Outcome Analysis in Elective Electrical Cardioversion of Atrial Fibrillation Patients: Development and Validation of a Machine Learning Prognostic Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Model
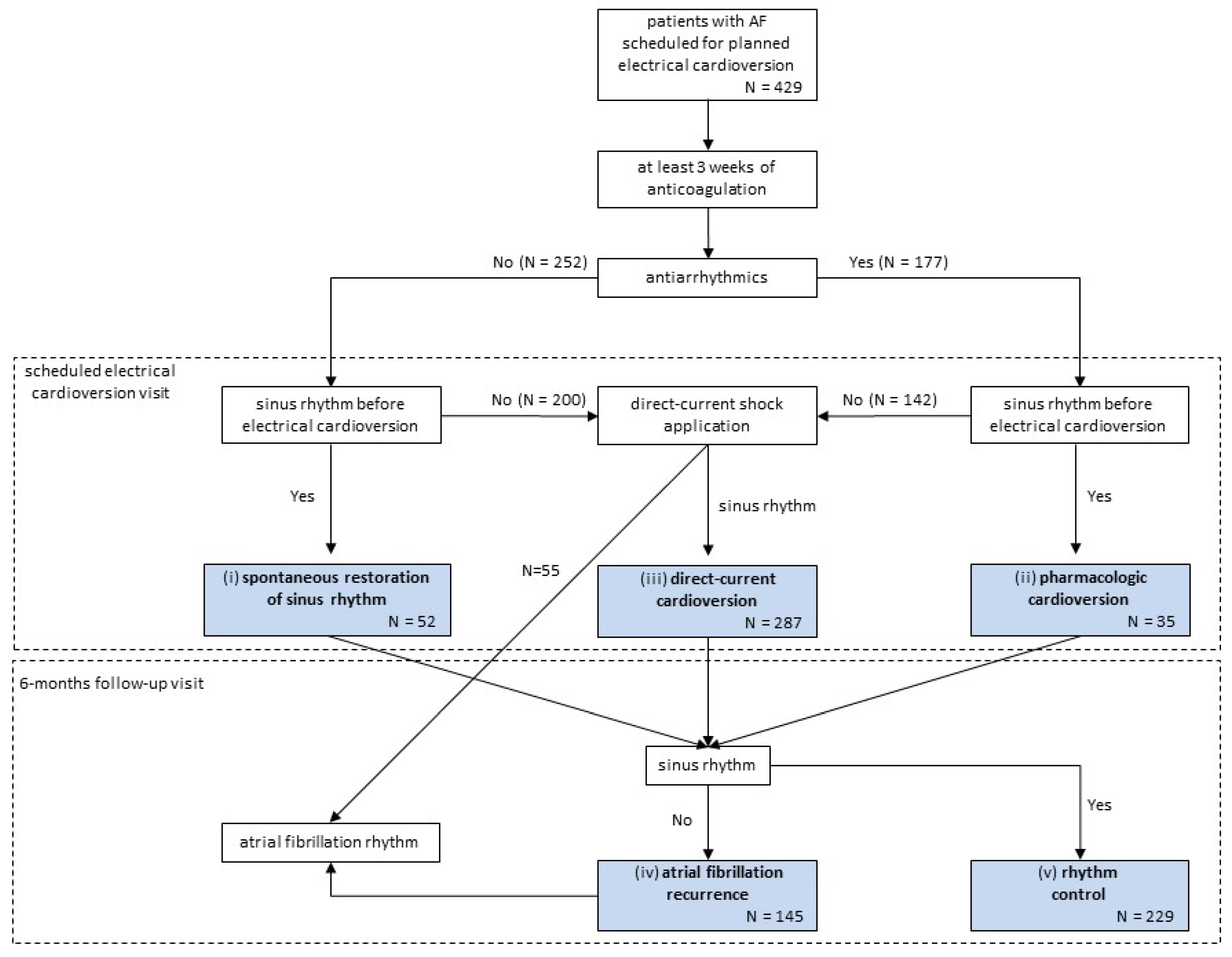
2.1.1. Task Definition and Clinical Pathways of Patients
2.1.2. Study Population
2.1.3. Data Collection and Preparation
2.2. Training the Model
2.2.1. Machine Learning Classifiers
2.2.2. Hyperparameter Tuning
2.3. Evaluating the Model
2.3.1. Evaluation Scheme
2.3.2. Comparison with Standard Successful Cardioversion Risk Scores
2.3.3. Feature Analysis
2.3.4. Open-Source Software
3. Results
3.1. Characteristics and Flow of the Study Population
3.2. Comparison of Prediction Models for Each Pathway
3.3. Feature Importance
3.4. Machine-Learning Models Deployment in a Calculator
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Section/Topic | Item | Checklist Item | Page | |
|---|---|---|---|---|
| Title and abstract | ||||
| 1 | D;V | Identify the study as developing and/or validating a multivariable prediction model, the target population, and the outcome to be predicted. | 1 | |
| Abstract | 2 | D;V | Provide a summary of objectives, study design, setting, participants, sample size, predictors, outcome, statistical analysis, results, and conclusions. | 2 |
| Introduction | ||||
| Background and objectives | 3a | D;V | Explain the medical context (including whether diagnostic or prognostic) and rationale for developing or validating the multivariable prediction model, including references to existing models. | 3 |
| 3b | D;V | Specify the objectives, including whether the study describes the development or validation of the model or both. | 3 | |
| Methods | ||||
| Source of data | 4a | D;V | Describe the study design or source of data (e.g., randomized trial, cohort, or registry data), separately for the development and validation data sets, if applicable. | 2 |
| 4b | D;V | Specify the key study dates, including start of accrual; end of accrual; and, if applicable, end of follow-up. | 3 | |
| Participants | 5a | D;V | Specify key elements of the study setting (e.g., primary care, secondary care, general population) including number and location of centers. | 3 |
| 5b | D;V | Describe eligibility criteria for participants. | 3 | |
| 5c | D;V | Give details of treatments received, if relevant. | 4 | |
| Outcome | 6a | D;V | Clearly define the outcome that is predicted by the prediction model, including how and when assessed. | 2, 3 |
| 6b | D;V | Report any actions to blind assessment of the outcome to be predicted. | 3 | |
| Predictors | 7a | D;V | Clearly define all predictors used in developing or validating the multivariable prediction model, including how and when they were measured. | Table 2 |
| 7b | D;V | Report any actions to blind assessment of predictors for the outcome and other predictors. | 4 | |
| Sample size | 8 | D;V | Explain how the study size was arrived at. | 4 |
| Missing data | 9 | D;V | Describe how missing data were handled (e.g., complete-case analysis, single imputation, multiple imputation) with details of any imputation method. | 4 |
| Statistical analysis methods | 10a | D | Describe how predictors were handled in the analyses. | 4 |
| 10b | D | Specify type of model, all model-building procedures (including any predictor selection), and method for internal validation. | 3, 4 | |
| 10c | V | For validation, describe how the predictions were calculated. | 4 | |
| 10d | D;V | Specify all measures used to assess model performance and, if relevant, to compare multiple models. | 4 | |
| 10e | V | Describe any model updating (e.g., recalibration) arising from the validation, if done. | NA | |
| Risk groups | 11 | D;V | Provide details on how risk groups were created, if done. | 4 |
| Development vs. validation | 12 | V | For validation, identify any differences from the development data in setting, eligibility criteria, outcome, and predictors. | 4 |
| Results | ||||
| Participants | 13a | D;V | Describe the flow of participants through the study, including the number of participants with and without the outcome and, if applicable, a summary of the follow-up time. A diagram may be helpful. | Figure 2 |
