Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Patient-Reported Outcomes Assessments
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Effect of Mobocertinib Treatment on Core Symptoms of Lung Cancer
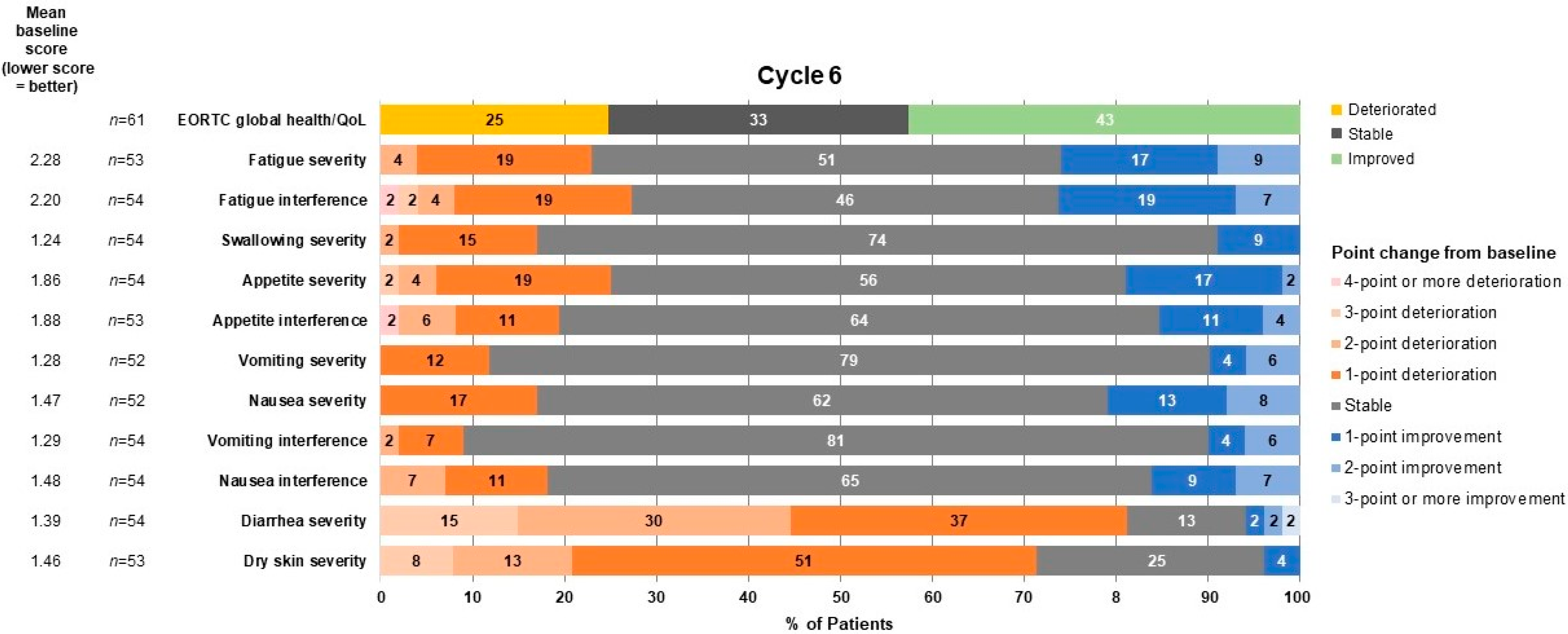
3.3. Effect of Mobocertinib Treatment on Global Health Status/QoL and Other Functions and Symptoms
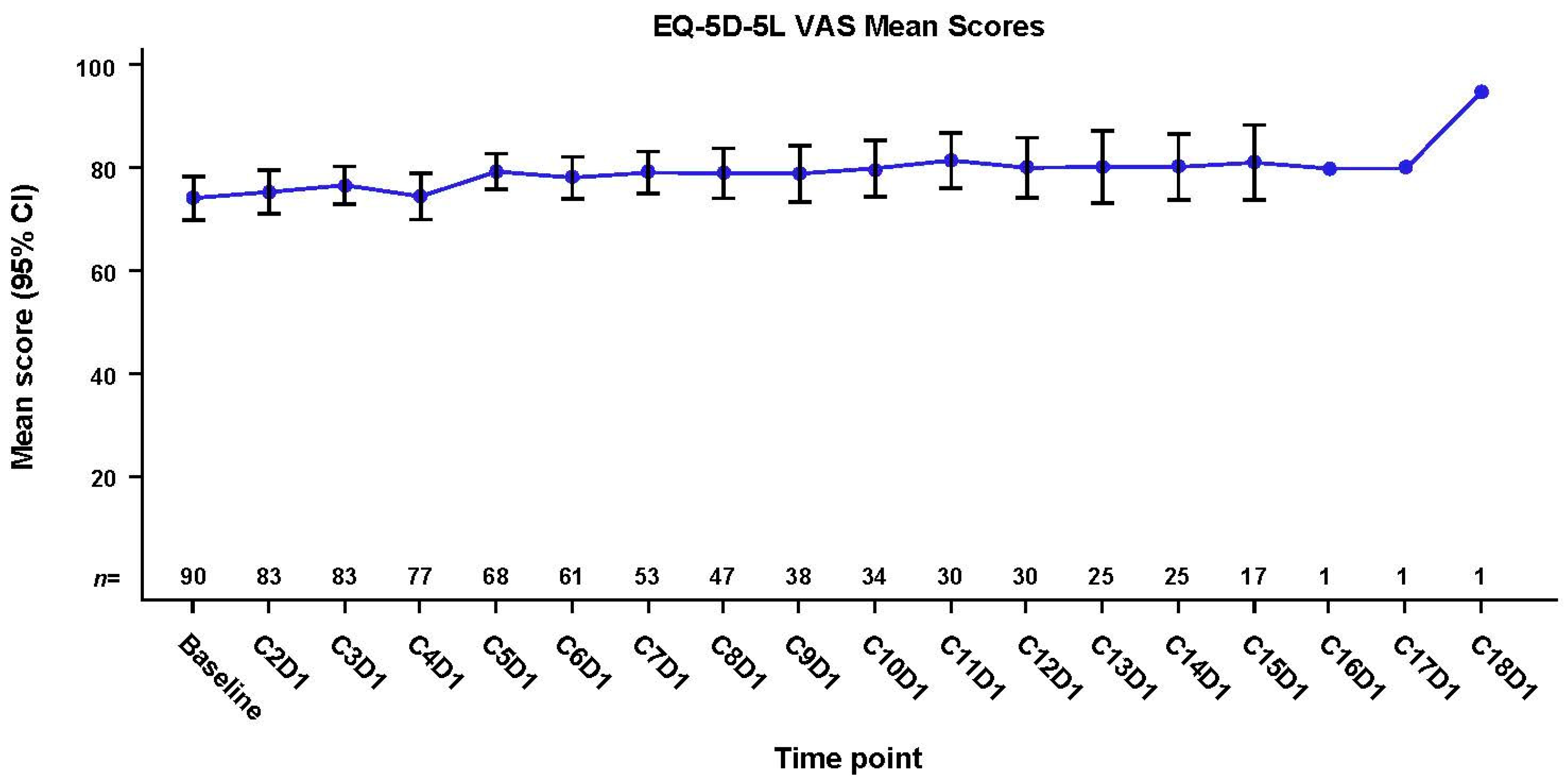
3.4. Effect of Mobocertinib Treatment on General HRQoL
3.5. Patient-Reported Symptomatic Treatment-Related Toxicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gralla, R.J.; Hollen, P.J.; Msaouel, P.; Davis, B.V.; Petersen, J. An Evidence-Based Determination of Issues Affecting Quality of Life and Patient-Reported Outcomes in Lung Cancer: Results of a Survey of 660 Patients. J. Thorac. Oncol. 2014, 9, 1243–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, P.T.; Vyse, S.; Huang, P.H. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin. Cancer Biol. 2020, 61, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, H.; Matsumoto, S.; Ohe, Y.; Satouchi, M.; Furuya, N.; Kim, Y.H.; Seto, T.; Soejima, K.; Hayakawa, D.; Kato, T.; et al. OA07.03 Clinical Outcome of Non-Small Cell Lung Cancer with EGFR/HER2 Exon 20 Insertions Identified in the LC-SCRUM-Japan. J. Thorac. Oncol. 2019, 14, S224. [Google Scholar] [CrossRef]
- Riess, J.W.; Gandara, D.R.; Frampton, G.M.; Madison, R.; Peled, N.; Bufill, J.A.; Dy, G.K.; Ou, S.-H.I.; Stephens, P.J.; McPherson, J.D.; et al. Diverse EGFR Exon 20 Insertions and Co-Occurring Molecular Alterations Identified by Comprehensive Genomic Profiling of NSCLC. J. Thorac. Oncol. 2018, 13, 1560–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, W.; Huang, Y.; Hong, S.; Zhang, Z.; Wang, M.; Gan, J.; Wang, W.; Guo, H.; Wang, K.; Zhang, L. EGFR exon 20 insertion mutations and response to osimertinib in non-small-cell lung cancer. BMC Cancer 2019, 19, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byeon, S.; Kim, Y.; Lim, S.W.; Cho, J.H.; Park, S.; Lee, J.; Sun, J.