Electrophysiology. Neurotox. Res. 2008, 14, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.F.; Gould, T.J. The Neural and Genetic Basis of Executive Function: Attention, Cognitive Flexibility, and Response Inhibition. Pharmacol. Biochem. Behav. 2014, 123, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Castañé, A.; Theobald, D.E.; Robbins, T.W. Selective Lesions of the Dorsomedial Striatum Impair Serial Spatial Reversal Learning in Rats. Behav. Brain Res. 2010, 210, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, H.F.; Robbins, T.W.; Roberts, A.C. Lesions of the Medial Striatum in Monkeys Produce Perseverative Impairments during Reversal Learning Similar to Those Produced by Lesions of the Orbitofrontal Cortex. J. Neurosci. 2008, 28, 10972–10982. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.; Li, Q.; Geng, H.; Chen, L.; Ip, N.Y.; Ke, Y.; Yung, W.-H. Dopamine Receptors Mediate Strategy Abandoning via Modulation of a Specific Prelimbic Cortex—Nucleus Accumbens Pathway in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, E4890–E4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferenczi, E.A.; Zalocusky, K.A.; Liston, C.; Grosenick, L.; Warden, M.R.; Amatya, D.; Katovich, K.; Mehta, H.; Patenaude, B.; Ramakrishnan, C.; et al. Prefrontal Cortical Regulation of Brainwide Circuit Dynamics and Reward-Related Behavior. Science 2016, 351, aac9698. [Google Scholar] [CrossRef] [Green Version]
- Nagano-Saito, A.; Leyton, M.; Monchi, O.; Goldberg, Y.K.; He, Y.; Dagher, A. Dopamine Depletion Impairs Frontostriatal Functional Connectivity during a Set-Shifting Task. J. Neurosci. 2008, 28, 3697–3706. [Google Scholar] [CrossRef]
- Grospe, G.M.; Baker, P.M.; Ragozzino, M.E. Cognitive Flexibility Deficits Following 6-OHDA Lesions of the Rat Dorsomedial Striatum. Neuroscience 2018, 374, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Tait, D.S.; Phillips, J.M.; Blackwell, A.D.; Brown, V.J. Effects of Lesions of the Subthalamic Nucleus/Zona Incerta Area and Dorsomedial Striatum on Attentional Set-Shifting in the Rat. Neuroscience 2017, 345, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Verharen, J.P.H.; de Jong, J.W.; Roelofs, T.J.M.; Huffels, C.F.M.; van Zessen, R.; Luijendijk, M.C.M.; Hamelink, R.; Willuhn, I.; Ouden, H.E.M.D.; van der Plasse, G.; et al. A Neuronal Mechanism Underlying Decision-Making Deficits during Hyperdopaminergic States. Nat. Commun. 2018, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Floresco, S.B.; Magyar, O.; Ghods-Sharifi, S.; Vexelman, C.; Tse, M.T.L. Multiple Dopamine Receptor Subtypes in the Medial Prefrontal Cortex of the Rat Regulate Set-Shifting. Neuropsychopharmacology 2006, 31, 297–309. [Google Scholar] [CrossRef]
- Clarke, H.F.; Walker, S.C.; Dalley, J.W.; Robbins, T.W.; Roberts, A.C. Cognitive Inflexibility after Prefrontal Serotonin Depletion Is Behaviorally and Neurochemically Specific. Cereb. Cortex 2007, 17, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.F.; Walker, S.C.; Crofts, H.S.; Dalley, J.W.; Robbins, T.W.; Roberts, A.C. Prefrontal Serotonin Depletion Affects Reversal Learning but Not Attentional Set Shifting. J. Neurosci. 2005, 25, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Alsiö, J.; Lehmann, O.; McKenzie, C.; Theobald, D.E.; Searle, L.; Xia, J.; Dalley, J.; Robbins, T. Serotonergic Innervations of the Orbitofrontal and Medial-prefrontal Cortices are Differentially Involved in Visual Discrimination and Reversal Learning in Rats. Cerebral Cortex 2021, 31, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Fudge, J.L.; Paulus, M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat. Rev. Neurosci. 2009, 10, 573–584. [Google Scholar] [CrossRef]
