The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions under High Tension: Evidence from Animal Studies and Clinical Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Research on Negative Pressure Treatment of Tension Wounds in a Porcine Model
2.2. Histological Evaluation
2.3. Immunohistological Staining and Evaluation
2.4. RNA Isolation and Quantitative Real-Time PCR (RT–PCR)
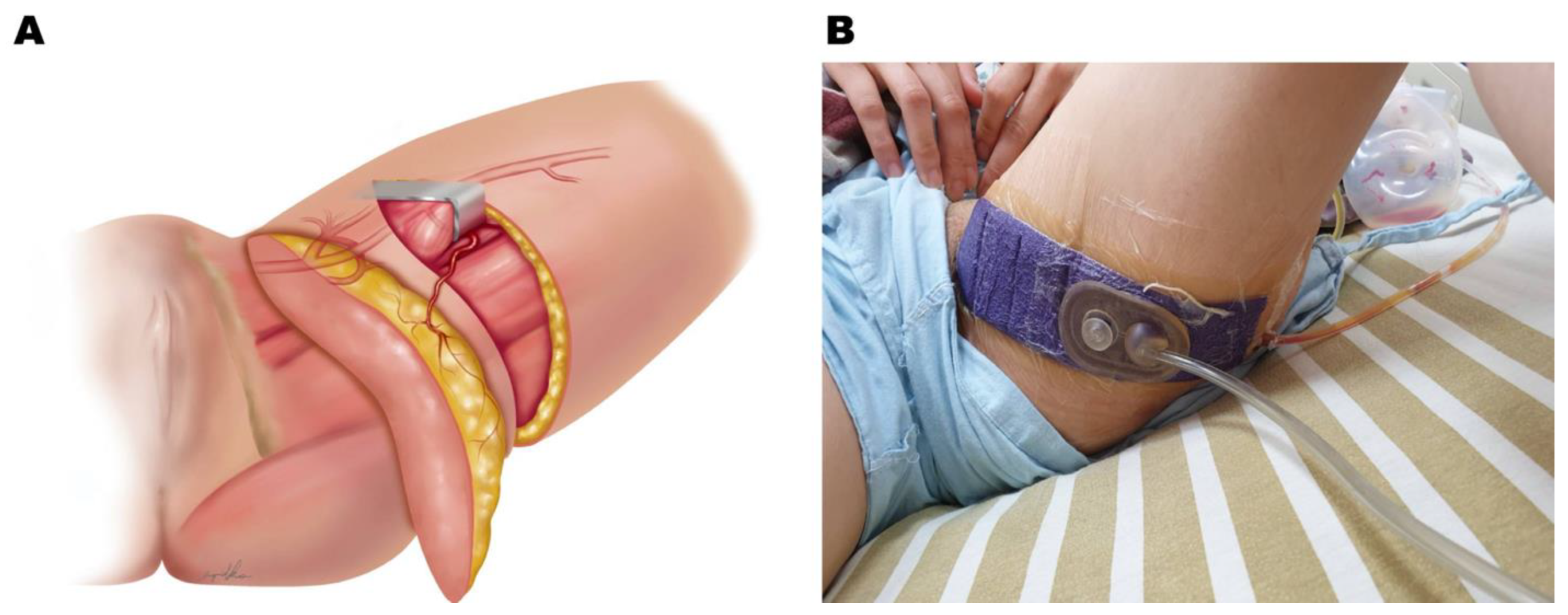
2.5. Clinical Application of Negative Pressure at the Donor Site of the PAP Flap
2.6. Application of iNPWT
2.7. Statistical Analysis
3. Results
3.1. Negative Pressure Enhanced Wound Healing under Tension in the Animal Study
3.2. Negative Pressure Enhanced Collagen Deposition in the Animal Study
| Control | NP Treatment | |
|---|---|---|
| Epidermal regeneration | ++ (3/4) | +++ (4/4) |
| Granulation tissue | ++ | + |
| Inflammatory cell infiltration | ++ | + |
| Angiogenesis | + | +++ |
| Fibrotic tissue | ++ | + |
| Collagen deposition | ++ | + |
3.3. Negative Pressure Promoted Angiogenesis in the Animal Study
3.4. Negative Pressure Treatment Decreased the Inflammatory Response in the Animal Study
3.5. Negative Pressure Treatment Enhanced Lymphangiogenesis in the Animal Study
3.6. Negative Pressure Treatment in the Clinical Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Leavitt, T.; Bayer, L.R.; Orgill, D.P. Effect of negative pressure wound therapy on wound healing. Curr. Probl. Surg. 2014, 51, 301–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, L.A.; Marshall, C.D.; Leavitt, T.; Hu, M.S.; Moore, A.L.; Gonzalez, J.G.; Longaker, M.T.; Gurtner, G.C. Mechanical Forces in Cutaneous Wound Healing: Emerging Therapies to Minimize Scar Formation. Adv. Wound Care 2018, 7, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamamoto, F.; Lima, A.L.M.; de Rezende, M.R.; Mattar-Junior, R.; Leonhardt, M.D.C.; Kojima, K.E.; dos Santos, C.C. A new low-cost negative-pressure wound therapy versus a commercially available therapy device widely used to treat complex traumatic injuries: A prospective, randomized, non-inferiority trial. Clinics 2017, 72, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet 2005, 366, 1704–1710. [Google Scholar] [CrossRef]
- Semsarzadeh, N.N.; Tadisina, K.K.; Maddox, J.; Chopra, K.; Singh, D.P. Closed Incision Negative-Pressure Therapy Is Associated with Decreased Surgical-Site Infections: A Meta-Analysis. Plast. Reconstr. Surg. 2015, 136, 592–602. [Google Scholar] [CrossRef]
- Wilkes, R.P.; Kilpad, D.V.; Zhao, Y.; Kazala, R.; McNulty, A. Closed incision management with negative pressure wound therapy (CIM): Biomechanics. Surg. Innov. 2012, 19, 67–75. [Google Scholar] [CrossRef]
