Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnostic Procedures and Imaging Protocols
2.2. Image Analysis and Postprocessing
2.3. Image Grading
2.4. Texture Analysis
2.5. Fissure Classification
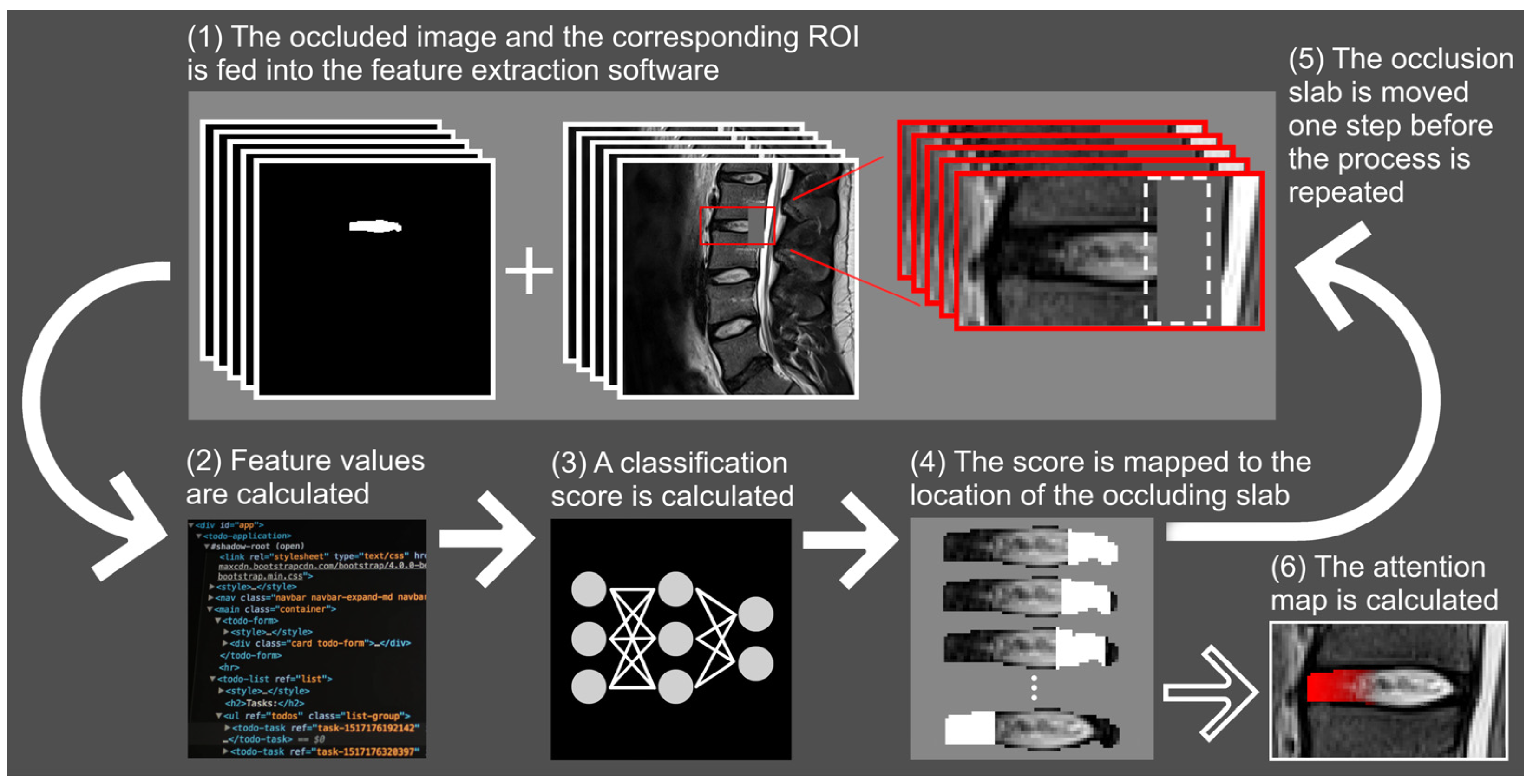
2.6. Fissure Localization with Attention Mapping
2.7. Statistical Analysis
3. Results
3.1. Fissure Classification
3.2. Fissure Localization with Attention Mapping
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Texture Analysis
Appendix A.2. Fissure Classification
- net.trainParam.epochs = 5000;
- net.trainParam.max_fail = 500;
- net.trainParam.min_grad = 1 × 10−10;
- net.trainParam.lr = 0.10;
- net.trainParam.lr_inc = 1.30;
- net.trainParam.lr_dec = 0.9;
- net.trainParam.max_perf_inc = 1.5.

