Real-Life Early Anthropometric, Lipid and Liver Changes after Direct-Acting Antiviral Therapy in PLWHIV with HCV Co-Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
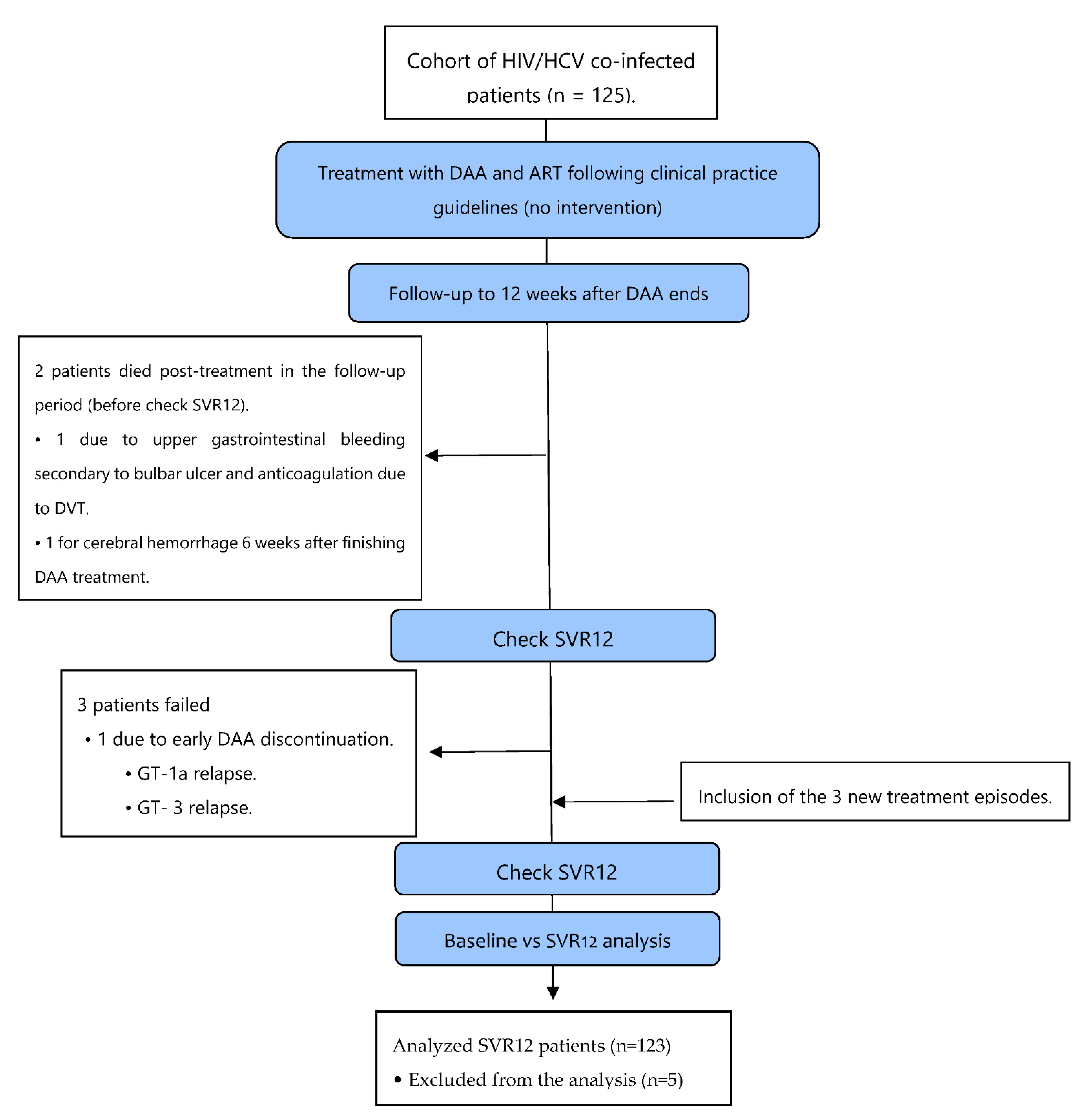
2.2. Patient Recruitment
2.3. Clinical Data Assessment
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis C. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 11 February 2022).
