Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects
Abstract
1. Introduction
2. Physiology and Function of the Peritoneum
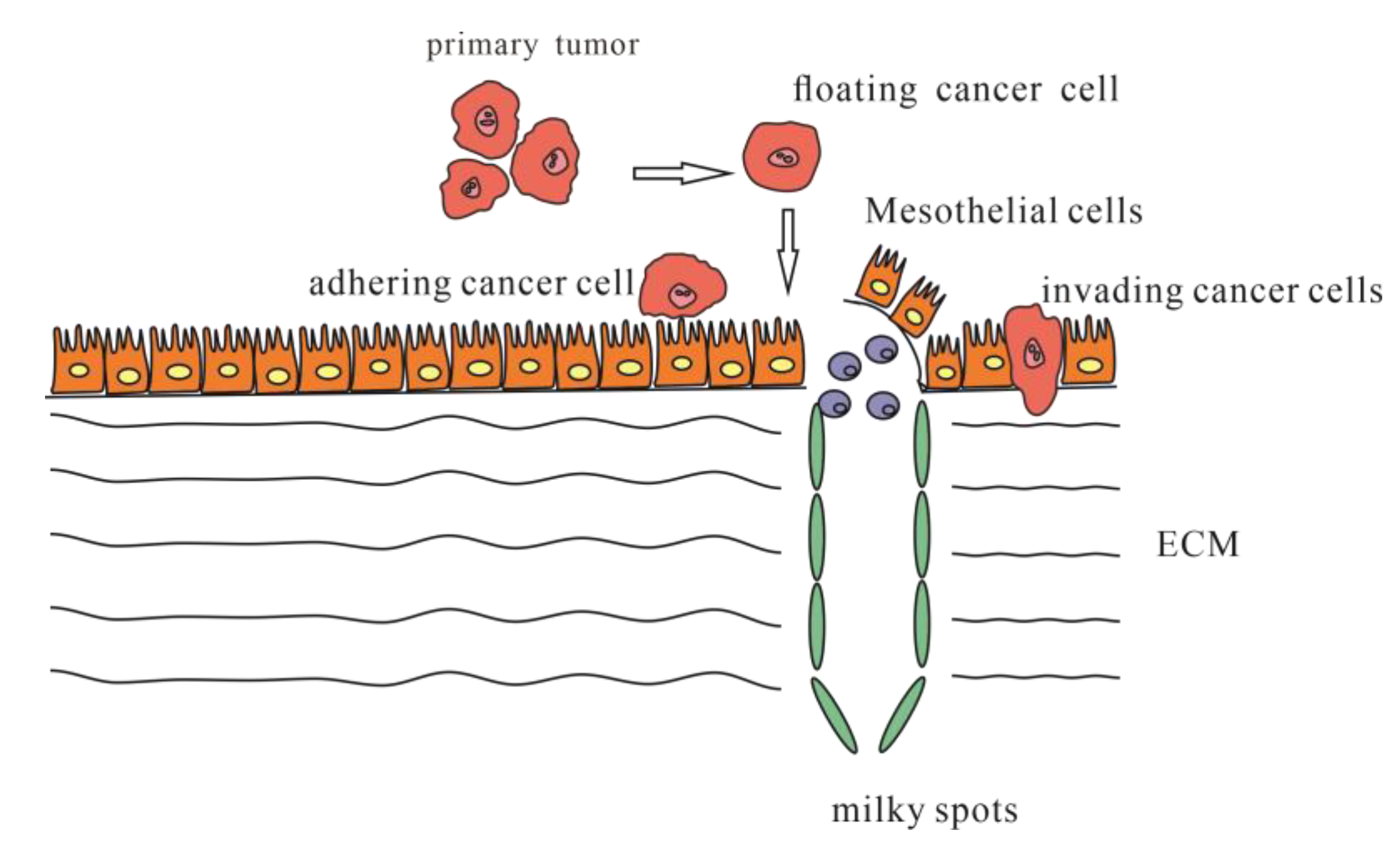
3. Peritoneal Metastasis
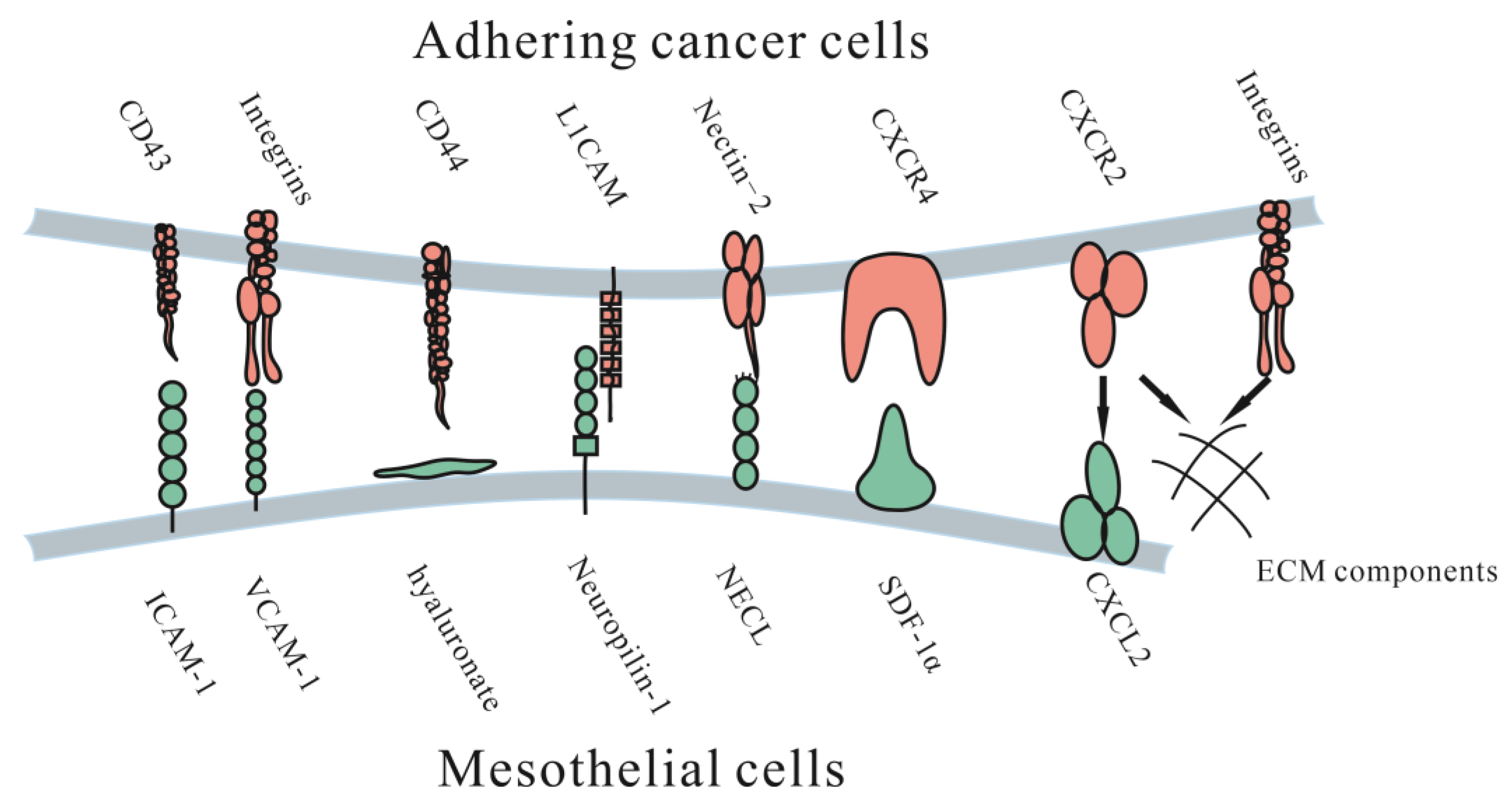
3.1. Adhesion to the Peritoneum
3.1.1. Immunoglobulin Superfamily
3.1.2. Proteoglycans
3.1.3. Integrins
3.1.4. CXC Subfamily
3.1.5. Other Molecules
3.2. Invasion into the Peritoneum
4. Diagnosis and Evaluation of Peritoneal Metastasis
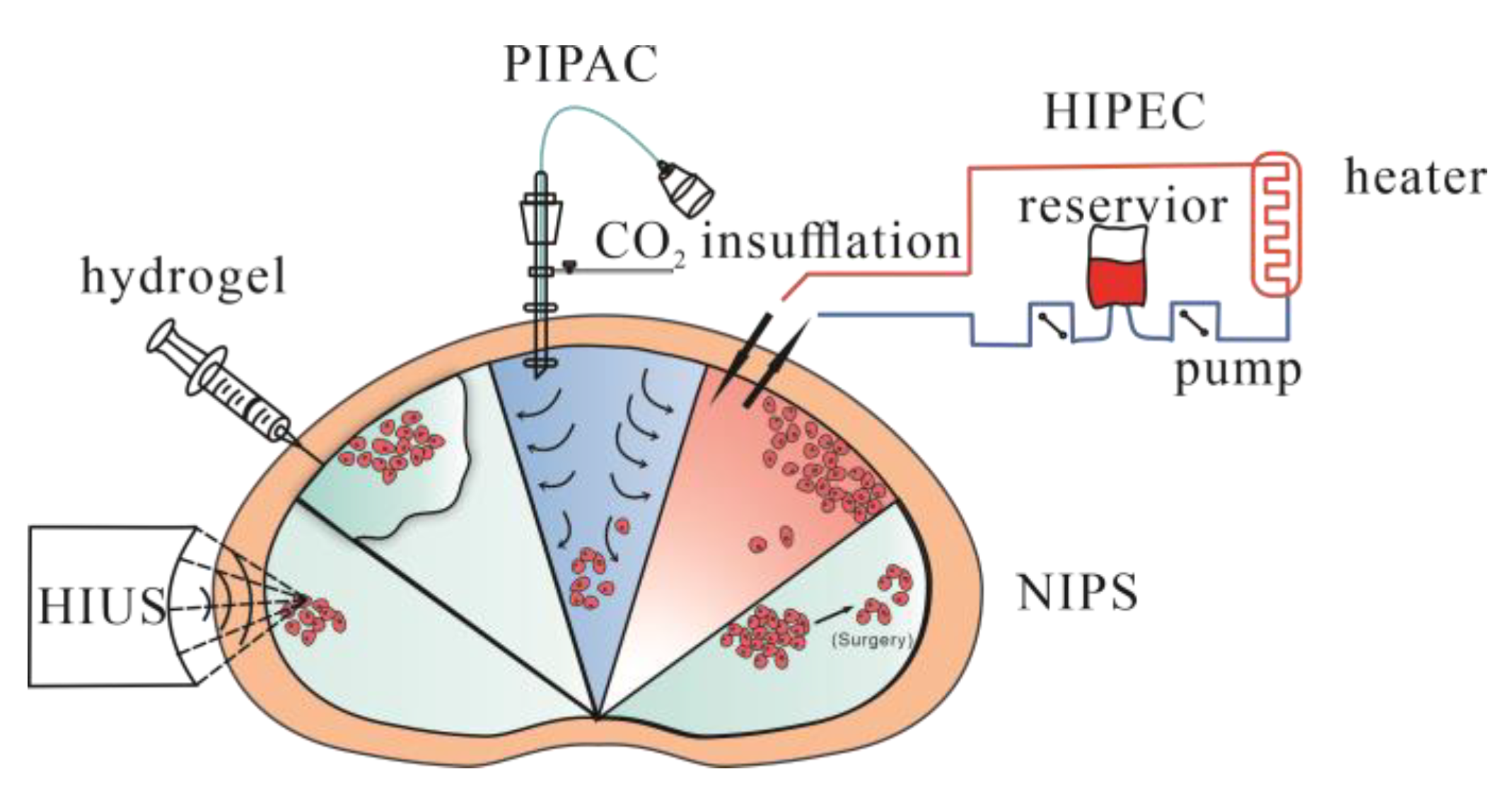
5. Treatment to Peritoneal Metastasis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzì, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. WJG 2013, 19, 6979–6994. [Google Scholar] [CrossRef]
- Raptopoulos, V.; Gourtsoyiannis, N. Peritoneal carcinomatosis. Eur. Radiol. 2001, 11, 2195–2206. [Google Scholar] [CrossRef]
- Anwar, A.; Kasi, A. Peritoneal Cancer; StatPearls: Tampa, FL, USA, 2020. [Google Scholar]
- Quere, P.; Facy, O.; Manfredi, S.; Jooste, V.; Faivre, J.; Lepage, C.; Bouvier, A.-M. Epidemiology, Management, and Survival of Peritoneal Carcinomatosis from Colorectal Cancer: A Population-Based Study. Dis. Colon Rectum 2015, 58, 743–752. [Google Scholar] [CrossRef]
- Feferman, Y.; Solomon, D.; Bhagwandin, S.; Kim, J.; Aycart, S.N.; Feingold, D.; Sarpel, U.; Labow, D.M. Sites of Recurrence After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Carcinomatosis from Colorectal and Appendiceal Adenocarcinoma: A Tertiary Center Experience. Ann. Surg. Oncol. 2019, 26, 482–489. [Google Scholar] [CrossRef]
- Birgisson, H.; Enblad, M.; Artursson, S.; Ghanipour, L.; Cashin, P.; Graf, W. Patients with colorectal peritoneal metastases and high peritoneal cancer index may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. (EJSO) 2020, 46, 2283–2291. [Google Scholar] [CrossRef]
- Deraco, M.; Santoro, N.; Carraro, O.; Inglese, M.G.; Rebuffoni, G.; Guadagni, S.; Somers, D.C.; Vaglini, M. Peritoneal Carcinomatosis: Feature of Dissemination a Review. Tumori J. 1999, 85, 1–5. [Google Scholar] [CrossRef]
- Kastelein, A.W.; Vos, L.M.C.; de Jong, K.H.; van Baal, J.O.A.M.; Nieuwland, R.; van Noorden, C.J.F.; Roovers, J.-P.W.R.; Lok, C.A.R. Embryology, anatomy, physiology and pathophysiology of the peritoneum and the peritoneal vasculature. Semin. Cell Dev. Biol. 2018, 92, 27–36. [Google Scholar] [CrossRef]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef]
- Sarfarazi, A.; Lee, G.; Mirjalili, S.A.; Phillips, A.R.; Windsor, J.A.; Trevaskis, N.L. Therapeutic delivery to the peritoneal lymphatics: Current understanding, potential treatment benefits and future prospects. Int. J. Pharm. 2019, 567, 118456. [Google Scholar] [CrossRef]
- Ceelen, W.P.; Bracke, M.E. Peritoneal minimal residual disease in colorectal cancer: Mechanisms, prevention, and treatment. Lancet Oncol. 2009, 10, 72–79. [Google Scholar] [CrossRef]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.G.; Bleichrodt, R.P. Peritoneal Carcinomatosis of Colorectal Origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef]
- Takebayashi, K.; Murata, S.; Yamamoto, H.; Ishida, M.; Yamaguchi, T.; Kojima, M.; Shimizu, T.; Shiomi, H.; Sonoda, H.; Naka, S.; et al. Surgery-Induced Peritoneal Cancer Cells in Patients Who Have Undergone Curative Gastrectomy for Gastric Cancer. Ann. Surg. Oncol. 2014, 21, 1991–1997. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Kim, H.J.; Park, E.J.; Turri, G.; Zagolin, G.; Foppa, C.; Baik, S.H.; Spolverato, G.; Spinelli, A.; Choi, G.S. Does laparoscopy increase the risk of peritoneal recurrence after resection for pT4 colon cancer? Results of a propensity score-matched analysis from an international cohort. Eur. J. Surg. Oncol. 2022, 48, 1823–1830. [Google Scholar] [CrossRef]
- Du, S.; Miao, J.; Zhu, Z.; Xu, E.; Shi, L.; Ai, S.; Wang, F.; Kang, X.; Chen, H.; Lu, X.; et al. NADPH oxidase 4 regulates anoikis resistance of gastric cancer cells through the generation of reactive oxygen species and the induction of EGFR. Cell Death Dis. 2018, 9, 948. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, B.; Su, F.; Gu, B.; Xiang, L.; Gao, L.; Zheng, P.; Li, X.-M.; Chen, H. TCF7L2 promotes anoikis resistance and metastasis of gastric cancer by transcriptionally activating PLAUR. Int. J. Biol. Sci. 2022, 18, 4560–4577. [Google Scholar] [CrossRef]