Appendix A.3. Fissure Localization with Attention Mapping
References
- Huber, F.A.; Guggenberger, R. AI MSK clinical applications: Spine imaging. Skeletal Radiol. 2022, 51, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Waldenberg, C.; Hebelka, H.; Brisby, H.; Lagerstrand, K.M. Differences in IVD characteristics between low back pain patients and controls associated with HIZ as revealed with quantitative MRI. PLoS ONE 2019, 14, e0220952. [Google Scholar] [CrossRef] [PubMed]
- Waldenberg, C.; Hebelka, H.; Brisby, H.; Lagerstrand, K.M. MRI histogram analysis enables objective and continuous classification of intervertebral disc degeneration. Eur. Spine J. 2017, 27, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Waldenberg, C.; Torén, L.; Grimby-Ekman, A.; Brisby, H.; Hebelka, H.; Lagerstrand, K. Texture Analysis of Magnetic Resonance Images Enables Phenotyping of Potentially Painful Annular Fissures. Spine 2021, 47, 430–437. [Google Scholar] [CrossRef]
- Hansson, E.K.; Hansson, T.H. The costs for persons sick-listed more than one month because of low back or neck problems. A two-year prospective study of Swedish patients. Eur. Spine J. 2005, 14, 337–345. [Google Scholar] [CrossRef]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef]
- Videman, T.; Nurminen, M. The occurrence of anular tears and their relation to lifetime back pain history: A cadaveric study using barium sulfate discography. Spine 2004, 29, 2668–2676. [Google Scholar] [CrossRef]
- Lim, C.-H.; Jee, W.-H.; Son, B.C.; Kim, D.-H.; Ha, K.-Y.; Park, C.-K. Discogenic lumbar pain: Association with MR imaging and CT discography. Eur. J. Radiol. 2005, 54, 431–437. [Google Scholar] [CrossRef]
- Sharma, A.; Pilgram, T.; Wippold, F.J. Association between Annular Tears and Disk Degeneration: A Longitudinal Study. Am. J. Neuroradiol. 2009, 30, 500–506. [Google Scholar] [CrossRef]
- Adams, M.A. Biomechanics of back pain. Acupunct. Med. 2004, 22, 178–188. [Google Scholar] [CrossRef]
- Deneuville, J.-P.; Yushchenko, M.; Vendeuvre, T.; Germaneau, A.; Billot, M.; Roulaud, M.; Sarracanie, M.; Salameh, N.; Rigoard, P. Quantitative MRI to Characterize the Nucleus Pulposus Morphological and Biomechanical Variation According to Sagittal Bending Load and Radial Fissure, an ex vivo Ovine Specimen Proof-of-Concept Study. Front. Bioeng. Biotechnol. 2021, 9, 676003. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hou, S.; Wu, W.; Zhang, C.; Yang, Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur. Spine J. 2006, 15, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.N.; Ross, E.R.S.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Aprill, C.; Bogduk, N. High-intensity zone: A diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br. J. Radiol. 1992, 65, 361–369. [Google Scholar] [CrossRef]
- Berger-Roscher, N.; Galbusera, F.; Rasche, V.; Wilke, H.-J. Intervertebral disc lesions: Visualisation with ultra-high field MRI at 11.7 T. Eur. Spine J. 2015, 24, 2488–2495. [Google Scholar] [CrossRef]