| 13b | D;V | Describe the characteristics of the participants (basic demographics, clinical features, available predictors), including the number of participants with missing data for predictors and the outcome. | Table 2 | |
| 13c | V | For validation, show a comparison with the development data of the distribution of important variables (demographics, predictors, and outcome). | Table 2 and Table 6 | |
| Model development | 14a | D | Specify the number of participants and outcome events in each analysis. | Figure 2 |
| 14b | D | If done, report the unadjusted association between each candidate predictor and outcome. | NA | |
| Model specification | 15a | D | Present the full prediction model to allow predictions for individuals (i.e., all regression coefficients and model intercept or baseline survival at a given time point). | 6 |
| 15b | D | Explain how to use the prediction model. | 6 | |
| Model performance | 16 | D;V | Report performance measures (with CIs) for the prediction model. | Table 3, Table 4 and Table 5 |
| Model-updating | 17 | V | If done, report the results from any model updating (i.e., model specification, model performance). | NA |
| Discussion | ||||
| Limitations | 18 | D;V | Discuss any limitations of the study (such as nonrepresentative sample, few events per predictor, missing data). | 14, 15 |
| Interpretation | 19a | V | For validation, discuss the results with reference to the performance of the development data and any other validation data. | 13, 14 |
| 19b | D;V | Give an overall interpretation of the results, considering objectives, limitations, results from similar studies, and other relevant evidence. | 13, 14 | |
| Implications | 20 | D;V | Discuss the potential clinical use of the model and implications for future research. | 13,14 |
| Other information | ||||
| Supplementary information | 21 | D;V | Provide information about the availability of supplementary resources, such as study protocol, Web calculator, and data sets. | 7, 14 |
| Funding | 22 | D;V | Give the source of funding and the role of the funders for the present study. | 15 |
References
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Ellinor, P.T., Jr.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Giubilato, S.; Di Fusco, S.A.; Piccioni, L.; Rao, C.M.; Iorio, A.; Cipolletta, L.; D’elia, E.; Gelsomino, S.; Rossini, R.; et al. Anticoagulation in Atrial Fibrillation Cardioversion: What Is Crucial to Take into Account. J. Clin. Med. 2021, 10, 3212. [Google Scholar] [CrossRef] [PubMed]
- Tieleman RG, Van Gelder IC, Crijns HJ, De Kam PJ, Van Den Berg MP, Haaksma J, Van Der Woude HJ, Allessie MA. Early recurrences of atrial fibrillation after electrical cardioversion: A result of fibrillation-induced electrical remodeling of the atria? J. Am. Coll. Cardiol. 1998, 31, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.; Frykman, V.; Jensen-Urstad, M.; Ostergren, J.; Rosenqvist, M. Factors predicting success rate and recurrence of atrial fibrillation after first electrical cardioversion in patients with persistent atrial fibrillation. Clin. Cardiol. 2001, 24, 238–244. [Google Scholar] [CrossRef]
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.; et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420. [Google Scholar] [CrossRef]
- Ortiz De Murua, J.A.; del Carmen Avila, M.; Ochoa, C.; de La Fuente, L.; Moreno De Vega, J.C.; del Campo, F.; Villafranca, J.L. Independent predictive factors of acute and first year success after electrical cardioversion in patients with chronic atrial fibrillation. Rev. Esp. Cardiol. 2001, 54, 958–964. [Google Scholar]
- Kuppahally, S.S.; Foster, E.; Shoor, S.; Steimle, A.E. Short-term and long-term success of electrical cardioversion in atrial fibrillation in managed care system. Int. Arch. Med. 2009, 2, 39. [Google Scholar] [CrossRef] [Green Version]
- Cappato, R.; Ezekowitz, M.D.; Klein, A.L.; Camm, A.J.; Ma, C.-S.; Le Heuzey, J.-Y.; Talajic, M.; Scanavacca, M.; Vardas, P.E.; Kirchhof, P.; et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur. Heart J. 2014, 35, 3346–3355. [Google Scholar] [CrossRef] [Green Version]