- Ye, G.; Yang, Q.; Lei, X.; Zhu, X.; Li, F.; He, J.; Chen, H.; Ling, R.; Zhang, H.; Lin, T.; et al. Nuclear MYH9-induced CTNNB1 transcription, targeted by staurosporin, promotes gastric cancer cell anoikis resistance and metastasis. Theranostics 2020, 10, 7545–7560. [Google Scholar] [CrossRef]
- Dolinschek, R.; Hingerl, J.; Benge, A.; Zafiu, C.; Schüren, E.; Ehmoser, E.K.; Lössner, D.; Reuning, U. Constitutive activation of integrin αvβ3 contributes to anoikis resistance of ovarian cancer cells. Mol. Oncol. 2021, 15, 503–522. [Google Scholar] [CrossRef]
- Shen, W.; Chen, D.; Fu, H.; Liu, S.; Sun, K.; Sun, X. S100A4 protects gastric cancer cells from anoikis through regulation of αv and α5 integrin. Cancer Sci. 2011, 102, 1014–1018. [Google Scholar] [CrossRef]
- Ogishima, J.; Taguchi, A.; Kawata, A.; Kawana, K.; Yoshida, M.; Yoshimatsu, Y.; Sato, M.; Nakamura, H.; Kawata, Y.; Nishijima, A.; et al. The oncogene KRAS promotes cancer cell dissemination by stabilizing spheroid formation via the MEK pathway. BMC Cancer 2018, 18, 1201. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, K.; Koizumi, K.; Kawashima, A.; Saitoh, Y.; Arita, Y.; Shinohara, K.; Minami, T.; Nakayama, T.; Sakurai, H.; Takahashi, Y.; et al. Role of the CXCL12/CXCR4 Axis in Peritoneal Carcinomatosis of Gastric Cancer. Cancer Res. 2006, 66, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.-D.; Na, D.; Liu, F.-N.; Du, Z.-M.; Sun, Z.; Li, Z.; Ma, X.-Y.; Wang, Z.-N.; Xu, H.-M. Induction of gastric cancer cell adhesion through transforming growth factor-beta1-mediated peritoneal fibrosis. J. Exp. Clin. Cancer Res. 2010, 29, 139. [Google Scholar] [CrossRef]
- Falk, P.; Angenete, E.; Bergström, M.; Ivarsson, M.-L. TGF-β1 promotes transition of mesothelial cells into fibroblast phenotype in response to peritoneal injury in a cell culture model. Int. J. Surg. 2013, 11, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Hirakawa-Chung, K.Y.; Yashiro, M.; Inoue, T.; Matsuoka, T.; Fujihara, T.; Murahashi, K.; Sawada, T.; Nakata, B.; Jikihara, I.; et al. TGF-beta1 produced by gastric cancer cells affects mesothelial cell morphology in peritoneal dissemination. Int. J. Oncol. 1998, 12, 847–898. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dou, R.; Huang, S.; Han, L.; Fu, H.; Yang, C.; Song, J.; Zheng, J.; Zhang, X.; Liu, K.; et al. LAMC1-mediated preadipocytes differentiation promoted peritoneum pre-metastatic niche formation and gastric cancer metastasis. Int. J. Biol. Sci. 2022, 18, 3082–3101. [Google Scholar] [CrossRef]
- Xuan, Y.; Wang, H.; Yung, M.M.H.; Chen, F.; Chan, W.-S.; Chan, Y.-S.; Tsui, S.K.W.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics 2022, 12, 3534–3552. [Google Scholar] [CrossRef]
- Oosterling, S.J.; van der Bij, G.J.; Bögels, M.; van der Sijp, J.R.M.; Beelen, R.H.J.; Meijer, S.; van Egmond, M. Insufficient ability of omental milky spots to prevent peritoneal tumor outgrowth supports omentectomy in minimal residual disease. Cancer Immunol. Immunother. 2005, 55, 1043–1051. [Google Scholar] [CrossRef]
- Miao, Z.-F.; Wang, Z.-N.; Zhao, T.-T.; Xu, Y.-Y.; Gao, J.; Miao, F.; Xu, H.-M. Peritoneal Milky Spots Serve as a Hypoxic Niche and Favor Gastric Cancer Stem/Progenitor Cell Peritoneal Dissemination Through Hypoxia-Inducible Factor 1α. Stem Cells 2014, 32, 3062–3074. [Google Scholar] [CrossRef]
- Cao, L.; Hu, X.; Zhang, J.; Huang, G.; Zhang, Y. The role of the CCL22-CCR4 axis in the metastasis of gastric cancer cells into omental milky spots. J. Transl. Med. 2014, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Ziprin, P.; Ridgway, P.F.; Pfistermüller, K.L.; Peck, D.H.; Darzi, A.W. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: A potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun. Adhes. 2003, 10, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yashiro, M.; Sunami, T.; Ohira, M.; Hirakawa-YS, C.K. Lipid-mediated gene transfection of intercellular adhesion molecule-1 suppresses the peritoneal metastasis of gastric carcinoma. Int. J. Mol. Med. 2002, 10, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Jones, L.M.; Catterall, J.B.; Turner, G.A. Expression of cell adhesion molecules on ovarian tumour cell lines and mesothelial cells, in relation to ovarian cancer metastasis. Cancer Lett. 1995, 91, 229–234. [Google Scholar] [CrossRef]