- Foster, A.L.; Gaisa, M.M.; Hijdra, R.M.; Turner, S.S.; Morey, T.J.; Jacobson, K.B.; Fierer, D.S. Shedding of Hepatitis C Virus into the Rectum of HIV-Infected Men Who Have Sex With Men. Clin. Infect. Dis. 2017, 64, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A.; Neukam, K. Acute Hepatitis C in the HIV-Infected Homosexual Male: A Second Wave of HIV/HCV Coinfection? Enferm. Infecc. Microbiol. Clínica 2015, 33, 1–2. [Google Scholar] [CrossRef] [PubMed]
- McCall, H.; Adams, N.; Mason, D.; Willis, J. What Is Chemsex and Why Does It Matter? BMJ 2015, 351, h5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blach, S.; Zeuzem, S.; Manns, M.; Altraif, I.; Duberg, A.-S.; Muljono, D.H.; Waked, I.; Alavian, S.M.; Lee, M.-H.; Negro, F.; et al. Global Prevalence and Genotype Distribution of Hepatitis C Virus Infection in 2015: A Modelling Study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Kirk, G.D.; Mehta, S.H.; Astemborski, J.; Galai, N.; Washington, J.; Higgins, Y.; Balagopal, A.; Thomas, D.L. HIV, Age, and the Severity of Hepatitis C Virus–Related Liver Disease. Ann. Intern. Med. 2013, 158, 658. [Google Scholar] [CrossRef] [Green Version]
- DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents—Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; OARAC: Bethesda, MD, USA, 2020. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/archive/AdultandAdolescentGL_2021_08_16.pdf (accessed on 11 February 2022).
- Burgess, S.V.; Hussaini, T.; Yoshida, E.M. Concordance of Sustained Virologic Response at Weeks 4, 12 and 24 Post-Treatment of Hepatitis c in the Era of New Oral Direct-Acting Antivirals: A Concise Review. Ann. Hepatol. 2016, 15, 154–159. [Google Scholar] [CrossRef]
- Labarga, P.; Soriano, V.; Vispo, M.E.; Pinilla, J.; Martín-Carbonero, L.; Castellares, C.; Casado, R.; Maida, I.; García-Gascó, P.; Barreiro, P. Hepatotoxicity of Antiretroviral Drugs Is Reduced after Successful Treatment of Chronic Hepatitis C in HIV-Infected Patients. J. Infect. Dis. 2007, 196, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Su, P.-S.; Su, C.-W.; Wu, S.-H.; Wei, T.-H.; Chu, C.-J.; Lin, C.-C.; Lee, S.-D.; Wang, Y.-J.; Lee, F.-Y.; Huang, Y.-H.; et al. Well Tolerability and Highly Effective Treatment Response for Hepatitis C Virus-Human Immunodeficiency Virus-Coinfected Patients Treated by All-Oral Direct-Acting Antivirals. J. Chin. Med. Assoc. 2021, 84, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Gil-Martin, Á.; Jarrin, I.; Moreno, A.; Dominguez, L.; Montes, M.; Aldámiz-Echevarría, T.; Téllez, M.J.; Santos, I.; Benitez, L.; et al. All-Oral Direct-Acting Antiviral Therapy against Hepatitis C Virus (HCV) in Human Immunodeficiency Virus/HCV-Coinfected Subjects in Real-World Practice: Madrid Coinfection Registry Findings. Hepatology 2018, 68, 32–47. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, H.; Omar, H.; Khalil, M.; Cordie, A.; Mohamed, R.; AbdAllah, M.; Abdel Maksoud, M.H.; el Garhy, N.; Ali, L.; el Serafy, M.; et al. Real-Life Experience of Treating HCV Co-Infection among HIV-Infected Population in Egypt: Single-Center Experience. Expert Rev. Anti-Infect. Ther. 2021, 20, 1–7. [Google Scholar] [CrossRef]
- Iossa, D.; Vitrone, M.; Gagliardi, M.; Falco, E.; Ragone, E.; Zampino, R.; Durante-Mangoni, E. Anthropometric Parameters and Liver Histology Influence Lipid Metabolic Changes in HCV Chronic Hepatitis on Direct-Acting Antiviral Treatment. Ann. Transl. Med. 2021, 9, 35. [Google Scholar] [CrossRef]
- Serfaty, L.; Andreani, T.; Giral, P.; Carbonell, N.; Chazouillères, O.; Poupon, R. Hepatitis C Virus Induced Hypobetalipoproteinemia: A Possible Mechanism for Steatosis in Chronic Hepatitis C. J. Hepatol. 2001, 34, 428–434. [Google Scholar] [CrossRef]
