3.1. Current Classification Systems Used in the Description of Laryngeal Mucosal Vascularization
Classification according to Ni et al. (2011) [2]
The first available classification system was described by Ni et al. This classification is widely used by many ENT clinicians and was originally designed to be used with the NBI technology. This classification can be used with other technologies such as IMAGE 1S (Karl Storz) with similar results [
3].
This system classifies endoscopy findings according to the changes of intrapapillary capillary loops (IPCLs) into five categories [
2]. Lesions in category I–IV are considered to be benign (
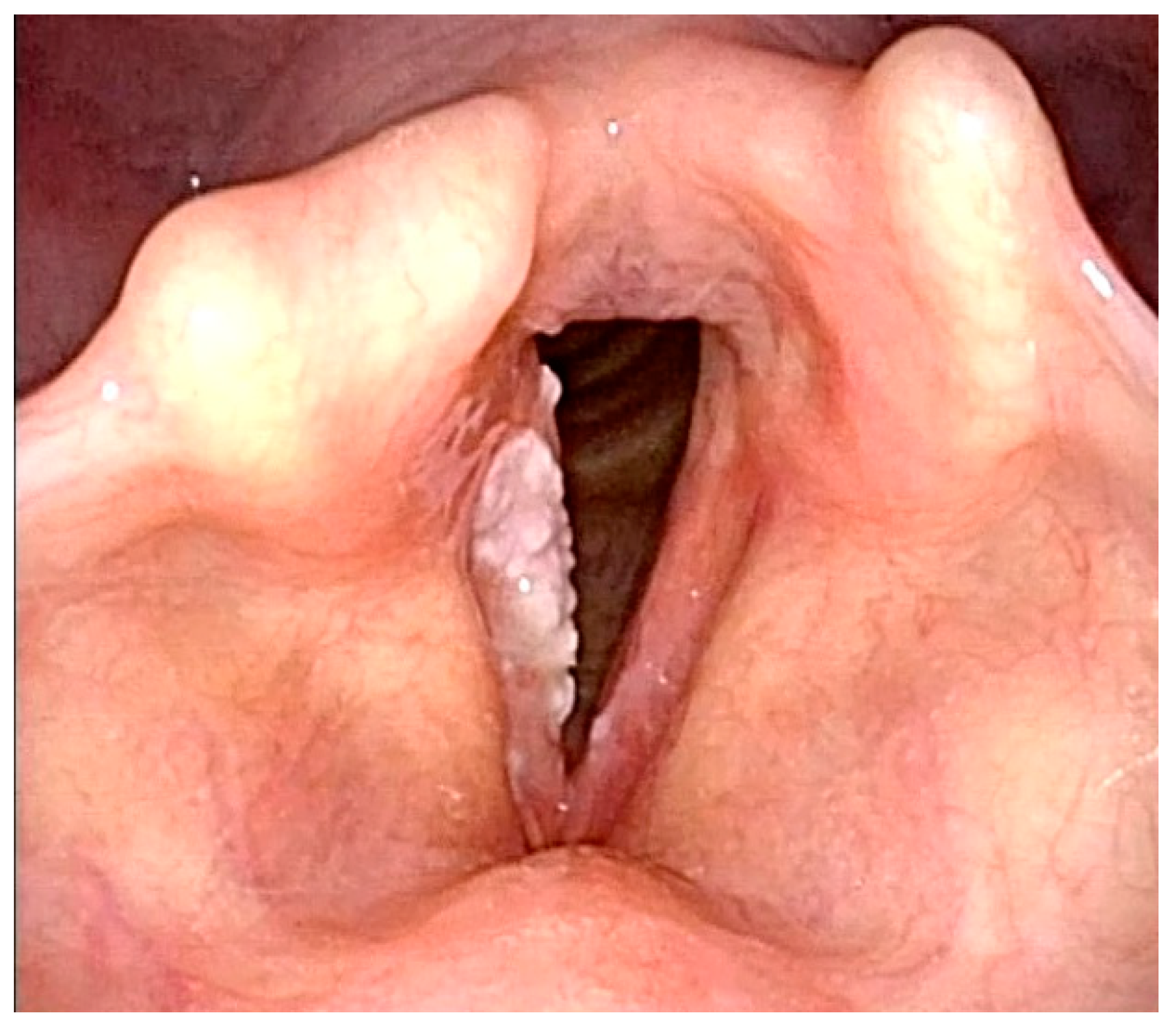
Figure 2) [
2]. Category V lesions are considered malignant lesions and are divided into three subcategories: Va, Vb, and Vc (
Figure 3) [
2]. Ni et al. reported a cancer lesion detection sensitivity of 88.9% and a specificity of 93.2% [
2]. Many subsequent studies and meta-analyses have confirmed the diagnostic value of this classification system [
4,
5,
6,
8]. The overview of this classification can be seen in
Table 1.