- Siyasi, M.; Mahjoubi, F.; Mahjoubi, B.; Shabani, S. Study of VCAM-1 Gene Expression in Normal and Tumoral Tissues in Patients with Colorectal Cancer. J. Biotechnol. Biomed. Sci. 2017, 1, 19–26. [Google Scholar] [CrossRef]
- Arlt, M.J.; Novak-Hofer, I.; Gast, D.; Gschwend, V.; Moldenhauer, G.; Grünberg, J.; Honer, M.; Schubiger, P.A.; Altevogt, P.; Krüger, A. Efficient Inhibition of Intra-Peritoneal Tumor Growth and Dissemination of Human Ovarian Carcinoma Cells in Nude Mice by Anti-L1-Cell Adhesion Molecule Monoclonal Antibody Treatment. Cancer Res. 2006, 66, 936–943. [Google Scholar] [CrossRef]
- Ichikawa, T.; Okugawa, Y.; Toiyama, Y.; Tanaka, K.; Yin, C.; Kitajima, T.; Kondo, S.; Shimura, T.; Ohi, M.; Araki, T.; et al. Clinical significance and biological role of L1 cell adhesion molecule in gastric cancer. Br. J. Cancer 2019, 121, 1058–1068. [Google Scholar] [CrossRef]
- Fournier, G.; Garrido-Urbani, S.; Reymond, N.; Lopez, M. Nectines et nectines-like. Med. Sci. (Paris) 2010, 26, 273–279. [Google Scholar] [CrossRef]
- Bekes, I.; Löb, S.; Holzheu, I.; Janni, W.; Baumann, L.; Wöckel, A.; Wulff, C. Nectin-2 in ovarian cancer: How is it expressed and what might be its functional role? Cancer Sci. 2019, 110, 1872–1882. [Google Scholar] [CrossRef]
- Casey, R.C.; Oegema, T.R., Jr.; Skubitz, K.M.; Pambuccian, S.E.; Grindle, S.M.; Skubitz, A.P.N. Cell membrane glycosylation mediates the adhesion, migration, and invasion of ovarian carcinoma cells. Clin. Exp. Metastasis 2003, 20, 143–152. [Google Scholar] [CrossRef]
- Nakamura, K.; Sawada, K.; Kinose, Y.; Yoshimura, A.; Toda, A.; Nakatsuka, E.; Hashimoto, K.; Mabuchi, S.; Morishige, K.-I.; Kurachi, H.; et al. Exosomes Promote Ovarian Cancer Cell Invasion through Transfer of CD44 to Peritoneal Mesothelial Cells. Mol. Cancer Res. 2017, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Tang, B.; Yu, P.-W.; Peng, Z.-H.; Qian, F.; Sun, G. Systemic and peritoneal inflammatory response after laparoscopic-assisted gastrectomy and the effect of inflammatory cytokines on adhesion of gastric cancer cells to peritoneal mesothelial cells. Surg. Endosc. 2010, 24, 2860–2870. [Google Scholar] [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Oosterling, S.J.; van der Bij, G.J.; Bögels, M.; Raa, S.T.; Post, J.A.; Meijer, G.A.; Beelen, R.H.; van Egmond, M. Anti-β1 Integrin Antibody Reduces Surgery-Induced Adhesion of Colon Carcinoma Cells to Traumatized Peritoneal Surfaces. Ann. Surg. 2008, 247, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, H.; Komatsu, S.; Sano, R.; Takada, Y.; Tsuji, T. Adhesion of Gastric Carcinoma Cells to Peritoneum Mediated by α3β1 Integrin (VLA-3). Cancer Res. 2004, 64, 6065–6070. [Google Scholar] [CrossRef] [PubMed]
- Scalici, J.M.; Harrer, C.; Allen, A.; Jazaeri, A.; Atkins, K.A.; McLachlan, K.R.; Slack-Davis, J.K. Inhibition of α4β1 integrin increases ovarian cancer response to carboplatin. Gynecol. Oncol. 2014, 132, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Che, X.; Yu, Y.; Cheng, Y.; Bai, M.; Yang, Z.; Guo, Q.; Xie, X.; Li, D.; Guo, M.; et al. Hypoxia-autophagy axis induces VEGFA by peritoneal mesothelial cells to promote gastric cancer peritoneal metastasis through an integrin α5-fibronectin pathway. J. Exp. Clin. Cancer Res. 2020, 39, 221. [Google Scholar] [CrossRef]
- Lepsenyi, M.; Algethami, N.; Al-Haidari, A.A.; Algaber, A.; Syk, I.; Rahman, M.; Thorlacius, H. CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin. Exp. Metastasis 2021, 38, 401–410. [Google Scholar] [CrossRef]
- Asem, M.; Young, A.M.; Oyama, C.; De La Zerda, A.C.; Liu, Y.; Yang, J.; Hilliard, T.S.; Johnson, J.; Harper, E.I.; Guldner, I.; et al. Host Wnt5a Potentiates Microenvironmental Regulation of Ovarian Cancer Metastasis. Cancer Res. 2020, 80, 1156–1170. [Google Scholar] [CrossRef]
- Volz, J.; Köster, S.; Spacek, Z.; Paweletz, N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg. Endosc. 1999, 13, 611–614. [Google Scholar] [CrossRef]
