Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction
Abstract
:1. Introduction
2. Materials and Methods
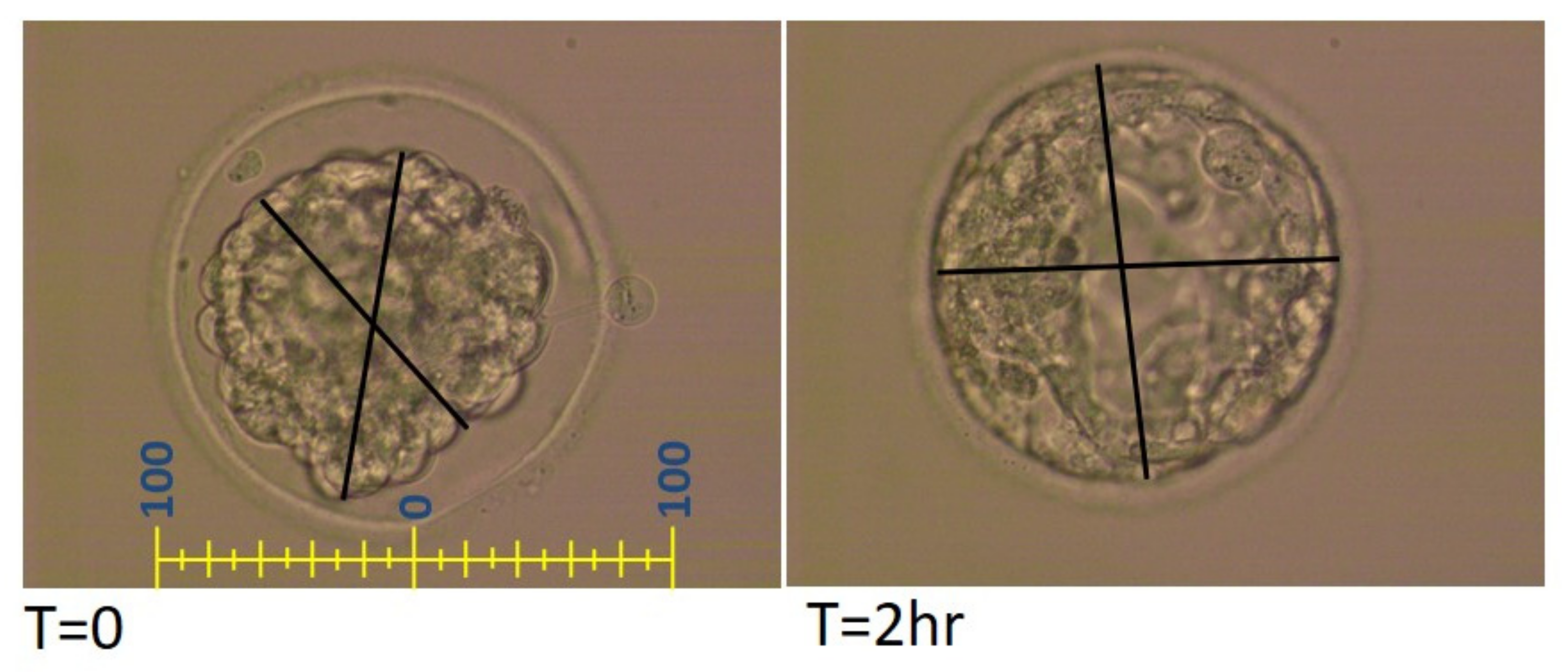
2.1. Embryo Vitrification Protocol and Embryonic Data
2.2. Endometrial Preparation Protocol
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study Group * (n) | Infertility Treatment Indication n (%) | |||||
|---|---|---|---|---|---|---|
| Male | Ovulatory Dysfunction | Mechanical | Unexplained | Uterine | Other | |
| 1 (37) | 8 (21.6) | 6 (16.2) | 6 (16.2) | 8 (21.6) | 1 (2.7) | 8 (21.6) |
| 2 (37) | 11 (29.7) | 5 (13.5) | 10 (27.0) | 5 (13.5) | 0 (0.0) | 6 (16.2) |
| 3 (41) | 14 (34.1) | 4 (9.8) | 5 (12.2) | 7 (17.1) | 2 (4.9) | 9 (22.0) |
References
- Reh, A.; Fino, E.; Krey, L.; Berkeley, A.; Noyes, N.; Grifo, J. Optimizing embryo selection with day 5 transfer. Fertil. Steril. 2010, 93, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Haikin Herzberger, E.; Ghetler, Y.; Tamir Yaniv, R.; Berkovitz, A.; Gonen, O.; Cohen, I.; Shulman, A.; Wiser, A. Time lapse microscopy is useful for elective single-embryo transfer. Gynecol. Endocrinol. 2016, 32, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, M.; Maignien, C.; Pocate-Cheriet, K.; Plu Bureau, G.; Marcellin, L.; Patrat, C.; Chapron, C.; Santulli, P. The freeze-all strategy after IVF: Which indications? Reprod. Biomed. Online 2021, 42, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.K.; Schoolcraft, W.B. Culture and transfer of human blastocysts. Curr. Opin. Obstet. Gynecol. 1999, 11, 307–311. [Google Scholar] [CrossRef]
- Gardner, D.K.; Balaban, B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘omics’: Is looking good still important? Mol. Hum. Reprod. 2016, 22, 704–718. [Google Scholar] [CrossRef]
- Shu, Y.; Watt, J.; Gebhardt, J.; Dasig, J.; Appling, J.; Behr, B. The value of fast blastocoele re-expansion in the selection of a viable thawed blastocyst for transfer. Fertil. Steril. 2009, 91, 401–406. [Google Scholar] [CrossRef]
- Desai, N.; Goldfarb, J. Examination of frozen cycles with replacement of a single thawed blastocyst. Reprod. Biomed. Online 2005, 11, 349–354. [Google Scholar] [CrossRef]
- Hiraoka, K.; Hiraoka, K.; Kinutani, M.; Kinutani, K. Blastocoele collapse by micropipetting prior to vitrification gives excellent survival and pregnancy outcomes for human day 5 and 6 expanded blastocysts. Hum. Reprod. 2004, 19, 2884–2888. [Google Scholar] [CrossRef] [Green Version]
- Violette, M.I.; Madan, P.; Watson, A.J. Na+/K+ -ATPase regulates tight junction formation and function during mouse preimplantation development. Dev. Biol. 2006, 289, 406–419. [Google Scholar] [CrossRef] [Green Version]
- Watson, A.J.; Barcroft, L.C. Regulation of blastocyst formation. Front. Biosci. 2001, 6, D708–D730. [Google Scholar] [CrossRef] [Green Version]
- Ebner, T.; Vanderzwalmen, P.; Shebl, O.; Urdl, W.; Moser, M.; Zech, N.H.; Tews, G. Morphology of vitrified/warmed day-5 embryos predicts rates of implantation, pregnancy and live birth. Reprod. Biomed. Online 2009, 19, 72–78. [Google Scholar] [CrossRef]