- Scalise, A.; Calamita, R.; Tartaglione, C.; Pierangeli, M.; Bolletta, E.; Gioacchini, M.; Gesuita, R.; Di Benedetto, G. Improving wound healing and preventing surgical site complications of closed surgical incisions: A possible role of Incisional Negative Pressure Wound Therapy. A systematic review of the literature. Int. Wound J. 2016, 13, 1260–1281. [Google Scholar] [CrossRef]
- Abesamis, G.M.; Chopra, S.; Vickery, K.; Deva, A.K. A Comparative Trial of Incisional Negative-Pressure Wound Therapy in Abdominoplasty. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2141. [Google Scholar] [CrossRef]
- Willy, C.; Agarwal, A.; Andersen, C.A.; De Santis, G.; Gabriel, A.; Grauhan, O.; Guerra, O.M.; Lipsky, B.A.; Malas, M.B.; Mathiesen, L.L.; et al. Closed incision negative pressure therapy: International multidisciplinary consensus recommendations. Int. Wound J. 2017, 14, 385–398. [Google Scholar] [CrossRef] [Green Version]
- van der Valk, M.J.; de Graaf, E.J.; Doornebosch, P.G.; Vermaas, M. Incisional Negative-Pressure Wound Therapy for Perineal Wounds After Abdominoperineal Resection for Rectal Cancer, a Pilot Study. Adv. Wound Care 2017, 6, 425–429. [Google Scholar] [CrossRef]
- Zwanenburg, P.R.; Tol, B.T.; De Vries, F.E.; Boermeester, M.A. Incisional Negative Pressure Wound Therapy for Surgical Site Infection Prophylaxis in the Post-Antibiotic Era. Surg. Infect. 2018, 19, 821–830. [Google Scholar] [CrossRef]
- Dayan, J.; Allen, R.J. Lower Extremity Free Flaps for Breast Reconstruction. Plast. Reconstr. Surg. 2017, 140, 77S–86S. [Google Scholar] [CrossRef]
- Dayan, J.H.; Allen, R.J. Neurotized Diagonal Profunda Artery Perforator Flaps for Breast Reconstruction. Plast. Reconstr. Surg.—Glob. Open 2019, 7, e2463. [Google Scholar] [CrossRef]
- Lhuaire, M.; Haddad, K.; Wirz, F.-S.; Abedalthaqafi, S.; Obadia, D.; Derder, M.; Marchac, A.; Benjoar, M.D.; Hivelin, M.; Lantieri, L.; et al. Medium- and Large-Sized Autologous Breast Reconstruction using a Fleur-de-lys Profunda Femoris Artery Perforator Flap Design: A Report Comparing Results with the Horizontal Profunda Femoris Artery Perforator Flap. J. Reconstr. Microsurg. 2019, 35, 8–14. [Google Scholar] [CrossRef]
- Marsidi, N.; Vermeulen, S.A.; Horeman, T.; Genders, R.E. Measuring Forces in Suture Techniques for Wound Closure. J. Surg. Res. 2020, 255, 135–143. [Google Scholar] [CrossRef]
- Bartlett, L.C. Pressure necrosis is the primary cause of wound dehiscence. Can. J. Surg. 1985, 28, 27–30. [Google Scholar]
- Kyriazidis, I.; Ali, S.R.; Maklad, M.; Curtin, E. Wound Closure Under Tension: It Takes Brains, Not Brawn. Aesthetic Surg. J. 2019, 39, NP11–NP12. [Google Scholar] [CrossRef] [PubMed]