- Meissner, E.G.; Lee, Y.; Osinusi, A.; Sims, Z.; Qin, J.; Sturdevant, D.; McHutchison, J.; Subramanian, M.; Sampson, M.; Naggie, S.; et al. Effect of Sofosbuvir and Ribavirin Treatment on Peripheral and Hepatic Lipid Metabolism in Chronic Hepatitis C Virus, Genotype 1–Infected Patients. Hepatology 2015, 61, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Syed, G.H.; Amako, Y.; Siddiqui, A. Hepatitis C Virus Hijacks Host Lipid Metabolism. Trends Endocrinol. Metab. 2010, 21, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, Y. Chronic Hepatitis C Virus Infection and Lipoprotein Metabolism. World J. Gastroenterol. 2015, 21, 10299. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Domínguez, L.; Lagarde, M.; Bisbal, O.; Matarranz, M.; Rubio, R.; Pulido, F. Sustained Virological Response at Week 12 after Interferon-Free Therapy as Criterion for HCV Cure Definition: Validation in a Real-Life Cohort of HIV-Coinfected Patients. Enferm. Infecc. Microbiol. Clin. 2020, 38, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H.; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C: Final Update of the Series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- Laguno, M.; Martínez-Rebollar, M.; Casanova, M.; de Lazzari, E.; González-Cordón, A.; Torres, B.; Inciarte, A.; de la Mora, L.; Ugarte, A.; Ambrosioni, J.; et al. Long-Term Evolution in Liver Fibrosis and Immune Profile after Direct-Acting Antivirals Therapy in Hepatitis C Virus-Human Immunodeficiency Virus Co-Infected Patients. Clin. Microbiol. Infect. 2022, 28, 610.e1–610.e7. [Google Scholar] [CrossRef]
- Spaziante, M.; Taliani, G.; Marchetti, G.; Tavelli, A.; Lichtner, M.; Cingolani, A.; Cicalini, S.; Biliotti, E.; Girardi, E.; Antinori, A.; et al. Impact of HCV Eradication on Lipid Metabolism in HIV/HCV Coinfected Patients: Data from ICONA and HepaICONA Foundation Cohort Study. Viruses 2021, 13, 1402. [Google Scholar] [CrossRef]
- Malin, J.; Boesecke, C.; Schwarze-Zander, C.; Wasmuth, J.; Schlabe, S.; Trebicka, J.; Spengler, U.; Llibre, J.; Jou, T.; Vasylyev, M.; et al. Liver Stiffness Regression after Successful Hepatitis C Treatment Is Independent of HIV Coinfection. HIV Med. 2019, 20, 230–236. [Google Scholar] [CrossRef]
- Fabbri, G.; Mastrorosa, I.; Vergori, A.; Timelli, L.; Lorenzini, P.; Zaccarelli, M.; Cicalini, S.; Bellagamba, R.; Plazzi, M.; Mazzotta, V.; et al. Liver Stiffness Reduction and Serum Fibrosis Score Improvement in HIV/Hepatitis C Virus-Coinfected Patients Treated with Direct-Acting Antivirals. HIV Med. 2018, 19, 578–584. [Google Scholar] [CrossRef]
- Kronfli, N.; Young, J.; Wang, S.; Cox, J.; Walmsley, S.; Hull, M.; Cooper, C.; Martel-Laferriere, V.; Wong, A.; Pick, N.; et al. Liver Fibrosis in Human Immunodeficiency Virus (HIV)-Hepatitis C Virus (HCV) Coinfection Before and After Sustained Virologic Response: What Is the Best Noninvasive Marker for Monitoring Regression? Clin. Infect. Dis. 2021, 73, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, P.; Martin-Carbonero, L.; Nunez, M.; Rivas, P.; Morente, A.; Simarro, N.; Labarga, P.; Gonzalez-Lahoz, J.; Soriano, V. Predictors of Liver Fibrosis in HIV-Infected Patients with Chronic Hepatitis C Virus (HCV) Infection: Assessment Using Transient Elastometry and the Role of HCV Genotype 3. Clin. Infect. Dis. 2006, 42, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Macías, J.; Mancebo, M.; Márquez, M.; Merino, D.; Téllez, F.; Rivero, A.; von Wichmann, M.A.; López-Cortés, L.F.; Merchante, N.; Santos, J.; et al. Low Risk of Liver Decompensation among Human Immunodeficiency Virus/Hepatitis C Virus-Coinfected Patients with Mild Fibrosis in the Short Term. Hepatology 2015, 61, 1503–1511. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 11 February 2022).