- Hellman, T.; Kiviniemi, T.; Nuotio, I.; Vasankari, T.; Hartikainen, J.; Lip, G.Y.; Airaksinen, K.J. Intensity of anticoagulation and risk of thromboembolism after elective cardioversion of atrial fibrillation. Thromb Res. 2017, 156, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, D.; Talajic, M.; Dorian, P.; Connolly, S.; Eisenberg, M.J.; Green, M.; Kus, T.; Lambert, J.; Dubuc, M.; Gagné, P.; et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000, 342, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Longas-Tejero, M.A.; Bergmann, J.F.; Belmin, J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2012, CD005049. [Google Scholar] [CrossRef]
- Kirchhof, P.; Andresen, D.; Bosch, R.; Borggrefe, M.; Meinertz, T.; Parade, U.; Ravens, U.; Samol, A.; Steinbeck, G.; Treszl, A.; et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): A prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012, 380, 238–246. [Google Scholar] [CrossRef]
- Um, K.J.; McIntyre, W.F.; Healey, J.S.; Mendoza, P.A.; Koziarz, A.; Amit, G.; Chu, V.A.; Whitlock, R.P.; Belley-Côté, E.P. Pre- and post-treatment with amiodarone for elective electrical cardioversion of atrial fibrillation: A systematic review and meta-analysis. Europace 2019, 21, 856–863. [Google Scholar] [CrossRef]
- Gwag, H.B.; Chun, K.J.; Hwang, J.K.; Park, S.J.; Kim, J.S.; Park, K.M.; On, Y.K. Which antiarrhythmic drug to choose after electrical cardioversion: A study on non-valvular atrial fibrillation patients. PLoS ONE 2018, 13, e0197352. [Google Scholar] [CrossRef]
- El Amrani, A.; Viñolas, X.; Arias, M.A.; Bazan, V.; Valdovinos, P.; Alegret, J.M. Pharmacological Cardioversion after Pre-Treatment with Antiarrythmic Drugs Prior to Electrical Cardioversion in Persistent Atrial Fibrillation: Impact on Maintenance of Sinus Rhythm. J. Clin. Med. 2021, 10, 1029. [Google Scholar] [CrossRef]
- Doyle, B.; Reeves, M. “Wait and see” approach to the emergency department cardioversion of acute atrial fibrillation. Emerg. Med. Int. 2011, 2011, 545023. [Google Scholar] [CrossRef] [Green Version]
- Zohar, P.; Kovacic, M.; Brezocnik, M.; Podbregar, M. Prediction of maintenance of sinus rhythm after electrical cardioversion of atrial fibrillation by non-deterministic modelling. Europace 2005, 7, 500–507. [Google Scholar] [CrossRef]
- Raitt, M.H.; Volgman, A.S.; Zoble, R.G.; Charbonneau, L.; Padder, F.A.; O’Hara, G.E.; Kerr, D. Prediction of the recurrence of atrial fibrillation after cardioversion in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2006, 151, 390–396. [Google Scholar] [CrossRef]
- Falsetti, L.; Viticchi, G.; Tarquinio, N.; Silvestrini, M.; Capeci, W.; Balloni, A.; Catozzo, V.; Gentile, A.; Pellegrini, F. CHA2DS2-VASc in the prediction of early atrial fibrillation relapses after electrical or pharmacological cardioversion. J. Cardiovasc. Med. Hagerstown 2014, 15, 636–641. [Google Scholar] [CrossRef]
- Sterling, M.; Huang, D.T.; Ghoraani, B. Developing a New Computer-Aided Clinical Decision Support System for Prediction of Successful Postcardioversion Patients with Persistent Atrial Fibrillation. Comput. Math. Methods Med. 2015, 2015, 527815. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, S.; Lip, G.Y.; Biancari, F.; Nuotio, I.; Hartikainen, J.E.; Ylitalo, A.; Airaksinen, K.J. Predicting Unsuccessful Electrical Cardioversion for Acute Atrial Fibrillation (from the AF-CVS Score). Am. J. Cardiol. 2017, 119, 749–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, F.; Serenelli, M.; Airaksinen, J.; Pavasini, R.; Tomaszuk-Kazberuk, A.; Mlodawska, E.; Jaakkola, S.; Balla, C.; Falsetti, L.; Tarquinio, N.; et al. CHA2DS2-VASc score predicts atrial fibrillation recurrence after cardioversion: Systematic review and individual patient pooled meta-analysis. Clin. Cardiol. 2019, 42, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Wałek, P.; Sielski, J.; Gorczyca, I.; Roskal-Wałek, J.; Starzyk, K.; Jaskulska-Niedziela, E.; Bartkowiak, R.; Wożakowska-Kapłon, B. Left atrial mechanical remodelling assessed as the velocity of left atrium appendage wall motion during atrial fibrillation is associated with maintenance of sinus rhythm after electrical cardioversion in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0228239. [Google Scholar] [CrossRef]