- Maezawa, T.; Yamanaka, M.; Hashimoto, S.; Amo, A.; Ohgaki, A.; Nakaoka, Y.; Fukuda, A.; Ikeda, T.; Inoue, M.; Morimoto, Y. Possible selection of viable human blastocysts after vitrification by monitoring morphological changes. J. Assist. Reprod. Genet. 2014, 31, 1099–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coello, A.; Meseguer, M.; Galan, A.; Alegre, L.; Remohi, J.; Cobo, A. Analysis of the morphological dynamics of blastocysts after vitrification/warming: Defining new predictive variables of implantation. Fertil. Steril. 2017, 108, 659–666.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacic, B.; Taborin, M.; Vlaisavljevic, V. Artificial blastocoel collapse of human blastocysts before vitrification and its effect on re-expansion after warming—A prospective observational study using time-lapse microscopy. Reprod. Biomed. Online 2018, 36, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Giunco, H.; Connerney, M.; Boylan, C.; Koelper, N.; Mersereau, J.; Berger, D.S. Embryo re-expansion does not affect clinical pregnancy rates in frozen embryo transfer cycles: A retrospective study. J. Assist. Reprod. Genet. 2021, 38, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Iwasawa, T.; Takahashi, K.; Goto, M.; Anzai, M.; Shirasawa, H.; Sato, W.; Kumazawa, Y.; Terada, Y. Human frozen-thawed blastocyst morphokinetics observed using time-lapse cinematography reflects the number of trophectoderm cells. PLoS ONE 2019, 14, e0210992. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, A.; Westin, C.; Wikland, M.; Hardarson, T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum. Reprod. 2013, 28, 1199–1209. [Google Scholar] [CrossRef]
- Du, Q.Y.; Wang, E.Y.; Huang, Y.; Guo, X.Y.; Xiong, Y.J.; Yu, Y.P.; Yao, G.D.; Shi, S.L.; Sun, Y.P. Blastocoele expansion degree predicts live birth after single blastocyst transfer for fresh and vitrified/warmed single blastocyst transfer cycles. Fertil. Steril. 2016, 105, 910–919.e911. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Yan, Y.; Huang, X.; Sun, L.; Li, Y. Blastocoele expansion: An important parameter for predicting clinical success pregnancy after frozen-warmed blastocysts transfer. Reprod. Biol. Endocrinol. 2019, 17, 15. [Google Scholar] [CrossRef] [Green Version]
- Cimadomo, D.; Capalbo, A.; Levi-Setti, P.E.; Soscia, D.; Orlando, G.; Albani, E.; Parini, V.; Stoppa, M.; Dovere, L.; Tacconi, L.; et al. Associations of blastocyst features, trophectoderm biopsy and other laboratory practice with post-warming behavior and implantation. Hum. Reprod. 2018, 33, 1992–2001. [Google Scholar] [CrossRef]
- Petersen, B.M.; Boel, M.; Montag, M.; Gardner, D.K. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on day. Hum. Reprod. 2016, 31, 2231–2244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reignier, A.; Girard, J.M.; Lammers, J.; Chtourou, S.; Lefebvre, T.; Barriere, P.; Freour, T. Performance of Day 5 KIDScore™ morphokinetic prediction models of implantation and live birth after single blastocyst transfer. J. Assist. Reprod. Genet. 2019, 36, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Ferrick, L.; Lee, Y.S.L.; Gardner, D.K. Metabolic activity of human blastocysts correlates with their morphokinetics, morphological grade, kidscore and artificial intelligence ranking. Hum. Reprod. 2020, 35, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Feng, G.; Shu, J.; Zhang, B.; Zhou, H.; Gan, X.; Wang, C.; Chen, H. Blastocoele re-expansion time in vitrified-warmed cycles is a strong predictor of clinical pregnancy outcome. J. Obstet. Gynaecol. Res. 2017, 43, 689–695. [Google Scholar] [CrossRef]