- Florent, V.; Baroncini, M.; Jissendi-Tchofo, P.; Lopes, R.; Vanhoutte, M.; Rasika, S.; Pruvo, J.-P.; Vignau, J.; Verdun, S.; Johansen, J.E.; et al. Hypothalamic Structural and Functional Imbalances in Anorexia Nervosa. Neuroendocrinology 2020, 110, 552–562. [Google Scholar] [CrossRef]
- Simon, J.J.; Stopyra, M.A.; Mönning, E.; Sailer, S.C.; Lavandier, N.; Kihm, L.; Bendszus, M.; Preissl, H.; Herzog, W.; Friederich, H.-C. Neuroimaging of Hypothalamic Mechanisms Related to Glucose Metabolism in Anorexia Nervosa and Obesity. J. Clin. Investig. 2020, 130, 4094–4103. [Google Scholar] [CrossRef] [Green Version]
- Schebendach, J.E.; Uniacke, B.; Walsh, B.T.; Mayer, L.E.S.; Attia, E.; Steinglass, J. Fat Preference and Fat Intake in Individuals with and without Anorexia Nervosa. Appetite 2019, 139, 35–41. [Google Scholar] [CrossRef]
- Dalton, B.; Foerde, K.; Bartholdy, S.; McClelland, J.; Kekic, M.; Grycuk, L.; Campbell, I.C.; Schmidt, U.; Steinglass, J.E. The Effect of Repetitive Transcranial Magnetic Stimulation on Food Choice-Related Self-Control in Patients with Severe, Enduring Anorexia Nervosa. Int. J. Eat. Disord. 2020, 53, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- Cowdrey, F.A.; Finlayson, G.; Park, R.J. Liking Compared with Wanting for High- and Low-Calorie Foods in Anorexia Nervosa: Aberrant Food Reward Even after Weight Restoration. Am. J. Clin. Nutr. 2013, 97, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Mayer, L.E.; Schebendach, J.; Bodell, L.P.; Shingleton, R.M.; Walsh, B.T. Eating Behavior in Anorexia Nervosa: Before and after Treatment. Int. J. Eat. Disord. 2011, 45, 290–293. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.J.; O′Daly, O.G.; Uher, R.; Friederich, H.-C.; Giampietro, V.; Brammer, M.; Williams, S.; Schiöth, H.B.; Treasure, J.; Campbell, I.C. Differential Neural Responses to Food Images in Women with Bulimia versus Anorexia Nervosa. PLoS ONE 2011, 6, e22259. [Google Scholar] [CrossRef] [PubMed]
- Cowdrey, F.A.; Park, R.J.; Harmer, C.; McCabe, C. Increased Neural Processing of Rewarding and Aversive Food Stimuli in Recovered Anorexia Nervosa. Biol. Psychiatry 2011, 70, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Aizenstein, H.; Mazurkewicz, L.; Fudge, J.; Frank, G.; Putnam, K.; Bailer, U.F.; Fischer, L.; Kaye, W.H. Altered Insula Response to Taste Stimuli in Individuals Recovered from Restricting-Type Anorexia Nervosa. Neuropsychopharmacology 2008, 33, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Nordbø, R.H.S.; Espeset, E.M.S.; Gulliksen, K.S.; Skårderud, F.; Holte, A. The Meaning of Self-Starvation: Qualitative Study of Patients’ Perception of Anorexia Nervosa. Int. J. Eat. Disord. 2006, 39, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Soussignan, R.; Rigaud, D.; Schaal, B. Pleasure for Visual and Olfactory Stimuli Evoking Energy-Dense Foods Is Decreased in Anorexia Nervosa. Psychiatry Res. 2010, 180, 42–47. [Google Scholar] [CrossRef]