- Singh, J.P. It Is Time for Us to Get Artificially Intelligent! JACC Clin. Electrophysiol. 2019, 5, 263–265. [Google Scholar] [CrossRef]
- Dorado-Díaz, P.I.; Sampedro-Gómez, J.; Vicente-Palacios, V.; Sánchez, P.L. Applications of Artificial Intelligence in Cardiology. The Future is Already Here. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 1065–1075. [Google Scholar] [CrossRef]
- Moawad, G.N.; Elkhalil, J.; Klebanoff, J.S.; Rahman, S.; Habib, N.; Alkatout, I. Augmented Realities, Artificial Intelligence, and Machine Learning: Clinical Implications and How Technology Is Shaping the Future of Medicine. J. Clin. Med. 2020, 9, 3811. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. Ann. Intern. Med. 2015, 162, 55-U103. [Google Scholar] [CrossRef] [Green Version]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Phyton. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Gallego-Delgado, M.; Villacorta, E.; Valenzuela-Vicente, M.C.; Walias-Sánchez, Á.; Ávila, C.; Velasco-Cañedo, M.J.; Cano-Mozo, M.T.; Martín-García, A.; García-Sánchez, M.J.; Sánchez, A.; et al. Start-up of a Cardiology Day Hospital: Activity, Quality Care and Cost-effectiveness Analysis of the First Year of Operation. Rev. Esp. Cardiol. Engl. Ed. 2019, 72, 130–137. [Google Scholar] [CrossRef]
- Varma, S.; Simon, R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. 2006, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.; Goadrich, M. The relationship between precision-recall and ROC curves. In Proceedings of the 23rd International Conference on Machine Learning (ICML-06), Pittsburgh, PA, USA, 25–29 June 2006; pp. 233–240. [Google Scholar]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadeau, C.; Bengio, Y. Inference for the generalization error. Mach. Learn. 2003, 52, 239–281. [Google Scholar] [CrossRef] [Green Version]
- Bouckaert, R. Choosing Between Two Learning Algorithms Based on Calibrated Tests. ICML 2003, 3, 51–58. [Google Scholar]
- De Vos, C.B.; Pisters, R.; Nieuwlaat, R.; Prins, M.H.; Tieleman, R.G.; Coelen, R.J.; van den Heijkant, A.C.; Allessie, M.A.; Crijns, H.J. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J. Am. Coll. Cardiol. 2010, 55, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Emren, S.V.; Kocabaş, U.; Duygu, H.; Levent, F.; Şimşek, E.Ç.; Emren, Z.Y.; Tülüce, S. The role of HATCH score in predicting the success rate of sinus rhythm following electrical cardioversion of atrial fibrillation. Kardiol. Pol. 2016, 74, 978–984. [Google Scholar] [CrossRef] [Green Version]
- Kerr, K.F.; Wang, Z.; Janes, H.; McClelland, R.L.; Psaty, B.M.; Pepe, M.S. Net reclassification indices for evaluating risk prediction instruments: A critical review. Epidemiology 2014, 25, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Celi, L.A.; Citi, L.; Ghassemi, M.; Pollard, T.J. The PLoS ONE collection on machine learning in health and biomedicine: Towards open code and open data. PLoS ONE 2019, 14, e0210232. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Oto, E.; Okutucu, S.; Katircioglu-Öztürk, D.; Güvenir, H.A.; Karaagaoglu, E.; Borggrefe, M.; Breithardt, G.; Goette, A.; Ravens, U.; Steinbeck, G.; et al. Predictors of sinus rhythm after electrical cardioversion of atrial fibrillation: Results from a data mining project on the Flec-SL trial data set. Europace 2017, 19, 921–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzelczyk, T.A.; Kaplan, R.M.; Medler, M.; Knight, B.P. Outcomes Associated with Electrical Cardioversion for Atrial Fibrillation when Performed Autonomously by an Advanced Practice Provider. JACC Clin. Electrophysiol. 2017, 3, 1447–1452. [Google Scholar] [CrossRef] [PubMed]



| Algorithm | Feature Selection | Hyperparameters |
|---|---|---|
| Boosted Trees | No selection Univariate selection RF feature importance | Number of trees: 25, 100, or 1000 Depth of the trees: 3, 5, or 7 Learning rate: 0.1 or 0.05 L1 regularization term weights: 0 or 1 L2 regularization term weight. 1 |