- Heath, R.M.; Jayne, D.G.; O’Leary, R.; Morrison, E.E.; Guillou, P.J. Tumour-induced apoptosis in human mesothelial cells: A mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br. J. Cancer 2004, 90, 1437–1442. [Google Scholar] [CrossRef]
- Yonemura, Y.; Endo, Y.; Fujita, H.; Kimura, K.; Sugiyama, K.; Momiyama, N.; Shimada, H.; Sasaki, T. Inhibition of peritoneal dissemination in human gastric cancer by MMP-7-specific antisense oligonucleotide. J. Exp. Clin. Cancer Res. 2001, 20, 205–212. [Google Scholar]
- Oku, T.; Shimada, K.; Kenmotsu, H.; Ando, Y.; Kurisaka, C.; Sano, R.; Tsuiji, M.; Hasegawa, S.; Fukui, T.; Tsuji, T. Stimulation of Peritoneal Mesothelial Cells to Secrete Matrix Metalloproteinase-9 (MMP-9) by TNF-α: A Role in the Invasion of Gastric Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 3961. [Google Scholar] [CrossRef] [PubMed]
- Koch, J.; Mönch, D.; Maaß, A.; Mangold, A.; Gužvić, M.; Mürdter, T.; Leibold, T.; Dahlke, M.-H.; Renner, P. Pharmacologic Targeting of MMP2/9 Decreases Peritoneal Metastasis Formation of Colorectal Cancer in a Human Ex Vivo Peritoneum Culture Model. Cancers 2022, 14, 3760. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, J.; Liu, S.; Ji, C.; Guan, W.; Chen, L.; Guan, Y.; Yang, X.; Zhou, Z. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur. Radiol. 2020, 30, 239–246. [Google Scholar] [CrossRef]
- Sun, P.; Li, X.; Wang, L.; Wang, R.; Du, X. Enhanced computed tomography imaging features predict tumor grade in pseudomyxoma peritonei. Quant. Imaging Med. Surg. 2022, 12, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of Preoperative Computed Tomography in Estimating Peritoneal Cancer Index in Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, M.-J.; Yun, M.J.; Oh, Y.T.; Kim, J.H.; Hwang, H.S.; Park, M.-S.; Cha, S.-W.; Lee, J.D.; Noh, S.H.; et al. Comparison of CT and 18F-FDG PET for Detecting Peritoneal Metastasis on the Preoperative Evaluation for Gastric Carcinoma. Korean J. Radiol. 2006, 7, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Chua, T.C. CT versus intraoperative peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: Importance of the difference between statistical significance and clinical relevance. Ann. Surg. Oncol. 2009, 16, 2662–2663. [Google Scholar] [CrossRef]
- Laghi, A.; Bellini, D.; Rengo, M.; Accarpio, F.; Caruso, D.; Biacchi, D.; Di Giorgio, A.; Sammartino, P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: Systematic review and meta-analysis. La Radiol. Med. 2016, 122, 1–15. [Google Scholar] [CrossRef]
- Sant, I.V.; Engbersen, M.P.; Bhairosing, P.A.; Lambregts, D.M.J.; Beets-Tan, R.G.H.; van Driel, W.J.; Aalbers, A.G.J.; Kok, N.F.M.; Lahaye, M.J. Diagnostic performance of imaging for the detection of peritoneal metastases: A meta-analysis. Eur. Radiol. 2020, 30, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Lee, S.-W. Diagnostic accuracy of 18F-FDG PET/CT for detection of peritoneal carcinomatosis; a systematic review and meta-analysis. Br. J. Radiol. 2018, 91, 20170519. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Chen, J.-H.; Liang, J.-A.; Huang, W.-S.; Cheng, K.-Y.; Kao, C.-H. PET or PET/CT for Detection of Peritoneal Carcinomatosis: A Meta-Analysis. Clin. Nucl. Med. 2013, 38, 623–629. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritoneal carcinomatosis of unknown primary site, a study of 25 patients over 30 years. Eur. J. Surg. Oncol. 2020, 46, 1908–1911. [Google Scholar] [CrossRef]
- Nakada, A.; Maruyama, T.; Kamiya, M.; Hanaoka, K.; Urano, Y. Rapid Visualization of Deeply Located Tumors In Vivo by Intravenous Administration of a γ-Glutamyltranspeptidase-Activated Fluorescent Probe. Bioconjugate Chem. 2022, 33, 523–529. [Google Scholar] [CrossRef]
- Llueca, A.; Escrig, J.; Serra-Rubert, A.; Gomez-Quiles, L.; Rivadulla, I.; Játiva-Porcar, R.; Moreno-Clarí, E.; Montañés-Pauls, B.; Granel-Villach, L.; Villegas-Cánovas, C.; et al. Prognostic value of peritoneal cancer index in primary advanced ovarian cancer. Eur. J. Surg. Oncol. 2018, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Yonemura, Y.; Mehta, S.; Benzerdjeb, N.; Kammar, P.; Parikh, L.; Prabhu, A.; Mishra, S.; Shah, M.; Shaikh, S.; et al. The Pathologic Peritoneal Cancer Index (PCI) Strongly Differs from the Surgical PCI in Peritoneal Metastases Arising From Various Primary Tumors. Ann. Surg. Oncol. 2020, 27, 2985–2996. [Google Scholar] [CrossRef] [PubMed]