- Steinglass, J.E.; Figner, B.; Berkowitz, S.; Simpson, H.B.; Weber, E.U.; Walsh, B.T. Increased Capacity to Delay Reward in Anorexia Nervosa. J. Int. Neuropsychol. Soc. 2012, 18, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Wierenga, C.E.; Bischoff-Grethe, A.; Melrose, A.J.; Irvine, Z.; Torres, L.; Bailer, U.F.; Simmons, A.; Fudge, J.L.; McClure, S.M.; Ely, A.; et al. Hunger Does Not Motivate Reward in Women Remitted from Anorexia Nervosa. Biol. Psychiatry 2015, 77, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, S.; Geisler, D.; Ritschel, F.; King, J.A.; Seidel, M.; Boehm, I.; Breier, M.; Clas, S.; Weiss, J.; Marxen, M.; et al. Elevated Cognitive Control over Reward Processing in Recovered Female Patients with Anorexia Nervosa. J. Psychiatry Neurosci. 2015, 40, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Harrison, A.; O’Brien, N.; Lopez, C.; Treasure, J. Sensitivity to Reward and Punishment in Eating Disorders. Psychiatry Res. 2010, 177, 1–11. [Google Scholar] [CrossRef]
- Kaye, W.H.; Frank, G.; McConaha, C.G.K. Altered Dopamine Activity after Recovery from Restricting-Type Anorexia Nervosa. Neuropsychopharmacology 1999, 21, 503–506. [Google Scholar] [CrossRef] [Green Version]
- Schalla, M.A.; Stengel, A. Activity Based Anorexia as an Animal Model for Anorexia Nervosa—A Systematic Review. Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaye, W.H.; Ebert, M.H.; Raleigh, M.; Lake, C.R. Abnormalities in CNS Monoamine Metabolism in Anorexia Nervosa. Arch. Gen. Psychiatry 1984, 41, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bailer, U.F.; Frank, G.K.; Price, J.C.; Meltzer, C.C.; Becker, C.; Mathis, C.A.; Wagner, A.; Barbarich-Marsteller, N.C.; Bloss, C.S.; Putnam, K.; et al. Interaction between Serotonin Transporter and Dopamine D2/D3 Receptor Radioligand Measures Is Associated with Harm Avoidant Symptoms in Anorexia and Bulimia Nervosa. Psychiatry Res. 2013, 211, 160–168. [Google Scholar] [CrossRef] [Green Version]
- Frank, G.K.; Bailer, U.F.; Henry, S.E.; Drevets, W.; Meltzer, C.C.; Price, J.C.; Mathis, C.A.; Wagner, A.; Hoge, J.; Ziolko, S.; et al. Increased Dopamine d2/d3 Receptor Binding after Recovery from Anorexia Nervosa Measured by Positron Emission Tomography and [11c]Raclopride. Biol. Psychiatry 2005, 58, 908–912. [Google Scholar] [CrossRef]
- Chen, X.; Voets, S.; Jenkinson, N.; Galea, J.M. Dopamine-Dependent Loss Aversion during Effort-Based Decision-Making. J. Neurosci. 2020, 40, 661–670. [Google Scholar] [CrossRef]
- Kaye, W.H.; Gwirtsman, H.E.; George, D.T.; Ebert, M.H. Altered Serotonin Activity in Anorexia Nervosa after Long-Term Weight Restoration: Does Elevated Cerebrospinal Fluid 5-Hydroxyindoleacetic Acid Level Correlate with Rigid and Obsessive Behavior? Arch. Gen. Psychiatry. 1991, 48, 556–562. [Google Scholar] [CrossRef]
- Audenaert, K.; Van Laere, K.; Dumont, F.; Vervaet, M.; Goethals, I.; Slegers, G.; Mertens, J.; Van Heeringen, C.; Dierckx, R.A. Decreased 5-HT2a Receptor Binding in Patients with Anorexia Nervosa. J. Nucl. Med. 2003, 44, 163–169. [Google Scholar]