| Random Forest | No selection Univariate selection Feature importance (random forest) | Number of trees: 100 or 1000 Number of features considered at each branch split: 1 or auto (square root of total features) Split criterion: Gini impurity or information gain. Max depth of trees: 1, 2, 5, or unbounded |
| Extremely Randomized Trees | No selection Univariate selection RF feature importance | Number of trees: 100 or 1000 Number of features considered at each branch split: 1 or auto (square root of total features) Split criterion: Gini impurity or information gain. Max depth of trees: 1, 2, 5, or unbounded |
| Logistic Regression | No selection Univariate selection RF feature importance | Regularization term: L1 or L2 |
| Development Dataset N = 316 | Validation Dataset N = 113 | |||
|---|---|---|---|---|
| Missing Values | Mean | Missing Values | Mean | |
| Demographics | ||||
| Age, years | 2 | 62.1 ± 11.9 | 4 | 63.0 ± 12.2 |
| Gender, male | - | 240 (75.9%) | - | 83 (73.5%) |
| Weight, kg | 30 | 84.0 ± 17.1 | 19 | 85.2 ± 20.1 |
| Height, cm | 39 | 170.0 ± 8.9 | 28 | 170.6 ± 9.9 |
| Body mass index, kg/m2 | 41 | 28.9 ± 5.0 | 28 | 29.0 ± 6.2 |
| Cardiovascular risk factors | ||||
| Hypertension | - | 172 (54.4%) | - | 62 (54.9%) |
| Dyslipidemia | - | 128 (40.5%) | - | 47 (41.6%) |
| Active smoking | - | 46 (14.6%) | - | 12 (10.6%) |
| Smoking history | - | 133 (42.1%) | - | 45 (39.8%) |
| Diabetes mellitus | - | 59 (18.7%) | - | 23 (20.4%) |
| Cardiovascular history | ||||
| Heart failure | - | 100 (31.6%) | - | 24 (21.2%) |
| Coronary artery disease | - | 52 (16.5%) | - | 11 (9.7%) |
| Previous direct-current shock application attempt | - | 22 (7.0%) | - | 15 (13.3%) |
| Previous transient ischemic attack or stroke | - | 19 (6.0%) | - | 6 (5.3%) |
| History of oral anticoagulation treatment | - | 158 (50.0%) | - | 33 (29.2%) |
| Peripheral vascular disease | - | 16 (5.1%) | - | 9 (8.0%) |
| Rheumatic heart disease | - | 7 (2.2%) | - | 1 (0.9%) |
| Other comorbidities | ||||
| Chronic obstructive pulmonary disease | - | 66 (20.9%) | - | 15 (13.3%) |
| Prior cancer | - | 28 (8.9%) | - | 10 (8.8%) |
| Prior bleeding | - | 11 (3.5%) | - | 3 (2.7%) |
| Venous thromboembolism | - | 10 (3.2%) | - | 1 (0.9%) |
| Impaired physical mobility | - | 8 (2.5%) | - | 6 (5.3%) |
| Clinical and biochemical variables | ||||
| NYHA functional class >I | - | 106 (33.5%) | - | 39 (34.5%) |
| NYHA functional class >II | - | 35 (11.1%) | - | 10 (8.8%) |
| NYHA functional class >III | - | 9 (2.8%) | - | 0.0 ± 0.0 |
| CHAD2DS2-VASc score | - | 2.2 ± 1.7 | - | 2.1 ± 1.6 |
| HATCH score | - | 1.6 ± 1.5 | - | 1.4 ± 1.2 |
| HASBLED score | - | 2.3 ± 1.1 | - | 2.1 ± 0.9 |
| Anemia | - | 35 (11.1%) | - | 14 (12.4%) |
| Creatinine, mg/dL | - | 1.0 ± 0.4 | 1 | 1.0 ± 0.3 |
| Glomerular filtration rate, mL/min/1.73 m2 | - | 75.6 ± 17.1 | 1 | 77.0 ± 17.0 |
| Atrial fibrillation classification | ||||
| Paroxysmal | - | 61 (19.3%) | - | 24 (21.2%) |
| Persistent | - | 250 (79.1%) | - | 89 (78.8%) |
| Long-standing persistent | - | 5 (1.6%) | - | 0 (0%) |
| Echocardiographic findings | ||||
| LV mass index, g/m2 | 42 | 102.3 ± 32.4 | 53 | 97.7 ± 28.4 |
| LVEF < 50% | - | 73 (23.1%) | - | 26 (23.0%) |
| LVEF < 40% | - | 42 (13.3%) | - | 13 (11.5%) |
| LVEF < 30% | - | 21 (6.6%) | - | 6 (5.3%) |
| Tricuspid regurgitant jet velocity, cm/sec | 149 | 257.7 ± 47.1 | 67 | 247.0 ± 50.9 |
| At least moderate probability pulmonary hypertension | - | 43 (13.6%) | - | 9 (8.0%) |
| High probability pulmonary hypertension | - | 10 (3.2%) | - | 2 (1.8%) |
| LA volume index, mL/m2 | 37 | 43.6 ± 17.4 | 44 | 44.9 ± 16.7 |
| LA volume index ≥ 35 mL/m2 | - | 182 (57.6%) | - | 51 (45.1%) |
| LA volume index ≥ 42 mL/m2 | - | 138 (43.7%) | - | 34 (30.1%) |
| LA volume index > 48 mL/m2 | - | 98 (31.0%) | - | 27 (23.9%) |
| Significant valvular heart disease | - | 68 (21.5%) | - | 21 (18.6%) |
| Mitral stenosis | - | 2 (0.6%) | - | 1 (0.9%) |
| Mitral regurgitation | - | 39 (12.3%) | - | 15 (13.3%) |
| Aortic stenosis | - | 2 (0.6%) | - | 3 (2.7%) |