- Avesani, G.; Arshad, M.; Lu, H.; Fotopoulou, C.; Cannone, F.; Melotti, R.; Aboagye, E.; Rockall, A. Radiological assessment of Peritoneal Cancer Index on preoperative CT in ovarian cancer is related to surgical outcome and survival. La Radiol. Med. 2020, 125, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Evrard, C.; Messina, S.; Sefrioui, D.; Frouin, É.; Auriault, M.-L.; Chautard, R.; Zaanan, A.; Jaffrelot, M.; De La Fouchardière, C.; Aparicio, T.; et al. Heterogeneity of Mismatch Repair Status and Microsatellite Instability between Primary Tumour and Metastasis and Its Implications for Immunotherapy in Colorectal Cancers. Int. J. Mol. Sci. 2022, 23, 4427. [Google Scholar] [CrossRef]
- Bhullar, D.S.; Barriuso, J.; Mullamitha, S.; Saunders, M.P.; O’Dwyer, S.T.; Aziz, O. Biomarker concordance between primary colorectal cancer and its metastases. EBioMedicine 2019, 40, 363–374. [Google Scholar] [CrossRef]
- Ubink, I.; van Eden, W.J.; Snaebjornsson, P.; Kok, N.F.M.; van Kuik, J.; van Grevenstein, W.M.U.; Laclé, M.M.; Sanders, J.; Fijneman, R.J.A.; Elias, S.G.; et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br. J. Surg. 2018, 105, e204–e211. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Yamamoto, G.; Takahashi, A.; Arai, Y.; Yamada, M.; Kakuta, M.; Yamaguchi, K.; Akagi, Y.; Nishimura, Y.; Sakamoto, H.; et al. High concordance rate of KRAS/BRAF mutations and MSI-H between primary colorectal cancer and corresponding metastases. Oncol. Rep. 2017, 37, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Vignot, S.; Lefebvre, C.; Frampton, G.M.; Meurice, G.; Yelensky, R.; Palmer, G.; Capron, F.; Lazar, V.; Hannoun, L.; Miller, V.A.; et al. Comparative analysis of primary tumour and matched metastases in colorectal cancer patients: Evaluation of concordance between genomic and transcriptional profiles. Eur. J. Cancer 2015, 51, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ariake, K.; Motoi, F.; Ohtsuka, H.; Fukase, K.; Masuda, K.; Mizuma, M.; Hayashi, H.; Nakagawa, K.; Morikawa, T.; Maeda, S.; et al. Predictive risk factors for peritoneal recurrence after pancreatic cancer resection and strategies for its prevention. Surg. Today 2017, 47, 1434–1442. [Google Scholar] [CrossRef]
- Takii, Y.; Mizusawa, J.; Kanemitsu, Y.; Komori, K.; Shiozawa, M.; Ohue, M.; Ikeda, S.; Takiguchi, N.; Kobatake, T.; Ike, H.; et al. The Conventional Technique Versus the No-touch Isolation Technique for Primary Tumor Resection in Patients with Colon Cancer (JCOG1006): A Multicenter, Open-Label, Randomized, Phase III Trial. Ann. Surg. 2022, 275, 849–855. [Google Scholar] [CrossRef]
- Carlier, C.; Mathys, A.; De Jaeghere, E.; Steuperaert, M.; De Wever, O.; Ceelen, W. Tumour tissue transport after intraperitoneal anticancer drug delivery. Int. J. Hyperth. 2017, 33, 534–542. [Google Scholar] [CrossRef]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for the Management of Peritoneal Carcinomatosis From Colorectal Cancer: A Multi-Institutional Study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Glehen, O.; Cotte, E.; Schreiber, V.; Sayag-Beaujard, A.C.; Vignal, J.; Gilly, F.N. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br. J. Surg. 2004, 91, 747–754. [Google Scholar] [CrossRef]
- Kecmanovic, D.M.; Pavlov, M.J.; Ceranic, M.S.; Sepetkovski, A.V.; Kovacevic, P.A.; Stamenkovic, A.B. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur. J. Surg. Oncol. (ESJO) 2005, 31, 147–152. [Google Scholar] [CrossRef]
- Rosa, F.; Galiandro, F.; Ricci, R.; Di Miceli, D.; Quero, G.; Fiorillo, C.; Cina, C.; Alfieri, S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases: Analysis of short- and long-term outcomes. Langenbeck’s Arch. Surg. 2021, 406, 2797–2805. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- González-Moreno, S.; González-Bayón, L.A.; Ortega-Pérez, G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J. Gastrointest. Oncol. 2010, 2, 68–75. [Google Scholar] [CrossRef] [PubMed]