- Pollhammer, M.S.; Duscher, D.; Schmidt, M.; Aitzetmüller, M.M.; Tschandl, P.; Froschauer, S.M.; Huemer, G.M. Double-Loop Dermal Suture: A Technique for High-Tension Wound Closure. Aesthetic Surg. J. 2016, 36, NP165–NP167. [Google Scholar] [CrossRef]
- Chen, C.-E.; Wang, T.-H. A modified technique for high-tension wound closure. J. Am. Acad. Dermatol. 2020, 83, e339–e340. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Liu, J.-W.; Brey, E.M.; Cheng, M.-H. The Effects of Negative Pressure by External Tissue Expansion Device on Epithelial Cell Proliferation, Neo-Vascularization and Hair Growth in a Porcine Model. PLoS ONE 2016, 11, e0154328. [Google Scholar] [CrossRef] [Green Version]
- Calomfiresco, A.; Wolski, V.; Grigoriu, T.; Bădulesco, E.; Peahă, M.; Ionesco, M.; Nitzoulesco, C. Correlation between the immunological level of the population and the circulation of C. diphtheriae during the stage of diphtheria eradication. Arch. Roum. Pathol. Exp. Microbiol. 1964, 23, 1053–1060. [Google Scholar] [PubMed]
- Muhammad, A.A.; Fakurazi, S.; Arulselvan, P.; See, C.P.; Abas, F. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des. Dev. Ther. 2016, 10, 1715–1730. [Google Scholar] [CrossRef] [Green Version]
- Borgquist, O.; Ingemansson, R.; Malmsjö, M. The Influence of Low and High Pressure Levels during Negative-Pressure Wound Therapy on Wound Contraction and Fluid Evacuation. Plast. Reconstr. Surg. 2011, 127, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.J.; Haddock, N.; Ahn, C.Y.; Sadeghi, A. Breast Reconstruction with the Profunda Artery Perforator Flap. Plast. Reconstr. Surg. 2012, 129, 16e–23e. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Sadeghi, A.; Allen, R.J. The Anatomic Basis of the Profunda Femoris Artery Perforator Flap: A New Option for Autologous Breast Reconstruction—A Cadaveric and Computer Tomography Angiogram Study. J. Reconstr. Microsurg. 2012, 28, 381–386. [Google Scholar] [CrossRef]
- Wallace, A.; Wood, D.A. Development of a simple procedure for the preparation of semipermeable antibody-containing microcapsules and their analytical performance in a radioimmunoassay for 17-hydroxyprogesterone. Clin. Chim. Acta 1984, 140, 203–212. [Google Scholar] [CrossRef]
- Cho, M.-J.; Teotia, S.S.; Haddock, N.T. Classification and Management of Donor-Site Wound Complications in the Profunda Artery Perforator Flap for Breast Reconstruction. J. Reconstr. Microsurg. 2020, 36, 110–115. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lessing, C.; Derrick, K. Healed Porcine Incisions Previously Treated With a Surgical Incision Management System: Mechanical, Histomorphometric, and Gene Expression Properties. Aesthetic Plast. Surg. 2014, 38, 767–778. [Google Scholar] [CrossRef]