| Aortic regurgitation | - | 9 (2.8%) | - | 4 (3.5%) |
| Tricuspid regurgitation | - | 23 (7.3%) | - | 5 (4.4%) |
| Mechanical prosthetic valve | - | 10 (3.2%) | - | 1 (0.9%) |
| Biological prosthetic valve | - | 9 (2.8%) | - | 5 (4.4%) |
| Oral anticoagulation | ||||
| Time under anticoagulation, days | - | 30.9 ± 23.2 | - | 27.4 ± 17.2 |
| K-vitamin antagonist | - | 102 (32.3%) | 1 | 14 (12.5%) |
| Direct oral anticoagulants | - | 214 (67.7%) | 1 | 98 (87.5%) |
| Dabigatran | - | 23 (7.3%) | - | 11 (9.7%) |
| Rivaroxaban | - | 93 (29.4%) | - | 17 (15.0%) |
| Apixaban | - | 79 (25.0%) | - | 49 (43.4%) |
| Edoxaban | - | 19 (6.0%) | - | 20 (17.7%) |
| Low-weight-molecular heparin | - | 0 (0%) | - | 2 (1.8%) |
| Antiarrhythmic drugs | ||||
| Antiarrhythmics before scheduled EC | - | 132 (41.8%) | - | 45 (39.8%) |
| Amiodarone before scheduled EC | - | 98 (31.0%) | - | 34 (30.1%) |
| Flecainide before scheduled EC | - | 30 (9.5%) | - | 11 (9.7%) |
| Dronedarone before scheduled EC | - | 4 (1.3%) | - | 0 (0%) |
| Antiarrhythmics after scheduled EC | - | 198 (62.7%) | - | 65 (57.5%) |
| Amiodarone after scheduled EC | - | 147 (46.5%) | - | 38 (33.6%) |
| Flecainide after scheduled EC | - | 45 (14.2%) | - | 27 (23.9%) |
| Dronedarone after scheduled EC | - | 6 (1.9%) | - | 0 (0%) |
| Concomitant medications | ||||
| Nonsteroidal anti-inflammatory drug | - | 5 (1.6%) | - | 0 (0%) |
| Aspirin | - | 39 (12.3%) | - | 7 (6.2%) |
| Dual antiplatelet therapy | - | 4 (1.3%) | - | 1 (0.9%) |
| Beta-blocker | - | 243 (76.9%) | - | 88 (77.9%) |
| ACE inhibitors/angiotensin II receptor blocker | - | 155 (49.1%) | - | 40 (35.4%) |
| Sacubitril-Valsartan | - | 2 (0.6%) | - | 1 (0.9%) |
| Calcium antagonist | - | 50 (15.8%) | - | 11 (9.7%) |
| Aldosterone receptor antagonist | 1 | 31 (9.8%) | - | 10 (8.8%) |
| Digoxin | - | 20 (6.3%) | - | 1 (0.9%) |
| Direct-current procedure | ||||
| Number of shocks | 76 | 1.4 ± 0.7 | 30 | 1.3 ± 0.6 |
| Applied maximal energy, J | 105 | 176.2 ± 102.6 | 42 | 165.6 ± 36.5 |
| Pathway | Predictions | Model | AUC-ROC | AUC-ROC Change | AUC-PR | AUC-PR Change | ||
|---|---|---|---|---|---|---|---|---|
| Spontaneous SR restoration | 1840 | CHA2DS2-VASc | 0.62 (0.50–0.73) | Baseline model | −7% | 0.33 (0.23–0.44) | Baseline model | −2% |
| HATCH | 0.69 (0.58–0.80) | +7% | Baseline model | 0.35 (0.25–0.45) | +2% | Baseline model | ||
| Regularized logistic regression | 0.81 (0.71–0.92) | +19% | +12% | 0.68 (0.53–0.82) | +35% | +33% | ||
| Random forest | 0.82 (0.72–0.92) | +20% | +13% | 0.67 (0.53–0.81) | +34% | +32% | ||
| Extremely randomized trees | 0.81 (0.71–0.92) | +19% | +12% | 0.68 (0.54–0.83) | +35% | +33% | ||
| Boosted trees | 0.80 (0.70–0.91) | +18% | +11% | 0.68 (0.53–0.82) | +35% | +33% | ||
| Pharmacologic cardioversion | 1320 | CHA2DS2–VASc | 0.53 (0.39–0.67) | Baseline model | −2% | 0.29 (0.20–0.37) | Baseline model | +2% |
| HATCH | 0.55 (0.43–0.67) | +2% | Baseline model | 0.27 (0.21–0.33) | −2% | Baseline model | ||
| Regularized logistic regression | 0.74 (0.60–0.87) | +21% | +19% | 0.64 (0.47–0.80) | +35% | +37% | ||
| Random forest | 0.67 (0.49–0.85) | +14% | +12% | 0.60 (0.42–0.77) | +31% | +33% | ||
| Extremely randomized trees | 0.68 (0.51–0.84) | +15% | +13% | 0.58 (0.41–0.75) | +29% | +31% | ||
| Boosted trees | 0.68 (0.53–0.84) | +15% | +13% | 0.61 (0.45–0.78) | +32% | +34% | ||
| Direct-current cardioversion | 2550 | CHA2DS2-VASc | 0.52 (0.42–0.62) | Baseline model | –6% | 0.85 (0.81–0.89) | Baseline model | −1% |
| HATCH | 0.58 (0.47–0.68) | +6% | Baseline model | 0.86 (0.82–0.90) | +1% | Baseline model | ||
| Regularized logistic regression | 0.51 (0.40–0.62) | −1% | −7% | 0.85 (0.80–0.89) | 0% | −1% | ||
| Random forest | 0.48 (0.38–0.59) | −4% | −10% | 0.85 (0.80–0.89) | 0% | −1% | ||
| Extremely randomized trees | 0.47 (0.35–0.58) | −5% | −11% | 0.84 (0.79–0.88) | −1% | −2% | ||
| Boosted trees | 0.46 (0.38–0.55) | −6% | −12% | 0.84 (0.80–0.87) | −1% | −2% | ||
| 6-month AF recurrence | 2730 | CHA2DS2-VASc | 0.54 (0.47–0.61) | Baseline model | −4% | 0.40 (0.35–0.46) | Baseline model | +2% |