- Carragee, E.J.; Don, A.S.; Hurwitz, E.L.; Cuellar, J.M.; Carrino, J.A.; Herzog, R. 2009 ISSLS Prize Winner: Does discography cause accelerated progression of degeneration changes in the lumbar disc: A ten-year matched cohort study. Spine 2009, 34, 2338–2345. [Google Scholar] [CrossRef]
- Hebelka, H.; Nilsson, A.; Hansson, T. Pressure Increase in Adjacent Discs During Clinical Discography Questions the Methods Validity. Spine 2014, 39, 893–899. [Google Scholar] [CrossRef]
- Eldaya, R.W.; Parsons, M.S.; Orlowski, H.L.P.; Reis, M.N.; Sharma, A. Evaluating the effect of a post-processing algorithm in detection of annular fissure on MR imaging. Eur. Spine J. 2021, 30, 2150–2156. [Google Scholar] [CrossRef]
- Raudner, M.; Schreiner, M.M.; Hilbert, T.; Kober, T.; Weber, M.; Szelényi, A.; Windhager, R.; Juras, V.; Trattnig, S. Clinical implementation of accelerated T2 mapping: Quantitative magnetic resonance imaging as a biomarker for annular tear and lumbar disc herniation. Eur. Radiol. 2021, 31, 3590–3599. [Google Scholar] [CrossRef]
- Torén, L.; Lagerstrand, K.; Waldenberg, C.; Brisby, H.; Hebelka, H. MRI During Spinal Loading Reveals Intervertebral Disc Behavior Corresponding to Discogram Findings of Annular Fissures and Pain Provocation. Spine 2020, 45, E1500–E1506. [Google Scholar] [CrossRef]
- Hebelka, H.; Hansson, T. HIZ’s relation to axial load and low back pain: Investigated with axial loaded MRI and pressure controlled discography. Eur. Spine J. 2013, 22, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Pfaehler, E.; Zwanenburg, A.; de Jong, J.R.; Boellaard, R. RaCaT: An open source and easy to use radiomics calculator tool. PLoS ONE 2019, 14, e0212223. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, A.; Leger, S.; Vallières, M.; Löck, S. Image biomarker standardisation initiative. arXiv 2016, arXiv:1612.07003. [Google Scholar]
- Sachs, B.L.; Vanharanta, H.; Spivey, M.A.; Guyer, R.D.; Videman, T.; Rashbaum, R.F.; Johnson, R.G.; Hochschuler, S.H.; Mooney, V. Dallas discogram description. A new classification of CT/discography in low-back disorders. Spine 1987, 12, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Derby, R.; Kim, B.-J.; Chen, Y.; Seo, K.-S.; Lee, S.-H. The Relation Between Annular Disruption on Computed Tomography Scan and Pressure-Controlled Diskography. Arch. Phys. Med. Rehabil. 2005, 86, 1534–1538. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Senck, S.; Trieb, K.; Kastner, J.; Hofstaetter, S.G.; Lugmayr, H.; Windisch, G. Visualization of intervertebral disc degeneration in a cadaveric human lumbar spine using microcomputed tomography. J. Anat. 2020, 236, 243–251. [Google Scholar] [CrossRef]
- Shan, Z.; Chen, H.; Liu, J.; Ren, H.; Zhang, X.; Zhao, F. Does the high-intensity zone (HIZ) of lumbar Intervertebral discs always represent an annular fissure? Eur. Radiol. 2017, 27, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, M.D.; Fergus, R. Visualizing and Understanding Convolutional Networks. In Proceedings of the European conference on computer vision, Zurich, Switzerland, 6–12 September 2014; pp. 818–833. [Google Scholar]