Classification proposed by the European Laryngological Society (2016) [9]
This classification system was published by Arens et al. in 2016 [
9]. It separates lesions according to their vascular architecture into two categories: longitudinal or perpendicular [
9]. Longitudinal vascularization passes parallel to the mucosa and is associated with benign lesions (
Figure 2) [
9]. Perpendicular vascularization runs upright in the mucosa and is interpreted as suspicious (
Figure 3) [
9]. Perpendicular vascularization is specific for papilloma, high-grade dysplastic lesions, carcinoma in situ, and invasive carcinoma [
9].
The high diagnostic yield of the classification has been confirmed by other authors [
10,
11]. Šifrer et al. studied 104 patients and described perpendicular vascularization in only 9.3% of benign lesions [
10]. Histologically verified papillomatosis and malignant lesions showed perpendicular vascularization in 96.2% of subjects [
10].
Table 2 overviews this classification.
Classification according to Puxxedu et al. (2016) [12]
This classification system was designed exclusively for enhanced contact endoscopy [
12]. This technology combines enhanced endoscopy imaging (such as NBI or IMAGE 1S) and a special magnifying endoscope with a magnification up to 150x. Magnification of the observed tissue allows precise description of the changes in vascular microarchitecture. This technology is suitable only for use under general anesthesia due to the lack of flexible magnifying endoscopes.
The classification separates mucosal findings into types 0-IV, where 0 means normal mucosa, type I is interpreted as an inflammatory lesion, and type II is hyperplasia or papillomatosis if the capillary loop is encased by mucosal papilloma (
Figure 4) [
12]. Type III implies mild to moderate dysplasia [
12]. Type IV should be interpreted as either high-grade dysplasia, carcinoma in situ, or invasive carcinoma (
Figure 5) [
12]. The results provided by Puxxedu et al. are promising and suggest that the sensitivity and specificity of the method in differentiating normal tissue vs. histological alterations is 100% [
12]. The same sensitivity and specificity were achieved for differentiation of normal and inflammatory lesions vs. invasive carcinoma [
12]. To differentiate between normal tissue and hyperplasia vs. dysplasia and invasive carcinoma, Puxxedu found a sensitivity and specificity of 97.6% [
12]. We could not find other studies that confirm or contradict the results of this study. The overview of this classification can be seen in
Table 3.
3.2. Classification Systems Used in Examination of Leukoplakia
Leukoplakia represents a specific diagnostic and therapeutic problem, and thus particular classification systems for describing this distinct pathology have been developed. Leukoplakia is a descriptive term used to name white patch-like lesions present on the mucosa [
13]. Leukoplakia of the larynx can be mostly observed on the vocal cords. It is caused by extensive irritation of the laryngeal mucosa by alcohol, smoking, voice overuse, or laryngopharyngeal reflux [
13]. The irritation causes formation of a keratin layer. Another cause of laryngeal leukoplakia is the use of inhalation corticosteroids [
14]. Even though the term leukoplakia has been used for decades, it is descriptive but not clinically useful because it does not provide the risk stratification of the lesion. Histologically, the lesions can vary from hyperkeratosis to invasive cancer [
15]. Therefore, early identification of the character of the lesion is crucial for a good prognosis and outcome of the treatment.
The pre-histological diagnosis of leukoplakia is difficult. Even though as much as 50% of the samples return as non-dysplastic lesions from the histopathology exam, a diagnosis of invasive cancer is made in 6–22% of the samples [
16,
17,
18]. Therefore, lesion biopsy under general anesthesia remains common practice.