| HATCH | 0.58 (0.50–0.65) | +4% | Baseline model | 0.38 (0.33–0.43) | −2% | Baseline model | ||
| Regularized logistic regression | 0.63 (0.55–0.71) | +9% | +5% | 0.55 (0.47–0.63) | +15% | +17% | ||
| Random forest | 0.67 (0.59–0.75) | +13% | +9% | 0.61 (0.52–0.70) | +21% | +23% | ||
| Extremely randomized trees | 0.68 (0.61–0.75) | +14% | +10% | 0.61 (0.52–0.70) | +21% | +23% | ||
| Boosted trees | 0.63 (0.55–0.71) | +9% | +5% | 0.57 (0.48–0.65) | +17% | +19% | ||
| 6-month rhythm control | 3160 | CHA2DS2-VASc | 0.55 (0.48–0.62) | Baseline model | −4% | 0.58 (0.52–0.63) | Baseline model | −2% |
| HATCH | 0.59 (0.52–0.69) | +4% | Baseline model | 0.60 (0.54–0.66) | +2% | Baseline model | ||
| Regularized logistic regression | 0.63 (0.57–0.70) | +8% | +4% | 0.69 (0.63–0.74) | +11% | +9% | ||
| Random forest | 0.68 (0.62–0.74) | +13% | +9% | 0.71 (0.65–0.77) | +13% | +11% | ||
| Extremely randomized trees | 0.69 (0.62–0.75) | +14% | +10% | 0.72 (0.65–0.78) | +14% | +12% | ||
| Boosted trees | 0.57 (0.51–0.64) | +2% | −2% | 0.63 (0.58–0.68) | +5% | +3% | ||
| Pathway | Predictions | Model | AUC-ROC | AUC-ROC Change | AUC-PR | AUC-PR Change | ||
|---|---|---|---|---|---|---|---|---|
| Spontaneous SR restoration | 68 | CHA2DS2-VASc | 0.57 (0.59–0.65) | Baseline model | −9% | 0.31 (0.24–0.39) | Baseline model | −7% |
| HATCH | 0.66 (0.59–0.73) | +9% | Baseline model | 0.38 (0.30–0.47) | +7% | Baseline model | ||
| Regularized logistic regression | 0.80 (0.75–0.86) | +23% | +14% | 0.52 (0.44–0.60) | +21% | +14% | ||
| Random forest | 0.72 (0.66–0.79) | +15% | +6% | 0.48 (0.39–0.56) | +17% | +10% | ||
| Extremely randomized trees | 0.79 (0.73–0.84) | +22% | +13% | 0.57 (0.49–0.64) | +26% | +19% | ||
| Boosted trees | 0.77 (0.71–0.83) | +20% | +11% | 0.56 (0.48–0.64) | +25% | +18% | ||
| Pharmacologic cardioversion | 45 | CHA2DS2-VASc | 0.45 (0.34–0.56) | Baseline model | −10% | 0.18 (0.09–0.27) | Baseline model | −5% |
| HATCH | 0.55 (0.45–0.66) | +10% | Baseline model | 0.23 (0.13–0.33) | +5% | Baseline model | ||
| Regularized logistic regression | 0.62 (0.52–0.72) | +17% | +7% | 0.43 (0.32–0.54) | +25% | +20% | ||
| Random forest | 0.66 (0.57–0.76) | +21% | +11% | 0.40 (0.29–0.51) | +22% | +17% | ||
| Extremely randomized trees | 0.71 (0.63–0.80) | +26% | +16% | 0.42 (0.31–0.53) | +24% | +19% | ||
| Boosted trees | 0.57 (0.46–0.67) | +12% | +2% | 0.30 (0.19–0.40) | +12% | +7% | ||
| Direct-current cardioversion | 87 | CHA2DS2-VASc | 0.57 (0.48–0.66) | Baseline model | +2% | 0.88 (0.81–0.94) | Baseline model | +1% |
| HATCH | 0.55 (0.46–0.65) | –2% | Baseline model | 0.87 (0.81–0.94) | –1% | Baseline model | ||
| Regularized logistic regression | 0.53 (0.44–0.62) | –4% | –2% | 0.87 (0.81–0.94) | –1% | 0% | ||
| Random forest | 0.41 (0.32–0.49) | –16% | –14% | 0.85 (0.77–0.92) | –3% | –2% | ||
| Extremely randomized trees | 0.48 (0.39–0.57) | –9% | –7% | 0.88 (0.81–0.94) | 0% | +1% | ||
| Boosted trees | 0.58 (0.48–0.67) | +1% | +3% | 0.91 (0.86–0.97) | +3% | +4% | ||
| 6-month AF recurrence | 101 | CHA2DS2-VASc | 0.52 (0.46–0.58) | Baseline model | +1% | 0.41 (0.35–0.47) | Baseline model | +1% |
| HATCH | 0.51 (0.45–0.56) | –1% | Baseline model | 0.40 (0.34–0.46) | –1% | Baseline model | ||
| Regularized logistic regression | 0.64 (0.59–0.70) | +12% | +13% | 0.49 (0.43–0.55) | +8% | +9% | ||
| Random forest | 0.61 (0.55–0.67) | +9% | +10% | 0.50 (0.44–0.56) | +9% | +10% | ||
| Extremely randomized trees | 0.62 (0.56–0.68) | +10% | +11% | 0.53 (0.47–0.59) | +12% | +13% | ||
| Boosted trees | 0.57 (0.51–0.63) | +5% | +6% | 0.48 (0.42–0.54) | +7% | +8% | ||
| 6-month rhythm control | 113 | CHA2DS2-VASc | 0.50 (0.45–0.56) | Baseline model | –1% | 0.54 (0.48–0.59) | Baseline model | 0% |
| HATCH | 0.51 (0.46–0.56) | +1% | Baseline model | 0.54 (0.49–0.60) | 0% | Baseline model | ||
| Regularized logistic regression | 0.66 (0.61–0.71) | +16% | +15% | 0.68 (0.63–0.73) | +14% | +14% | ||
| Random forest | 0.60 (0.54–0.65) | +10% | +9% | 0.62 (0.56–0.67) | +8% | +8% | ||
| Extremely randomized trees | 0.60 (0.55–0.65) | +10% | +9% | 0.61 (0.56–0.67) | +7% | +7% | ||