- Harn, H.I.; Ogawa, R.; Hsu, C.; Hughes, M.W.; Tang, M.; Chuong, C. The tension biology of wound healing. Exp. Dermatol. 2019, 28, 464–471. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Sumpio, B.J.; Tsay, C.; Swallow, M.; Dash, B.; Thorn, S.L.; Sinusas, A.J.; Koo, A.; Hsia, H.C.; Au, A. Incisional Negative Pressure Wound Therapy Augments Perfusion and Improves Wound Healing in a Swine Model Pilot Study. Ann. Plast. Surg. 2019, 82 (Suppl. S4), S222–S227. [Google Scholar] [CrossRef]
- Tanaka, T.; Panthee, N.; Itoda, Y.; Yamauchi, N.; Fukayama, M.; Ono, M. Negative pressure wound therapy induces early wound healing by increased and accelerated expression of vascular endothelial growth factor receptors. Eur. J. Plast. Surg. 2016, 39, 247–256. [Google Scholar] [CrossRef]
- Ma, Z.; Shou, K.; Li, Z.; Jian, C.; Qi, B.; Yu, A. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp. Ther. Med. 2016, 11, 1307–1317. [Google Scholar] [CrossRef] [Green Version]
- Cremers, N.A.J.; Suttorp, M.; Gerritsen, M.M.; Wong, R.J.; Breda, C.V.R.-V.; van Dam, G.M.; Brouwer, K.M.; Kuijpers-Jagtman, A.M.; Carels, C.E.L.; Lundvig, D.M.S.; et al. Mechanical Stress Changes the Complex Interplay Between HO-1, Inflammation and Fibrosis, During Excisional Wound Repair. Front. Med. 2015, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Vargo, D. Negative pressure wound therapy in the prevention of wound infection in high risk abdominal wound closures. Am. J. Surg. 2012, 204, 1021–1024. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.W.; Paterno, J.; Sorkin, M.; Glotzbach, J.P.; Levi, K.; Januszyk, M.; Rustad, K.C.; Longaker, M.T.; Gurtner, G.C. Mechanical force prolongs acute inflammation via T-cell-dependent pathways during scar formation. FASEB J. 2011, 25, 4498–4510. [Google Scholar] [CrossRef]








| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| β-Actin | CGCGAGAAGATGACCCAGAT | GGAGGGCATACCCCTCGTAG |
| Collagen I | GACCTGCGTGTACCCCACTC | TGGGAAGCCTCAGTGGACAT |
| Collagen III | AGAGGAGTTGCCGGAGAACC | TTCCAGGAGCACCGTCATTT |
| Collagen VI | GCACTCCGGGAGGTACACAG | GGTCTCCTCGGACACCCTCT |
| CD31 | TTCTCCTGAGCGAGGAGGTG | CAAATGGCCTGGGTGTCATT |
| αSMA | GAACACGGCATCATCACCAA | GGACAGCACCGCCTGAATAG |
| CD14 | AACTGACGCTCGAGGACCTG | TGCTTGCGCCACTTTCAGTA |
| TNFα | TGTGCCTCAGCCTCTTCTCC | TGTCCCTCGGCTTTGACATT |
| Control Group | NP Group | p Value | |
|---|---|---|---|
| n = 13 | n = 12 | ||
| Age (years) | 39.2 ± 8.9 | 38.2 ± 5.5 | 0.746 |
| BMI (kg/m2) | 21.8 ± 3.2 | 20.4 ± 1.1 | 0.145 |
| Smoking | 0 | 0 | |
| Hypertension | 0 | 0 | |
| Diabetes mellitus | 0 | 0 | |
| TNM staging | |||
| Stage 0 (DCIS) | 3 (23.1%) | 7 (58.3%) | 0.092 |
| Stage I | 3 (23.1%) | 4 (33.3%) | |
| Stage II | 4 (30.8%) | 1(8.3%) | |
| Stage III | 3 (23.1%) | 0 (0%) | |
| ER status | |||
| Positive | 8 (61.5%) | 6 (50%) | 0.561 |
| Negative | 5 (38.5%) | 6 (50%) | |