- Sharma, A.; Parsons, M.; Pilgram, T. Temporal interactions of degenerative changes in individual components of the lumbar intervertebral discs: A sequential magnetic resonance imaging study in patients less than 40 years of age. Spine 2011, 36, 1794–1800. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Jamaludin, A.; Kadir, T.; Zisserman, A. SpineNet: Automated classification and evidence visualization in spinal MRIs. Med. Image Anal. 2017, 41, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Hashia, B.; Mir, A.H. Texture features’ based classification of MR images of normal and herniated intervertebral discs. Multimed. Tools Appl. 2020, 79, 15171–15190. [Google Scholar] [CrossRef]
- Nilsson, M.; Lagerstrand, K.; Kasperska, I.; Brisby, H.; Hebelka, H. Axial loading during MRI influences T2-mapping values of lumbar discs: A feasibility study on patients with low back pain. Eur. Spine J. 2016, 25, 2856–2863. [Google Scholar] [CrossRef]
- Muriuki, M.G.; Havey, R.M.; Voronov, L.I.; Carandang, G.; Zindrick, M.R.; Lorenz, M.A.; Lomasney, L.; Patwardhan, A.G. Effects of motion segment level, Pfirrmann intervertebral disc degeneration grade and gender on lumbar spine kinematics. J. Orthop. Res. 2016, 34, 1389–1398. [Google Scholar] [CrossRef]



| Parameter | T1-Weighted MRI (TSE) a | T1-Weighted MRI (SE) a | T2-Weighted MRI (TSE) a | T2-Weighted MRI (TSE) a | CT b |
|---|---|---|---|---|---|
| Imaging plane | Sagittal | Axial | Sagittal | Axial | Sagittal, axial |
| Repetition time (ms) | 448 | 500 | 4862 | 5000 | |
| Echo time (ms) | 11 | 15 | 97 | 119 | |
| Echo train length | 9 | 1 | 21 | 25 | |
| Slice thickness (mm) | 4.0 | 4.0 | 4.0 | 4.0 | 0.75 (reconstructed) |
| Slice gap (mm) | 0.4 | 0.4 | 0.4 | 0.4 | |
| Number of averages | 4 | 2 | 2 | 4 | |
| Pixel bandwidth (Hz) | 200 | 100 | 190 | 190 | |
| Flip angle (degree) | 149 | 90 | 150 | 150 | |
| Acquisition matrix | 512 × 256 | 256 × 135 | 512 × 256 | 256 × 126 | |
| Reconstruction matrix | 512 × 512 | 384 × 512 | 512 × 512 | 360 × 512 | 512 × 512 |
| Field of view (mm2) | 300 × 300 | 135 × 180 | 300 × 300 | 127 × 180 | 162 × 162 |
| Convolution kernel | B45s |
| Feature Group | Feature Name |
|---|---|
| Morphology | Geary’s C |
| Statistics | maximum |
| range | |
| Intensity volume | int at vol fraction 90 |
| Intensity histogram | 10th percentile |
| mode | |
| glcmFeatures2Davg | difference average |
| dissimilarity | |
| glcmFeatures2DDmrg | difference average |
| difference variance | |
| contrast | |
| dissimilarity | |
| glcmFeatures2Dmrg | difference average |
| glcmFeatures2Dvmrg | difference variance |
| contrast | |
| dissimilarity | |
| glcmFeatures3Davg | difference average |
| difference variance | |
| dissimilarity | |
| ngtdmFeatures2Dmrg | complexity |
| ngtdmFeatures3D | complexity |
| ngldmFeatures3Dmrg | dependence count energy |
| Patient and IVD Characteristics | Included to Create Classification Model | Included to Create Attention Maps | |
|---|---|---|---|
| Age (years) | 45 ± 9 * | 45 ± 9 * | |
| No. of patients | 43 | 42 | |
| No. of females | 24 (56) | 23 (55) | |
| No. of IVDs | 123 | 104 | |
| IVD segment | L1–L2 | 2 (2) | 2 (2) |