- Brunner, E.; Bathke, A.C.; Konietschke, F. Rank and Pseudo-Rank Procedures for Independent Observations in Factorial Designs; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-030-02912-8. [Google Scholar]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.4.0. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 30 March 2022).
- Sidorkiewicz, M. Hepatitis C Virus Uses Host Lipids to Its Own Advantage. Metabolites 2021, 11, 273. [Google Scholar] [CrossRef]
- Gitto, S.; Cicero, A.F.G.; Loggi, E.; Giovannini, M.; Conti, F.; Grandini, E.; Guarneri, V.; Scuteri, A.; Vitale, G.; Cursaro, C.; et al. Worsening of Serum Lipid Profile after Direct Acting Antiviral Treatment. Ann. Hepatol. 2018, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Adinolfi, L.E.; Fracanzani, A.L.; Rini, F.; Caldarella, R.; Calvaruso, V.; Cammà, C.; Ciaccio, M.; di Marco, V.; Grimaudo, S.; et al. Hepatitis C Virus Eradication by Direct-Acting Antiviral Agents Improves Carotid Atherosclerosis in Patients with Severe Liver Fibrosis. J. Hepatol. 2018, 69, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Nahon, P.; Layese, R.; Blaise, L.; Desbois, A.C.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; et al. Prognostic Value of Viral Eradication for Major Adverse Cardiovascular Events in Hepatitis C Cirrhotic Patients. Am. Heart J. 2018, 198, 4–17. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hung, C.-H.; Lu, S.-N.; Chen, C.-H.; Lee, C.-M.; Hu, T.-H.; Wang, J.-H. Liver Stiffness Measurement as an Alternative to Fibrotic Stage in Risk Assessment of Hepatocellular Carcinoma Incidence for Chronic Hepatitis C Patients. Liver Int. 2013, 33, 756–761. [Google Scholar] [CrossRef]
- Pietsch, V.; Deterding, K.; Attia, D.; Ringe, K.I.; Heidrich, B.; Cornberg, M.; Gebel, M.; Manns, M.P.; Wedemeyer, H.; Potthoff, A. Long-term Changes in Liver Elasticity in Hepatitis C Virus-infected Patients with Sustained Virologic Response after Treatment with Direct-acting Antivirals. United Eur. Gastroenterol. J. 2018, 6, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.A.; Rao, M.N.; Grunfeld, C. The Effects of HIV Protease Inhibitors on Carbohydrate and Lipid Metabolism. Curr. HIV/AIDS Rep. 2005, 2, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L. Insulin Resistance and Liver Steatosis in Chronic Hepatitis C Infection Genotype 3. World J. Gastroenterol. 2014, 20, 15233. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Maida, M.; Macaluso, F.S.; di Marco, V.; Cammà, C.; Cabibi, D.; Craxì, A. The Severity of Steatosis Influences Liver Stiffness Measurement in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2015, 62, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Goñi Esarte, S.; Juanbeltz, R.; Zozaya, J.M.; Úriz, J.I.; Castilla, J.; Herrero, J.I. Modification of Liver Fibrosis, Glucose and Lipid Profile after Hepatitis C Virus Clearance with Direct-Acting Antiviral Agents. Gastroenterol. Hepatol. 2020, 43, 248–255. [Google Scholar] [CrossRef] [PubMed]



| n = 123 | Mean | SD |
|---|---|---|
| Age (years) | 51.3 | ±6.7 |
| Sex | n | % |
| Male | 86 | 69.9 |
| Female | 37 | 30.1 |
| HCV/HIV transmission route | n | % |