-M.; Choi, Y.-L.; Lee, S.-H.; Ahn, J.S.; et al. Clinical Outcomes of EGFR Exon 20 Insertion Mutations in Advanced Non-small Cell Lung Cancer in Korea. Cancer Res. Treat. 2019, 51, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yang, G.; Li, J.; Xu, H.; Yang, L.; Xu, F.; Yang, Y.; Xia, B.; Zhu, V.W.; Qiu, W.; et al. Real-world treatment outcome of advanced Chinese NSCLC EGFR exon 20 insertion patients. J. Clin. Oncol. 2019, 37, 9043. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Vincent, S.; Baker, T.E.; Gould, A.E.; Li, S.; Wardwell, S.D.; Nadworny, S.; Ning, Y.; Zhang, S.; Huang, W.-S.; et al. Mobocertinib (TAK-788): A Targeted Inhibitor of EGFR Exon 20 Insertion Mutants in Non–Small Cell Lung Cancer. Cancer Discov. 2021, 11, 1672–1687. [Google Scholar] [CrossRef] [PubMed]
- Takeda Pharmaceuticals America. Exkivity [Package Insert]; Takeda Pharmaceuticals America, Inc.: Lexington, MA, USA, 2021. [Google Scholar]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.-W.; Yang, J.C.-H.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Garcia Campelo, M.R.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients with EGFR Exon 20 Insertion–Positive Metastatic Non–Small Cell Lung Cancer: A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol. 2021, 7, e214761. [Google Scholar] [CrossRef] [PubMed]
- Riely, G.J.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; Bazhenova, L.; et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non–Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov. 2021, 11, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.; Aaronson, N.; Ahmedzai, S.; Kaasa, S.; Sullivan, M. The EORTC QLQ-LC13: A Modular Supplement to the EORTC Core Quality