- Bailer, U.F.; Frank, G.; Henry, S.E.; Price, J.C.; Meltzer, C.C.; Weissfeld, L.; Mathis, C.A.; Drevets, W.C.; Wagner, A.; Hoge, J.; et al. Altered Brain Serotonin 5-HT1A Receptor Binding after Recovery from Anorexia Nervosa Measured by Positron Emission Tomography and [Carbonyl11C]WAY-100635. Arch. Gen. Psychiatry 2005, 62, 1032–1041. [Google Scholar] [CrossRef] [Green Version]
- Lang, K.; Lloyd, S.; Khondoker, M.; Simic, M.; Treasure, J.; Tchanturia, K. Do Children and Adolescents with Anorexia Nervosa Display an Inefficient Cognitive Processing Style? PLoS ONE 2015, 10, e0131724. [Google Scholar] [CrossRef] [Green Version]
- Cederlöf, M.; Thornton, L.M.; Baker, J.; Lichtenstein, P.; Larsson, H.; Rück, C.; Bulik, C.M.; Mataix-Cols, D. Etiological Overlap between Obsessive-Compulsive Disorder and Anorexia Nervosa: A Longitudinal Cohort, Multigenerational Family and Twin Study. World Psychiatry 2015, 14, 333–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zastrow, A.; Kaiser, S.; Stippich, C.; Walther, S.; Herzog, W.; Tchanturia, K.; Belger, A.; Weisbrod, M.; Treasure, J.; Friederich, H.-C. Neural Correlates of Impaired Cognitive-Behavioral Flexibility in Anorexia Nervosa. Am. J. Psychiatry 2009, 166, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.; Gnatt, I.; Phillipou, A.; Nedeljkovic, M. Cognitive Flexibility in Acute Anorexia Nervosa and after Recovery: A Systematic Review. Clin. Psychol. Rev. 2020, 81, 101905. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, K.K.; Darcy, A.; Colborn, D.; Gudorf, C.; Lock, J. Set-Shifting among Adolescents with Anorexia Nervosa. Int. J. Eat. Disord. 2012, 45, 909–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchanturia, K.; Morris, R.G.; Anderluh, M.B.; Collier, D.A.; Nikolaou, V.; Treasure, J. Set Shifting in Anorexia Nervosa: An Examination before and after Weight Gain, in Full Recovery and Relationship to Childhood and Adult OCPD Traits. J. Psychiatr. Res. 2004, 38, 545–552. [Google Scholar] [CrossRef]
- Chowdhury, T.G.; Chen, Y.-W.; Aoki, C. Using the Activity-based Anorexia Rodent Model to Study the Neurobiological Basis of Anorexia Nervosa. J. Vis. Exp. 2015, 104, e52927. [Google Scholar] [CrossRef] [Green Version]
- Scharner, S.; Stengel, A. Animal Models for Anorexia Nervosa—A Systematic Review. Front. Hum. Neurosci. 2020, 14, 596381. [Google Scholar] [CrossRef]
- Boakes, R.; Mills, K.; Single, J. Sex Differences in the Relationship between Activity and Weight Loss in the Rat. Behav. Neurosci. 1999, 113, 1080–1089. [Google Scholar] [CrossRef]
- Beeler, J.A.; Mourra, D.; Zanca, R.M.; Kalmbach, A.; Gellman, C.; Klein, B.Y.; Ravenelle, R.; Serrano, P.; Moore, H.; Rayport, S.; et al. Vulnerable and Resilient Phenotypes in a Mouse Model of Anorexia Nervosa. Biol. Psychiatry 2020, 90, 829–842. [Google Scholar] [CrossRef]
- Hancock, S.; Grant, V. Early Maternal Separation Increases Symptoms of Activity-Based Anorexia in Male and Female Rats. J. Exp. Psychol. Anim. Behav. Process. 2009, 35, 394–406. [Google Scholar] [CrossRef]
- Carrera, O.; Gutiérrez, E.; Boakes, R.A. Early Handling Reduces Vulnerability of Rats to Activity-Based Anorexia. Dev. Psychobiol. 2006, 48, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.M.; Boakes, R.A. Activity-Based Anorexia in Rats as Failure to Adapt to a Feeding Schedule. Behav. Neurosci. 1997, 111, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats. Neuropsychopharmacology 2017, 42, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Pjetri, E.; De Haas, R.; De Jong, S.; Gelegen, C.; Oppelaar, H.; Verhagen, L.A.W.; Eijkemans, M.J.C.; Adan, R.A.; Olivier, B.; Kas, M. Identifying Predictors of Activity Based Anorexia Susceptibility in Diverse Genetic Rodent Populations. PLoS ONE 2012, 7, e50453. [Google Scholar] [CrossRef] [Green Version]