| L2–L3 | 15 (12) | 13 (13) | |
| L3–L4 | 40 (33) | 36 (35) | |
| L4–L5 | 40 (33) | 35 (34) | |
| L5–S1 | 26 (21) | 18 (17) | |
| Pfirrmann classification | Grade 1 | 0 (0) | 0 (0) |
| Grade 2 | 20 (16) | 20 (19) | |
| Grade 3 | 48 (39) | 43 (41) | |
| Grade 4 | 51 (41) | 41 (39) | |
| Grade 5 | 4 (3) | 0 (0) | |
| High-intensity zone presence | None | 62 (50) | 56 (54) |
| Ventral only | 2 (2) | 2 (2) | |
| Dorsal only | 57 (46) | 44 (42) | |
| Ventral and Dorsal | 2 (2) | 2 (2) | |
| Dallas discogram description | Grade 0 | 8 (7) | 8 (8) |
| Grade 1 | 21(17) | 21 (20) | |
| Grade 2 | 87 (71) | 69 (66) | |
| Grade 3 | 7 (6) | 6 (6) | |
| Annular fissure presence | None | - | 29 (28) |
| Ventral only | - | 3 (3) | |
| Dorsal only | - | 61 (59) | |
| Lateral only | - | 2 (2) | |
| Ventral and Dorsal | - | 9 (9) |
| IVD No. | True Fissure Position | Fissure Position Determined in the Attention Map | Segment | Pfirrmann Classification | HIZ Position | Dallas Discogram Description |
|---|---|---|---|---|---|---|
| 1 a | Dorsal only | Ventral (border zone NP AF) | L4–L5 | Grade 4 | Dorsal | Grade 3 |
| 2 | Dorsal only | Ventral and dorsal | L4–L5 | Grade 3 | None | Grade 2 |
| 3 | Dorsal only | Ventral | L2–L3 | Grade 3 | Dorsal | Grade 2 |
| 4 b | Lateral only | Ventral and dorsal (border zone NP AF) | L4–L5 | Grade 3 | None | Grade 2 |
| 5 | Lateral only | Dorsal (border zone NP AF) | L4–L5 | Grade 3 | None | Grade 2 |
| 6 c | Ventral and dorsal | Dorsal | L3–L4 | Grade 4 | Dorsal | Grade 2 |
| 7 c | Ventral and dorsal | Dorsal | L3–L4 | Grade 3 | None | Grade 2 |
| 8 c | Ventral and dorsal | Dorsal | L3–L4 | Grade 4 | Ventral | Grade 2 |
| 9 c | Ventral and dorsal | Dorsal | L5–S1 | Grade 3 | Dorsal | Grade 2 |
| 10 d | Ventral and dorsal | Dorsal | L2–L3 | Grade 3 | None | Grade 2 |
| 11 e | Ventral and dorsal | Dorsal | L2–L3 | Grade 4 | None | Grade 2 |
| 12 a | Ventral and dorsal | Ventral | L4–L5 | Grade 4 | None | Grade 2 |
| 13 | Ventral only | Dorsal | L3–L4 | Grade 3 | Ventral | Grade 2 |
| 14 f | None | Dorsal | L2–L3 | Grade 3 | None | Grade 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldenberg, C.; Eriksson, S.; Brisby, H.; Hebelka, H.; Lagerstrand, K.M. Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics. J. Clin. Med. 2023, 12, 11. https://doi.org/10.3390/jcm12010011
Waldenberg C, Eriksson S, Brisby H, Hebelka H, Lagerstrand KM. Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics. Journal of Clinical Medicine. 2023; 12(1):11. https://doi.org/10.3390/jcm12010011
Chicago/Turabian StyleWaldenberg, Christian, Stefanie Eriksson, Helena Brisby, Hanna Hebelka, and Kerstin Magdalena Lagerstrand. 2023. "Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics" Journal of Clinical Medicine 12, no. 1: 11. https://doi.org/10.3390/jcm12010011
APA StyleWaldenberg, C., Eriksson, S., Brisby, H., Hebelka, H., & Lagerstrand, K. M. (2023). Detection of Imperceptible Intervertebral Disc Fissures in Conventional MRI—An AI Strategy for Improved Diagnostics. Journal of Clinical Medicine, 12(1), 11. https://doi.org/10.3390/jcm12010011