of Life Questionnaire (QLQ-C30) for Use in Lung Cancer Clinical Trials. Eur. J. Cancer 1994, 30, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and Preliminary Testing of the New Five-level Version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, J.; Roberts, L.; Jean-Baptiste, M.; Revicki, D.; Salvatore, G.; Li, S.; Galaznik, A. P3.15-03 Capturing the Patient Experience for the Treatment of EGFR Exon 20 Mutations in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, S991–S992. [Google Scholar] [CrossRef] [Green Version]
- DeTora, L.M.; Toroser, D.; Sykes, A.; Vanderlinden, C.; Plunkett, F.J.; Lane, T.; Hanekamp, E.; Dormer, L.; DiBiasi, F.; Bridges, D.; et al. Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 Update. Ann. Intern. Med. 2022, 175, 1298–1304. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | EXCLAIM Cohort (N = 96) |
|---|---|
| Median age (range), y | 59 (27–80) |
| Female, n (%) | 62 (65) |
| Race, n (%) | |
| Asian | 66 (69) |
| White | 28 (29) |
| Black | 2 (2) |
| Other | 0 (0) |
| Histology, n (%) | |
| Adenocarcinoma | 95 (99) |
| Squamous carcinoma | 1 (1) |
| Large cell carcinoma | 0 (0) |
| ECOG PS, n (%) | |
| 0 | 28 (29) |
| 1 | 68 (71) |
| Smoking history, n (%) | |
| Never | 70 (73) |
| Current | 2 (2) |
| Former | 24 (25) |
| Prior systemic anticancer regimens, n (%) | |
| 1 | 49 (51) |
| 2 | 30 (31) |
| ≥3 | 17 (18) |
| Median number of prior regimens, n | 1 |
| Prior platinum-based chemotherapy, n (%) | 86 (90) |
| Prior immunotherapy, n (%) | 33 (34) |
| Prior EGFR TKI, n (%) | 30 (31) |
| Baseline stable brain metastases, n (%) | 33 (34) |
| LSM Change From Baseline [95% CI] | |||
|---|---|---|---|
| Timepoint | Dyspnea Subscale | Cough Subscale | Chest Pain Subscale |
| C2D1 | −4.4 [−7.6, −1.2] (n = 86) | −10.1 [−13.9, −6.3] (n = 86) | −10.9 [−14.3, −7.6] (n = 86) |