- Milton, L.K.; Patton, T.; O’Keeffe, M.; Oldfield, B.J.; Foldi, C.J. In Pursuit of Biomarkers for Predicting Susceptibility to Activity-Based Anorexia (ABA) in Adolescent Female Rats. Int. J. Eat. Disord. 2022. [Google Scholar] [CrossRef]
- Milton, L.K.; Oldfield, B.J.; Foldi, C.J. Evaluating Anhedonia in the Activity-Based Anorexia (ABA) Rat Model. Physiol. Behav. 2018, 194, 324–332. [Google Scholar] [CrossRef]
- Verhagen, L.A.W.; Luijendijk, M.C.M.; De Groot, J.-W.; Van Dommelen, L.P.G.; Klimstra, A.G.; Adan, R.A.H.; Roeling, T.A.P. Anticipation of Meals during Restricted Feeding Increases Activity in the Hypothalamus in Rats. Eur. J. Neurosci. 2011, 34, 1485–1491. [Google Scholar] [CrossRef]
- Barbarich-Marsteller, N.C.; Underwood, M.D.; Foltin, R.W.; Myers, M.M.; Walsh, B.T.; Barrett, J.S.; Marsteller, U.A. Identifying Novel Phenotypes of Vulnerability and Resistance to Activity-Based Anorexia in Adolescent Female Rats. Int. J. Eat. Disord. 2013, 46, 737–746. [Google Scholar] [CrossRef]
- Achamrah, N.; Nobis, S.; Goichon, A.; Breton, J.; Legrand, R.; Rego, J.L.D.; Déchelotte, P.; Fetissov, S.O.; Belmonte, L.; Coëffier, M. Sex Differences in Response to Activity-Based Anorexia Model in C57Bl/6 Mice. Physiol. Behav. 2017, 170, 1–5. [Google Scholar] [CrossRef]
- Franceschini, A.; Fattore, L. Gender-Specific Approach in Psychiatric Diseases: Because Sex Matters. Eur. J. Pharmacol. 2021, 896, 173895. [Google Scholar] [CrossRef]
- Doerries, L.E.; Stanley, E.Z.; Aravich, P.F. Activity-Based Anorexia: Relationship to Gender and Activity-Stress Ulcers. Physiol. Behav. 1991, 50, 945–949. [Google Scholar] [CrossRef]
- Klenotich, S.J.; Seiglie, M.P.; McMurray, M.S.; Roitman, J.D.; Le Grange, D.; Dugad, P.; Dulawa, S.C. Olanzapine, but Not Fluoxetine, Treatment Increases Survival in Activity-Based Anorexia in Mice. Neuropsychopharmacology 2012, 37, 1620–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atchley, D.P.; Eckel, L.A. Fenfluramine Treatment in Female Rats Accelerates the Weight Loss Associated with Activity-Based Anorexia. Pharmacol. Biochem. Behav. 2005, 80, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Atchley, D.; Eckel, L. Treatment with 8-OH-DPAT Attenuates the Weight Loss Associated with Activity-Based Anorexia in Female Rats. Pharmacol. Biochem. Behav. 2006, 83, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.E.; Clissold, K.A.; Lin, P.; Cain, A.E.; Ciesinski, A.F.; Hopkins, T.R.; Ilesanmi, A.O.; Kelly, E.A.; Pierce-Messick, Z.; Powell, D.S.; et al. A Systematic Investigation of the Differential Roles for Ventral Tegmentum Serotonin 1- and 2-Type Receptors on Food Intake in the Rat. Brain Res. 2016, 1648, 54–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klenotich, S.J.; Ho, E.V.; McMurray, M.S.; Server, C.H.; Dulawa, S.C. Dopamine D2/3 Receptor Antagonism Reduces Activity-Based Anorexia. Transl. Psychiatry 2015, 5, e613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, L.A.W.; Luijendijk, M.C.M.; Korte-Bouws, G.A.H.; Korte, S.M.; Adan, R.A.H. Dopamine and Serotonin Release in the Nucleus Accumbens during Starvation-Induced Hyperactivity. Eur. Neuropsychopharmacol. 