| PR status | |||
| Positive | 8 (61.5%) | 6 (50%) | 0.561 |
| Negative | 5 (38.5%) | 6 (50%) | |
| HER-2 status | |||
| Positive | 9 (69.2%) | 9 (75%) | 1.000 |
| Negative | 4 (30.8%) | 3 (25%) |
| Control Group | NP Group | p Value | |
|---|---|---|---|
| Variables | n = 13 | n = 12 | |
| Flap width (cm) | 7.7 ± 1.3 | 7.8 ± 0.9 | 0.895 |
| Flap length (cm) | 21.2 ± 3.6 | 20.5 ± 2.9 | 0.585 |
| Pedicle length (cm) | 5.7 ± 1.5 | 5.8 ± 1.5 | 0.817 |
| Perforator numbers | 1.6 ± 0.7 | 1.4 ± 0.7 | 0.459 |
| Harvest weight (g) | 293.4 ± 87.9 | 224.9 ± 59.5 | 0.034 * |
| Flap used weight (g) | 271.9 ± 78.5 | 209.3 ± 55.2 | 0.032 * |
| Flap used (%) | 93.3 ± 6.8 | 93.1 ± 4.0 | 0.958 |
| Mastectomy weight (g) | 264.8 ± 99.8 | 236.6 ± 99.1 | 0.505 |
| Flap used weight/mastectomy weight (%) | 107.4 ± 42.1 | 93.7 ± 27.7 | 0.371 |
| Control Group | NP Group | p Value | |
|---|---|---|---|
| Variables | n = 13 | n = 12 | |
| Off-bed time (days) | 5.5 ± 0.8 | 4.6 ± 1.1 | 0.028 * |
| Drainage amount (mL) | 166.8 ± 62.9 | 156.9 ± 57.3 | 0.701 |
| Vacuum ball removed timing (post-op days) | 8.4 ± 1.4 | 7.7 ± 1.5 | 0.269 |
| Vancouver scar scale | 5.3 ± 2.9 | 5.8 ± 2.5 | 0.693 |
| Re-exploration | 2 (15.4%) | 1 (8.3%) | 1.000 |
| Donor site acute complications (<30 days) | 3 (23.1%) | 0 | 0.22 |
| Wound breakdown required surgery | 2 | 0 | |
| Wound dehiscence with wound care | 1 | 0 | |
| Donor site long-term complications (>30 days) | 3 (23.1%) | 1 (8.3%) | 0.593 |
| Wound breakdown required surgery | 1 | 1 | |
| Wound dehiscence with wound care | 2 | 0 | |
| Donor-site revision | 3 (23.1%) | 2 (16.7%) | 1.000 |
| Lower limb lymphedema | 0 | 0 | |
| Distortion of major labia | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, H.-Y.; Hsieh, W.-C.; Chang, F.C.-S.; Liu, J.-W.; Kuo, W.-L.; Cheong, D.C.-F.; Huang, J.-J. The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions under High Tension: Evidence from Animal Studies and Clinical Experience. J. Clin. Med. 2023, 12, 106. https://doi.org/10.3390/jcm12010106
Hsiao H-Y, Hsieh W-C, Chang FC-S, Liu J-W, Kuo W-L, Cheong DC-F, Huang J-J. The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions under High Tension: Evidence from Animal Studies and Clinical Experience. Journal of Clinical Medicine. 2023; 12(1):106. https://doi.org/10.3390/jcm12010106
Chicago/Turabian StyleHsiao, Hui-Yi, Wei-Chuan Hsieh, Frank Chun-Shin Chang, Jia-Wei Liu, Wen-Ling Kuo, David Chon-Fok Cheong, and Jung-Ju Huang. 2023. "The Effect of Negative Pressure on Wound Healing and Regeneration in Closed Incisions under High Tension: Evidence from Animal Studies and Clinical Experience" Journal of Clinical Medicine 12, no. 1: 106. https://doi.org/10.3390/jcm12010106