| C3D1 | −5.2 [−8.4, −2.0] (n = 83) | −9.5 [−13.4, −5.7] (n = 83) | −9.0 [−12.4, −5.6] (n = 83) |
| C4D1 | −3.8 [−7.1, −0.5] (n = 77) | −8.5 [−12.4, −4.6] (n = 77) | −8.6 [−12.1, −5.1] (n = 77) |
| C5D1 | −4.9 [−8.3, −1.5] (n = 68) | −7.5 [−11.5, −3.4] (n = 68) | −8.2 [−11.8, −4.5] (n = 68) |
| C6D1 | −3.9 [−7.4, −0.4] (n = 61) | −7.4 [−11.7, −3.2] (n = 61) | −7.7 [−11.5, −3.9] (n = 61) |
| C7D1 | −4.3 [−7.9, −0.7] (n = 54) | −9.9 [−14.3, −5.5] (n = 54) | −6.5 [−10.5, −2.5] (n = 54) |
| C8D1 | −2.9 [−6.7, 0.9] (n = 47) | −12.0 [−16.6, −7.4] (n = 47) | −6.1 [−10.3, −1.9] (n = 47) |
| C9D1 | −1.9 [−5.9, 2.1] (n = 38) | −9.9 [−14.8, −4.9] (n = 38) | −8.0 [−12.6, −3.5] (n = 38) |
| C10D1 | −3.5 [−7.7, 0.7] (n = 34) | −12.3 [−17.4, −7.1] (n = 34) | −10.3 [−15.0, −5.5] (n = 34) |
| C11D1 | −3.7 [−8.1, 0.7] (n = 30) | −12.6 [−18.0, −7.2] (n = 30) | −9.3 [−14.3, −4.3] (n = 30) |
| C12D1 | −1.2 [−5.6, 3.2] (n = 30) | −13.7 [−19.1, −8.3] (n = 30) | −6.4 [−11.5, −1.4] (n = 30) |
| C13D1 | −3.5 [−8.2, 1.1] (n = 25) | −13.0 [−18.8, −7.2] (n = 25) | −8.3 [−13.7, −2.9] (n = 25) |
| C14D1 | −3.4 [−8.2, 1.3] (n = 24) | −11.8 [−17.6, −5.9] (n = 24) | −11.1 [−16.6, −5.6] (n = 24) |
| C15D1 | −3.7 (−9.0, 1.7) (n = 17) | −11.0 [−17.7, −4.3] (n = 17) | −8.0 [−14.3, −1.6] (n = 17) |
| C16D1 | −7.5 (−27.1, 12.1) (n = 1) | −13.2 [−38.4, 11.9] (n = 1) | −9.8 [−34.5, 14.9] (n = 1) |
| C17D1 | −7.5 (−27.1, 12.1) (n = 1) | −13.2 [−38.4, 11.9] (n = 1) | −9.8 [−34.5, 14.9] (n = 1) |
| C18D1 | −3.9 (−23.5, 15.7) (n = 1) | −25.2 [−50.3, 0] (n = 1) | −8.8 [−33.6, 15.9] (n = 1) |
| EOT | 3.3 (−0.9, 7.4) (n = 35) | −8.0 [−13.1, −2.9] (n = 35) | −6.3 [−11.1, −1.5] (n = 34) |
| 30D ALD | 4.9 (−0.2, 10.1) (n = 20) | −1.8 [−8.2, 4.6] (n = 20) | −1.0 [−7.0, 5.1] (n = 20) |
| Measure | N | LSM (SE) | 95% CI | p Value |
|---|---|---|---|---|
| Global health status/QoL | 86 | −1.8 (1.50) | (−4.8, 1.2) | 0.235 |