| IDU | 107 | 87.0 |
| HTX | 10 | 8.1 |
| MSM | 4 | 3.3 |
| Other (blood transfusion) | 2 | 1.6 |
| Opioid substitution therapy (OST) | n | % |
| no | 99 | 80.5 |
| yes | 24 | 19.5 |
| Baseline Child-Turcotte-Pugh (CTP) score | n | % |
| A | 116 | 94.3 |
| B | 7 | 5.7 |
| C | 0 | 0 |
| HCV Genotype (GT) | n | % |
| GT-1a | 34 | 27.6 |
| GT-1b | 12 | 9.8 |
| GT-1-other | 34 | 27.6 |
| GT-2 | 1 | 0.8 |
| GT-3 | 21 | 17.1 |
| GT-4 | 21 | 17.1 |
| DAA combination | n | % |
| SOF/DCV ± RBV | 14 | 11.4 |
| SOF/SMV ± RBV | 21 | 17.1 |
| 3D ± RBV | 25 | 20.3 |
| 2D ± RBV | 3 | 2.4 |
| SOF/LEDV ± RBV | 34 | 27.6 |
| GZP/ELB | 5 | 4.1 |
| SOF/VEL | 15 | 12.2 |
| G/P | 6 | 4.9 |
| ART combination | n | % |
| ABC/3TC + ATV | 2 | 1.6 |
| ABC/3TC + DTG | 20 | 16.3 |
| ABC/3TC + RAL | 2 | 1.6 |
| ATV + RAL | 1 | 0.8 |
| TDF/FTC + ATV | 3 | 2.4 |
| DRV | 2 | 1.6 |
| DRV+3TC, INI or MVC | 7 | 5.7 |
| TDF/FTC/RPV | 29 | 23.6 |
| TDF/FTC + RAL | 16 | 13.0 |
| EVG/c/FTC/TDF | 5 | 4.1 |
| DRV/b+FTC/TDF | 3 | 2.4 |
| FTC/TDF + DTG | 5 | 4.1 |
| FTC/TDF/ EFV | 3 | 2.4 |
| Other | 25 | 20.3 |
| NS3/NS4A PI | NS5A, NS5B Inh. | Total | |
|---|---|---|---|
| PI | 6 (4.9) | 13 (10.6) | 19 (15.4) |
| INI | 28 (22.8) | 26 (21.1) | 54 (43.9) |
| NNRTI | 23 (18.7) | 12 (9.8) | 35 (28.5) |
| Free | 3 (2.4) | 12 (9.8) | 15 (12.2) |
| Total | 60 (48.8) | 63 (51.2) | 123 (100.0) |
| Baseline Variables | Mean | SD | SVR12 Variables | Mean | SD | p-Value |
|---|---|---|---|---|---|---|
| Baseline weight | 69.2 | 14.7 | SVR12 weight | 70.4 | 14.3 | p = 0.317 |
| Baseline BMI | 23.9 | 4.0 | SVR12 BMI | 24.7 | 5.6 | p = 0.165 |
| Baseline cholesterol | 161.3 | 41.0 | SVR12 cholesterol | 183.3 | 41.6 | p < 0.01 |
| Baseline LDLc | 84.6 | 35.0 | SVR12 LDLc | 108.6 | 35.1 | p < 0.01 |
| Baseline ALT | 58.2 | 34.0 | SVR12 ALT | 22.0 | 16.0 | p < 0.01 |
| Baseline albumin | 4.2 | 0.4 | SVR12 albumin | 4.3 | 0.3 | p < 0.01 |
| Baseline bilirubin | 0.8 | 0.6 | SVR12 bilirubin | 0.6 | 0.5 | p < 0.05 |
| Baseline CTP score | 5.2 | 0.6 | SVR12 CTP score | 5.1 | 0.3 | p < 0.01 |
| Baseline TE | 13.7 | 13.3 | SVR12 TE | 11.8 | 12.1 | p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferra-Murcia, S.; Collado-Romacho, A.R.; Nievas-Soriano, B.J.; Reche-Lorite, F.; Parrón-Carreño, T. Real-Life Early Anthropometric, Lipid and Liver Changes after Direct-Acting Antiviral Therapy in PLWHIV with HCV Co-Infection. J. Clin. Med. 2022, 11, 2639. https://doi.org/10.3390/jcm11092639
Ferra-Murcia S, Collado-Romacho AR, Nievas-Soriano BJ, Reche-Lorite F, Parrón-Carreño T. Real-Life Early Anthropometric, Lipid and Liver Changes after Direct-Acting Antiviral Therapy in PLWHIV with HCV Co-Infection. Journal of Clinical Medicine. 2022; 11(9):2639. https://doi.org/10.3390/jcm11092639
Chicago/Turabian StyleFerra-Murcia, Sergio, Antonio Ramón Collado-Romacho, Bruno José Nievas-Soriano, Fernando Reche-Lorite, and Tesifón Parrón-Carreño. 2022. "Real-Life Early Anthropometric, Lipid and Liver Changes after Direct-Acting Antiviral Therapy in PLWHIV with HCV Co-Infection" Journal of Clinical Medicine 11, no. 9: 2639. https://doi.org/10.3390/jcm11092639