| Boosted trees | 0.58 (0.53–0.63) | +8% | +7% | 0.63 (0.58–0.68) | +9% | +9% | ||
| Pathway/Model | TP | FP | TN | FN | R | S | P | NPV | Net Reclassification Index |
|---|---|---|---|---|---|---|---|---|---|
| Spontaneous SR restoration | |||||||||
| Extremely randomized trees | 12 | 16 | 35 | 5 | 70.6% | 68.6% | 42.9% | 87.5% | +5.9% |
| CHA2DS2-VASc ≤ 1 | 9 | 20 | 31 | 8 | 52.9% | 60.8% | 31% | 79.5% | −19.6% |
| HATCH ≤ 0 | 9 | 10 | 41 | 8 | 52.9% | 80.4% | 47.4% | 83.7% | Baseline model |
| Pharmacologic cardioversion | |||||||||
| Extremely randomized trees | 4 | 3 | 33 | 5 | 44.4% | 91.7% | 57.1% | 86.8% | +38.8% |
| CHA2DS2-VASc ≤ 2 | 6 | 25 | 11 | 3 | 66.7% | 30.6% | 19.4% | 78.6% | Baseline model |
| HATCH ≤ 2 | 8 | 36 | 0 | 1 | 88.9% | 0% | 18.2% | 0% | −8.4% |
| Direct-current cardioversion | |||||||||
| Extremely randomized trees | 73 | 10 | 2 | 2 | 97.3% | 16.7% | 88% | 50% | −0.6% |
| CHA2DS2-VASc ≤ 0 | 10 | 3 | 9 | 65 | 13.3% | 75% | 76.9% | 12.1% | −26.3% |
| HATCH ≤ 1 | 61 | 8 | 4 | 14 | 81.3% | 33.3% | 88.4% | 22.2% | Baseline model |
| 6-month AF recurrence | |||||||||
| Extremely randomized trees | 16 | 14 | 47 | 24 | 40% | 77% | 53.3% | 66.2% | +14.8% |
| CHA2DS2-VASc >2 | 14 | 20 | 41 | 26 | 35% | 67.2% | 41.2% | 61.2% | Baseline model |
| HATCH >1 | 15 | 23 | 38 | 25 | 37.5% | 62.3% | 39.5% | 60.3% | −2.4% |
| 6-month rhythm control | |||||||||
| Extremely randomized trees | 45 | 24 | 28 | 16 | 73.8% | 53.8% | 65.2% | 63.6% | +22.1% |
| CHA2DS2-VASc ≤ 2 | 41 | 32 | 20 | 20 | 67.2% | 38.5% | 56.2% | 50% | Baseline model |
| HATCH ≤ 1 | 38 | 31 | 21 | 23 | 62.3% | 40.4% | 55.1% | 47.7% | −2.8% |
| Pathway | Variable | Score |
|---|---|---|
| Spontaneous SR restoration | Paroxysmal atrial fibrillation | 1 |
| History of oral anticoagulation treatment | 0.316 | |
| LA volume index ≥ 42 mL/m2 | 0.257 | |
| ACE inhibitors/Angiotensin II receptor blockers | 0.150 | |
| LVEF < 50% | 0.065 | |
| Pharmacologic cardioversion | Paroxysmal atrial fibrillation | 1 |
| Heart failure | 0.111 | |
| Dyslipidemia | 0.085 | |
| Glomerular filtration rate | 0.066 | |
| Peripheral vascular disease | 0.064 | |
| Direct-current cardioversion | Chronic obstructive pulmonary disease | 1 |
| Long-standing persistent AF | 0.693 | |
| Heart Failure | 0.411 | |
| Beta blockers | 0.297 | |
| LA volume index ≥ 35 mL/m2 | 0.277 | |
| 6-month AF recurrence | Spontaneous SR restoration | 1 |
| History of oral anticoagulation treatment | 0.857 | |
| Hypertension | 0.849 | |
| ACE inhibitors/angiotensin II receptor blockers | 0.827 | |
| NYHA functional class >II | 0.818 | |
| 6-month rhythm control | LA volume index ≥ 35 mL/m2 | 1 |
| Paroxysmal atrial fibrillation | 0.577 | |
| History of oral anticoagulation treatment | 0.468 | |
| LA volume index ≥ 48 mL/m2 | 0.446 | |
| Smoking history | 0.423 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez-Garcia, J.C.; Sánchez-Puente, A.; Sampedro-Gómez, J.; Vicente-Palacios, V.; Jiménez-Navarro, M.; Oterino-Manzanas, A.; Jiménez-Candil, J.; Dorado-Diaz, P.I.; Sánchez, P.L. Outcome Analysis in Elective Electrical Cardioversion of Atrial Fibrillation Patients: Development and Validation of a Machine Learning Prognostic Model. J. Clin. Med. 2022, 11, 2636. https://doi.org/10.3390/jcm11092636
Nuñez-Garcia JC, Sánchez-Puente A, Sampedro-Gómez J, Vicente-Palacios V, Jiménez-Navarro M, Oterino-Manzanas A, Jiménez-Candil J, Dorado-Diaz PI, Sánchez PL. Outcome Analysis in Elective Electrical Cardioversion of Atrial Fibrillation Patients: Development and Validation of a Machine Learning Prognostic Model. Journal of Clinical Medicine. 2022; 11(9):2636. https://doi.org/10.3390/jcm11092636
Chicago/Turabian StyleNuñez-Garcia, Jean C., Antonio Sánchez-Puente, Jesús Sampedro-Gómez, Victor Vicente-Palacios, Manuel Jiménez-Navarro, Armando Oterino-Manzanas, Javier Jiménez-Candil, P. Ignacio Dorado-Diaz, and Pedro L. Sánchez. 2022. "Outcome Analysis in Elective Electrical Cardioversion of Atrial Fibrillation Patients: Development and Validation of a Machine Learning Prognostic Model" Journal of Clinical Medicine 11, no. 9: 2636. https://doi.org/10.3390/jcm11092636