2009, 19, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.G.; Fenton, A.A.; Aoki, C. Effects of Adolescent Experience of Food Restriction and Exercise on Spatial Learning and Open Field Exploration of Female Rats. Hippocampus 2021, 31, 170–188. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Liesbrock, J.; Paulukat, L.; Kas, M.J.; Tolba, R.; Konrad, K.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J. Establishment of a Chronic Activity-Based Anorexia Rat Model. J. Neurosci. Methods 2018, 293, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Milton, L.K.; Mirabella, P.N.; Greaves, E.; Spanswick, D.C.; van den Buuse, M.; Oldfield, B.J.; Foldi, C.J. Suppression of Corticostriatal Circuit Activity Improves Cognitive Flexibility and Prevents Body Weight Loss in Activity-Based Anorexia in Rats. Biol. Psychiatry 2020, 90, 819–828. [Google Scholar] [CrossRef]
- Rivalan, M.; Munawar, H.; Fuchs, A.; Winter, Y. An Automated, Experimenter-Free Method for the Standardised, Operant Cognitive Testing of Rats. PLoS ONE 2017, 12, e0169476. [Google Scholar]
- Caglayan, A.; Stumpenhorst, K.; Winter, Y. Learning Set Formation and Reversal Learning in Mice during High-Throughput Home-Cage-Based Olfactory Discrimination. Front. Behav. Neurosci. 2021, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Horner, A.E.; Heath, C.J.; Hvoslef-Eide, M.; Kent, B.A.; Kim, C.H.; Nilsson, S.R.; Alsiö, J.; Oomen, C.A.; Holmes, A.; Saksida, L.M.; et al. The Touchscreen Operant Platform for Testing Learning and Memory in Rats and Mice. Nat. Protoc. 2013, 8, 1961–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.J.; Need, A.; Cirulli, E.T.; Chiba-Falek, O.; Attix, D.K. A Comparison of the Cambridge Automated Neuropsychological Test Battery (CANTAB) with “Traditional” Neuropsychological Testing Instruments. J. Clin. Exp. Neuropsychol. 2013, 35, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Winter, Y.; Schaefers, A.T.U. A Sorting System with Automated Gates Permits Individual Operant Experiments with Mice from a Social Home Cage. J. Neurosci. Methods 2011, 196, 276–280. [Google Scholar] [CrossRef]
- Costa, R.; Tamascia, M.L.; Nogueira, M.D.; Casarini, D.E.; Marcondes, F.K. Handling of Adolescent Rats Improves Learning and Memory and Decreases Anxiety. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 548–553. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Foldi, C.J. How Can Animal Models Inform the Understanding of Cognitive Inflexibility in Patients with Anorexia Nervosa? J. Clin. Med. 2022, 11, 2594. https://doi.org/10.3390/jcm11092594
Huang K, Foldi CJ. How Can Animal Models Inform the Understanding of Cognitive Inflexibility in Patients with Anorexia Nervosa? Journal of Clinical Medicine. 2022; 11(9):2594. https://doi.org/10.3390/jcm11092594
Chicago/Turabian StyleHuang, Kaixin, and Claire J. Foldi. 2022. "How Can Animal Models Inform the Understanding of Cognitive Inflexibility in Patients with Anorexia Nervosa?" Journal of Clinical Medicine 11, no. 9: 2594. https://doi.org/10.3390/jcm11092594