- Ebner, T.; Oppelt, P.; Radler, E.; Allerstorfer, C.; Habelsberger, A.; Mayer, R.B.; Shebl, O. Morphokinetics of vitrified and warmed blastocysts predicts implantation potential. J. Assist. Reprod. Genet. 2017, 34, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, K.; Berkkanoglu, M.; Bulut, H.; Donmez, L.; Isikli, A.; Coetzee, K. Blastocyst age, expansion, trophectoderm morphology, and number cryopreserved are variables predicting clinical implantation in single blastocyst frozen embryo transfers in freeze-only-IVF. J. Assist. Reprod. Genet. 2021, 38, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, E.; Andershed, A.N. Morphology vs morphokinetics: A retrospective comparison of inter-observer and intra-observer agreement between embryologists on blastocysts with known implantation outcome. JBRA Assist. Reprod. 2018, 22, 228–237. [Google Scholar] [CrossRef]


| Study Group * (n) | Pre-Freeze Gardner’s Expansion Score | KID Score Day 3 | KID Score Day 5 | |||
|---|---|---|---|---|---|---|
| 2 n (%) | 3 n (%) | 4 n (%) | 5 n (%) | |||
| 1 (37) | 5 (13.5) | 7 (18.9) | 24 (64.9) | 1 (2.7) | 4.27 ± 1.26 | 6.92 ± 1.35 |
| 2 (37) | 8 (21.6) | 10 (27.0) | 17 (45.9) | 2 (5.4) | 4.27 ± 1.17 | 6.48 ± 1.79 |
| 3 (41) | 2 (4.9) | 8 (19.5) | 26 (63.4) | 5 (12.2) | 4.51 ± 0.87 | 6.94 ± 1.62 |
| p-value | 0.17 | 0.54 | 0.37 | |||
| Study Group* (n) | Age (at Oocyte Retrieval) | N Oocytes Aspirated | Endometrial Lining, mm | Fertilization Method (%) | Clinical Pregnancy | |
|---|---|---|---|---|---|---|
| IVF | ICSI | |||||
| 1 (37) | 33.65 ± 6.01 | 12.38 ± 6.08 | 8.6 ± 2.1 | 54.1 | 45.9 | 7 (18.9%) |
| 2 (37) | 32.89 ± 5.04 | 13.89 ± 7.12 | 9.4 ± 1.9 | 67.6 | 32.4 | 10 (27.0%) |
| 3 (41) | 33.63 ± 6.07 | 14.22 ± 8.31 | 9.1 ± 1.5 | 57.9 | 42.1 | 21 (51.2%) |
| p-value | 0.88 | 0.50 | 0.16 | 0.49 | 0.007 | |
| Study Group * | Age < 35 Years | Age ≥ 35 Years |
|---|---|---|
| Clinical Pregnancy n (%) | Clinical Pregnancy n (%) | |
| Group 1 | 20 (25) | 17 (11.8) |
| Group 2 | 23 (34.8) | 14 (14.3) |
| Group 3 | 21 (52.4) | 20 (50.0) |
| p-value | 0.07 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hershko-Klement, A.; Raviv, S.; Nemerovsky, L.; Rom, T.; Itskovich, A.; Bakhshi, D.; Shulman, A.; Ghetler, Y. Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction. J. Clin. Med. 2022, 11, 2673. https://doi.org/10.3390/jcm11092673
Hershko-Klement A, Raviv S, Nemerovsky L, Rom T, Itskovich A, Bakhshi D, Shulman A, Ghetler Y. Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction. Journal of Clinical Medicine. 2022; 11(9):2673. https://doi.org/10.3390/jcm11092673
Chicago/Turabian StyleHershko-Klement, Anat, Shaul Raviv, Luba Nemerovsky, Tal Rom, Ayelet Itskovich, Danit Bakhshi, Adrian Shulman, and Yehudith Ghetler. 2022. "Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction" Journal of Clinical Medicine 11, no. 9: 2673. https://doi.org/10.3390/jcm11092673
APA StyleHershko-Klement, A., Raviv, S., Nemerovsky, L., Rom, T., Itskovich, A., Bakhshi, D., Shulman, A., & Ghetler, Y. (2022). Standardization of Post-Vitrification Human Blastocyst Expansion as a Tool for Implantation Prediction. Journal of Clinical Medicine, 11(9), 2673. https://doi.org/10.3390/jcm11092673