- De Bree, E.; Michelakis, D.; Stamatiou, D.; Romanos, J.; Zoras, O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum 2017, 2, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.D.; Levine, E.A.; Mangieri, C.W.; Gawdi, R.; Moaven, O.; Russell, G.; Lundy, M.E.; Perry, K.C.; Votanopoulos, K.I.; Shen, P. Repeat Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Cancers with Peritoneal Metastasis: A 30-year Institutional Experience. Ann. Surg. Oncol. 2022, 29, 3436–3445. [Google Scholar] [CrossRef]
- Moukarzel, L.A.; Ferrando, L.; Dopeso, H.; Stylianou, A.; Basili, T.; Pareja, F.; Paula, A.D.C.; Zoppoli, G.; Abu-Rustum, N.R.; Reis-Filho, J.S.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) with carboplatin induces distinct transcriptomic changes in ovarian tumor and normal tissues. Gynecol. Oncol. 2022, 165, 239–247. [Google Scholar] [CrossRef]
- Chia, C.S.; The Big Renape Group; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.-M.; Arvieux, C.; Boschetti, G.; et al. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Nadiradze, G.; Horvath, P.; Sautkin, Y.; Archid, R.; Weinreich, F.-J.; Königsrainer, A.; Reymond, M.A. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers 2019, 12, 34. [Google Scholar] [CrossRef]
- Kepenekian, V.; Péron, J.; You, B.; Bonnefoy, I.; Villeneuve, L.; Alyami, M.; Bakrin, N.; Rousset, P.; Benzerdjeb, N.; Glehen, O. Non-resectable Malignant Peritoneal Mesothelioma Treated with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Plus Systemic Chemotherapy Could Lead to Secondary Complete Cytoreductive Surgery: A Cohort Study. Ann. Surg. Oncol. 2021, 29, 2104–2113. [Google Scholar] [CrossRef]
- Alyami, M.; Mercier, F.; Siebert, M.; Bonnot, P.-E.; Laplace, N.; Villeneuve, L.; Passot, G.; Glehen, O.; Bakrin, N.; Kepenekian, V. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 128–133. [Google Scholar] [CrossRef]
- Taibi, A.; Sgarbura, O.; Hübner, M.; Bardet, S.M.; Alyami, M.; Bakrin, N.; Fontanier, S.D.; Eveno, C.; Gagniere, J.; Pache, B.; et al. Feasibility and Safety of Oxaliplatin-Based Pressurized Intraperitoneal Aerosol Chemotherapy with or without Intraoperative Intravenous 5-Fluorouracil and Leucovorin for Colorectal Peritoneal Metastases: A Multicenter Comparative Cohort Study. Ann. Surg. Oncol. 2022, 29, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Wassenaar, E.C.E.; Lurvink, R.J.; Creemers, G.-J.M.; Burger, J.W.A.; Los, M.; Huysentruyt, C.J.R.; van Lijnschoten, G.; Nederend, J.; Lahaye, M.J.; et al. Pressurized Intraperitoneal Aerosol Chemotherapy (Oxaliplatin) for Unresectable Colorectal Peritoneal Metastases: A Multicenter, Single-Arm, Phase II Trial (CRC-PIPAC). Ann. Surg. Oncol. 2021, 28, 5311–5326. [Google Scholar] [CrossRef] [PubMed]
- Kakchekeeva, T.; Demtröder, C.; Herath, N.I.; Griffiths, D.; Torkington, J.; Solaß, W.; Dutreix, M.; Reymond, M.A. In Vivo Feasibility of Electrostatic Precipitation as an Adjunct to Pressurized Intraperitoneal Aerosol Chemotherapy (ePIPAC). Ann. Surg. Oncol. 2016, 23, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Taibi, A.; Farinha, H.T.; Fontanier, S.D.; Sayedalamin, Z.; Hübner, M.; Sgarbura, O. Pressurized Intraperitoneal Aerosol Chemotherapy Enhanced by Electrostatic Precipitation (ePIPAC) for Patients with Peritoneal Metastases. Ann. Surg. Oncol. 2021, 28, 3852–3860. [Google Scholar] [CrossRef] [PubMed]
- Graversen, M.; Detlefsen, S.; Ellebaek, S.B.; Fristrup, C.W.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumor response in peritoneal metastasis is insufficient. Eur. J. Surg. Oncol. 2020, 46, 155–159. [Google Scholar] [CrossRef]
- Bachmann, C.; Sautkin, I.; Nadiradze, G.; Archid, R.; Weinreich, F.J.; Königsrainer, A.; Reymond, M.A. Technology development of hyperthermic pressurized intraperitoneal aerosol chemotherapy (hPIPAC). Surg. Endosc. 2021, 35, 6358–6365. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Khosrawipour, T.; Martino, A.; Kulas, J.; Pieczka, M.; Zacharski, M.; Nicpon, J.; Khosrawipour, V. Enabling Microparticle Imprinting to Achieve Penetration and Local Endurance in the Peritoneum via High-Intensity Ultrasound (HIUS) for the Treatment of Peritoneal Metastasis. Int. J. Surg. Oncol. 2020, 2020, 9679385. [Google Scholar] [CrossRef]