A few classification systems have been developed, and some of them can be used with white light endoscopy while others require enhanced imagining such as NBI. However, the proper NBI examination is difficult and sometimes impossible due to the “umbrella effect” [
13]. This phenomenon causes the reflection of the light emitted from the light source. Therefore, the emitted light does not reach the IPCLs in the mucosa, which limits examination [
13]. Nevertheless, vascularization around the leukoplakia can be observed and can yield important information about the observed lesion. It can be classified according to one of the available classifications. According to multiple authors, changes in the vascular architecture surrounding the primary lesion yield valuable information about the features of the lesion [
13,
19]. Stanikova et al. reported that perpendicular vascularization surrounding the leukoplakia was associated with malignant lesions (carcinoma in situ or invasive carcinoma). This was histologically confirmed in 84.6% of cases [
19]. Leukoplakia surrounded by longitudinal type of vascularization was histologically benign (hyperkeratosis or low-grade dysplasia) in 83.8% of cases [
19]. The authors also suggest that leukoplakia with favorable surrounding findings in NBI endoscopy can be followed conservatively without surgical intervention [
19].
Clinical scoring of leukoplakia according to Young et al. (2014) [20]
Young et al. proposed a scoring system of vocal cord leukoplakia based on their macroscopical appearance during white light endoscopy [
20]. His classification stratifies leukoplakia by seven macroscopical features: color, texture, size, hyperemia, thickness, symmetry, and oedema [
20]. Color, texture, size, and hyperemia significantly correlated with final histopathology and therefore were proposed as one of the possible ways to select high-risk patients. Interrater reliability of the classification was found to be from 68 to 79% [
20]. Lesions with lower scores had very high probability to be less aggressive and should be managed conservatively [
20]. Unfortunately, the study did not provide an optimal cut-off point that could be used to differentiate between low-risk and high-risk lesions. The overview of this classification can be seen in
Table 4.
Clinical scoring of leukoplakia by Fang et al. (2016) [21]
Fang et al. continued the previous research and removed one of the criteria (edema) from the Young et al. scoring system. Therefore, a six-tier system was established. Observed morphological features of the leukoplakia were useful in differentiation between malignant and benign lesions [
21]. The morphological features were color, texture, size, hyperemia, thickness, and symmetry. The scoring system achieved good sensitivity (80.4%) and specificity (81.5%) with good interrater reliability [
21]. Unfortunately, this study did not provide a specific cut-off that could be used to differentiate between benign and malignant lesion. Rather, the authors advised clinicians to set the cut-off point for each institution individually [
21]. The overview of this classification can be seen in
Table 5.
Laryngoscopic classification of vocal cord leukoplakia by Zhang et al. (2017) [17]
Zhang et al. tried to simplify classifications mentioned before by stratifying vocal cord leukoplakia into three subtypes: type I—flat and smooth; type II—bulged and smooth; and type III—bulged and rough [
17]. According to the results, type I is mostly histologically interpreted as keratinization or hyperplasia without dysplastic changes (
Figure 6) [
17]. In type II, the dominant histology was mild to moderate dysplasia [
17]. Type III presented the highest incidence of cancerous lesion (carcinoma in situ or invasive carcinoma), while incidence of non-cancerous lesions (keratosis or hyperplasia) was the lowest from all types (
Figure 7) [
17]. The authors further proposed conservative treatment in type I leukoplakia and surgical resection in type III leukoplakia [
17]. Type II remains a grey zone, but the authors stated that leukoplakia in this stage is irreversible and may contain moderate or severe dysplasia [
17]. The overview of this classification can be seen in
Table 6.
A similar classification system was also proposed by Chen et al. [
22]. This classification also used a three-tier classification system with similar categories: flat and smooth, elevated and smooth, and rough leukoplakia [
22]. This study included 375 patients treated for vocal cord leukoplakia and confirmed that the morphology of the leukoplakia correlates significantly with the final histology examination [
22].
Narrow-Band Imaging endoscopic classification of laryngeal leukoplakia according to Ni et al. (2019) [23]
Attempts to introduce advanced endoscopic methods used the modified Ni et al. classification. This classification stratifies leukoplakia into six types. Types 1–3 indicate benign leukoplakia (
Figure 8) and types 4–6 suggest possibility of malignancy (
Figure 9) [
23]. The accuracy of the classification in judging the pathological nature of the leukoplakia was 90.8% [
23]. The overview of this classification can be seen in
Table 7.
An examination that can provide additional information about the lesion is laryngeal videostroboscopy. According to Rzepakowska et al., non-invasive leukoplakia (parakeratosis, low-grade dysplasia, etc.) tends to preserve the mucosal wave of the vocal cord [
24]. On the other hand, the mucosal wave tends to diminish in the case of an invasive form of leukoplakia (high-grade dysplasia, invasive carcinoma, etc.) [
24]. As stated by El-Demerdash, the overall accuracy of laryngeal videostroboscopy versus histology was 95% [
25]. Those results were further confirmed by studies by other authors [
25,
26,
27].