| Physical functioning | 86 | 0 (1.33) | (−2.7, 2.6) | 0.986 |
| Role functioning | 86 | −2.4 (1.76) | (−5.9, 1.1) | 0.178 |
| Emotional functioning | 86 | 0.1 (1.26) | (−2.4, 2.6) | 0.920 |
| Cognitive functioning | 86 | −2.3 (1.29) | (−4.9, 0.2) | 0.072 |
| Social functioning | 86 | 1.0 (1.80) | (−2.6, 4.6) | 0.576 |
| Fatigue | 86 | 1.5 (1.6) | (−1.6, 4.7) | 0.335 |
| Nausea and vomiting | 86 | 0.6 (1.12) | (−1.6, 2.9) | 0.576 |
| Pain | 86 | −3.3 (1.74) | (−6.8, 0.1) | 0.060 |
| Dyspnea | 86 | −5.1 (1.54) | (−8.1, −2.0) | 0.002 |
| Insomnia | 86 | −6.5 (1.93) | (−10.4, −2.7) | 0.001 |
| Appetite loss | 86 | 6.6 (2.19) | (2.2, 10.9) | 0.004 |
| Constipation | 86 | −5.7 (1.08) | (−7.9, −3.6) | <0.001 |
| Diarrhea | 86 | 34.1 (2.10) | (29.9, 38.2) | <0.001 |
| Financial difficulties | 86 | 0.5 (1.85) | (−3.2, 4.2) | 0.786 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Campelo, M.R.; Zhou, C.; Ramalingam, S.S.; Lin, H.M.; Kim, T.M.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Goodman, E.; Mehta, M.; et al. Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort. J. Clin. Med. 2023, 12, 112. https://doi.org/10.3390/jcm12010112
Garcia Campelo MR, Zhou C, Ramalingam SS, Lin HM, Kim TM, Riely GJ, Mekhail T, Nguyen D, Goodman E, Mehta M, et al. Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort. Journal of Clinical Medicine. 2023; 12(1):112. https://doi.org/10.3390/jcm12010112
Chicago/Turabian StyleGarcia Campelo, Maria Rosario, Caicun Zhou, Suresh S. Ramalingam, Huamao M. Lin, Tae Min Kim, Gregory J. Riely, Tarek Mekhail, Danny Nguyen, Erin Goodman, Minal Mehta, and et al. 2023. "Mobocertinib (TAK-788) in EGFR Exon 20 Insertion+ Metastatic NSCLC: Patient-Reported Outcomes from EXCLAIM Extension Cohort" Journal of Clinical Medicine 12, no. 1: 112. https://doi.org/10.3390/jcm12010112