- Lau, H.; Khosrawipour, T.; Mikolajczyk, A.; Frelkiewicz, P.; Nicpon, J.; Arafkas, M.; Pigazzi, A.; Knoefel, W.T.; Khosrawipour, V. Intraperitoneal chemotherapy of the peritoneal surface using high-intensity ultrasound (HIUS): Investigation of technical feasibility, safety and possible limitations. J. Cancer 2020, 11, 7209–7215. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, P.; Zhu, Z.; Zhang, J.; Huang, J.; Wang, T.; Chen, J.; Xu, H. Benefits of Surgery After Neoadjuvant Intraperitoneal and Systemic Chemotherapy for Gastric Cancer Patients with Peritoneal Metastasis: A Meta-Analysis. J. Surg. Res. 2020, 245, 234–243. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takiguchi, S.; Nakajima, K.; Miyata, H.; Yamasaki, M.; Kurokawa, Y.; Okada, K.; Mori, M.; Doki, Y. Neoadjuvant Intraperitoneal and Systemic Chemotherapy for Gastric Cancer Patients with Peritoneal Dissemination. Ann. Surg. Oncol. 2011, 18, 3726–3731. [Google Scholar] [CrossRef]
- Zang, D.; Zhang, C.; Li, C.; Fan, Y.; Li, Z.; Hou, K.; Che, X.; Liu, Y.; Qu, X. LPPR4 promotes peritoneal metastasis via Sp1/integrin α/FAK signaling in gastric cancer. Am. J. Cancer Res. 2020, 10, 1026–1044. [Google Scholar] [PubMed]
- Daniel, S.K.; Seo, Y.D.; Pillarisetty, V.G. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal malignancies. Semin. Cancer Biol. 2020, 65, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Mandai, M.; Hamanishi, J.; Yoshioka, Y.; Matsumura, N.; Baba, T.; Yamaguchi, K.; Murakami, R.; Yamamoto, A.; Kharma, B.; et al. PD-L1 on Tumor Cells Is Induced in Ascites and Promotes Peritoneal Dissemination of Ovarian Cancer through CTL Dysfunction. Clin. Cancer Res. 2013, 19, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Lemke-Miltner, C.D.; Blackwell, S.; Tomanek-Chalkley, A.; Gibson-Corely, K.N.; Coleman, K.L.; Weiner, G.J.; Chan, C.H.F. Intraperitoneal CMP-001: A Novel Immunotherapy for Treating Peritoneal Carcinomatosis of Gastrointestinal and Pancreaticobiliary Cancer. Ann. Surg. Oncol. 2021, 28, 1187–1197. [Google Scholar] [CrossRef]
- Sabree, S.A.; Voigt, A.P.; Blackwell, S.E.; Vishwakarma, A.; Chimenti, M.S.; Salem, A.K.; Weiner, G.J. Direct and indirect immune effects of CMP-001, a virus-like particle containing a TLR9 agonist. J. Immunother. Cancer 2021, 9, e002484. [Google Scholar] [CrossRef]
- Engeland, C.E.; Bell, J.C. Introduction to Oncolytic Virotherapy. In Oncolytic Viruses; Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2058, pp. 1–6. [Google Scholar] [CrossRef]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, W.S.; Kim, C.W.; Lee, S.J.; Yang, H.; Kong, S.J.; Ning, J.; Yang, K.-M.; Kang, B.; Kim, W.R.; et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J. Immunother. Cancer 2020, 8, e000857. [Google Scholar] [CrossRef]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, M.S.; Lee, J.H.; Hwang, J.E.; Shim, H.J.; Cho, S.H.; Kim, D.-E.; Ko, H.M.; Cho, C.-S.; Park, I.-K.; et al. Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials 2013, 34, 1433–1441. [Google Scholar] [CrossRef]
- Qian, H.; Qian, K.; Cai, J.; Yang, Y.; Zhu, L.; Liu, B. Therapy for Gastric Cancer with Peritoneal Metastasis Using Injectable Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles. ACS Biomater. Sci. Eng. 2019, 5, 1100–1112. [Google Scholar] [CrossRef]
- Xu, S.; Fan, H.; Yin, L.; Zhang, J.; Dong, A.; Deng, L.; Tang, H. Thermosensitive hydrogel system assembled by PTX-loaded copolymer nanoparticles for sustained intraperitoneal chemotherapy of peritoneal carcinomatosis. Eur. J. Pharm. Biopharm. 2016, 104, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Hiramoto, S.; Amano, Y.; Emoto, S.; Yamaguchi, H.; Ishigami, H.; Kitayama, J.; Ito, T. Intraperitoneal Delivery of Cisplatin via a Hyaluronan-Based Nanogel/in Situ Cross-Linkable Hydrogel Hybrid System for Peritoneal Dissemination of Gastric Cancer. Mol. Pharm. 2017, 14, 3105–3113. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Y.; Yang, Z.; Peng, H.; Wei, R.; Wang, C.; Feng, M. Tunneling Nanotubular Expressways for Ultrafast and Accurate M1 Macrophage Delivery of Anticancer Drugs to Metastatic Ovarian Carcinoma. ACS Nano 2019, 13, 1078–1096. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, M.V.; Cano-Garrido, O.; Álamo, P.; Sala, R.; Gallardo, A.; Serna, N.; Falgàs, A.; Voltà-Durán, E.; Casanova, I.; Sánchez-Chardi, A.; et al. Engineering Secretory Amyloids for Remote and Highly Selective Destruction of Metastatic Foci. Adv. Mater. 2020, 32, e1907348. [Google Scholar] [CrossRef]
- Van De Sande, L.; Cosyns, S.; Willaert, W.; Ceelen, W. Albumin-based cancer therapeutics for intraperitoneal drug delivery: A review. Drug Deliv. 2020, 27, 40–53. [Google Scholar] [CrossRef]
| Ref. | Disease | Type | Group | Survival | Death and Complication | Recurrence |
|---|---|---|---|---|---|---|
| Glehen [78] | colorectal cancer | retrospective multicenter study | 506 | overall median survival: 19.2 months complete CRS vs. not complete CRS: 32.4 months vs. 8.4 months (p < 0.001) | complication rate: 22.9% death rate: 4% | 371 recurrence (73.3% with 158 (41.9%) peritoneal recurrence |
| Glehen [79] | colorectal cancer | retrospective study | 53 | median overall survival: 12.8 months CCR-0 vs. CCR-1 vs. CCR-2: 32.9 vs. 12.5 vs. 8.1 (p < 0.001) | complication rate: 23% death rate: 4% | – |
| Kecmanovic [80] | colorectal cancer | retrospective study | 18 | median overall survival: 15 months | complication: 8 | 3 live with cancer progress, 3 died of it |
| Rosa [81] | colorectal cancer | retrospective study | 67 | median overall survival: 41 months 3-year overall survival: 43% | complication rate: 35.8% | – |
| François Quénet [82] | colorectal cancer | PRODIGE 7 a multicenter, randomized, open-label, phase 3 trial | 133 (CRS plus HIPEC) vs. 132(CRS) | median overall survival: 41.7 months (CRS plus HIPEC) vs. 41.2 months (CRS) | – | |
| Driel [83] | ovarian cancer | a multicenter, open-label, phase 3 trial | 123 (Surgery) vs. 122 (Surgery plus HIPEC) | median overall survival: 33.9 months (Surgery) vs. 45.7 months. (Surgery plus HIPEC) 3-year overall: 48% (Surgery) vs. 62% (Surgery plus HIPEC) | Complication: 122 (Surgery) vs. 118 (Surgery plus HIPEC) death: 62% (Surgery) vs. 50% (Surgery plus HIPEC) | recurrence or death: 89% (Surgery) vs. 81% (Surgery plus HIPEC) |
| Hydrogels | Drugs | In Vitro | In Vivo | Highlight |
|---|---|---|---|---|
| linoleic acid-coupled Pluronic F-127 (Plu-CLA) [111] | Docetaxel | Gastric cancer cells TMK1 | peritoneal metastasis from gastric cancer | docetaxel–Plu-CLA synergistically inhibits peritoneal metastasis and prolongs survival in a peritoneal gastric cancer model. |
| Albumin Hydrogel Hybridized with Paclitaxel-Loaded Red Blood Cell Membrane Nanoparticles [112] | Paclitaxel | Gastric cancer cells | peritoneal metastasis from gastric cancer | the hydrogel possesses good tumor growth suppression properties after a single injection. |
| PTX/PECT (gel) [113] | PTX | Colorectal cancer cells CT-26 | peritoneal metastasis from colorectal cancer | sustained drug concentration at peritoneal levels in combination with drug in the form of nanoparticle contributes to the enhanced anti-tumor efficacy. |
| HA nanogels [114] | Cisplatin | – | peritoneal metastasis from gastric cancer (MKN45P cells) | led to a significantly decreased number of peritoneal nodules, especially those smaller than 1.0 mm. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Xie, X.; Min, T.; Sun, T.; Wang, H.; Zhang, Y.; Dang, C.; Zhang, H. Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects. J. Clin. Med. 2023, 12, 103. https://doi.org/10.3390/jcm12010103
Ren K, Xie X, Min T, Sun T, Wang H, Zhang Y, Dang C, Zhang H. Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects. Journal of Clinical Medicine. 2023; 12(1):103. https://doi.org/10.3390/jcm12010103
Chicago/Turabian StyleRen, Kaijie, Xin Xie, Tianhao Min, Tuanhe Sun, Haonan Wang, Yong Zhang, Chengxue Dang, and Hao Zhang. 2023. "Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects" Journal of Clinical Medicine 12, no. 1: 103. https://doi.org/10.3390/jcm12010103
APA StyleRen, K., Xie, X., Min, T., Sun, T., Wang, H., Zhang, Y., Dang, C., & Zhang, H. (2023). Development of the Peritoneal Metastasis: A Review of Back-Grounds, Mechanisms, Treatments and Prospects. Journal of Clinical Medicine, 12(1), 103. https://doi.org/10.3390/jcm12010103




