The Cystic Anechoic Zone of Uterine Cavity Newly Observed during Controlled Ovarian Hyperstimulation Affects Pregnancy Outcomes of Fresh Embryo Transfer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. IVF/ICSI-ET Procedures
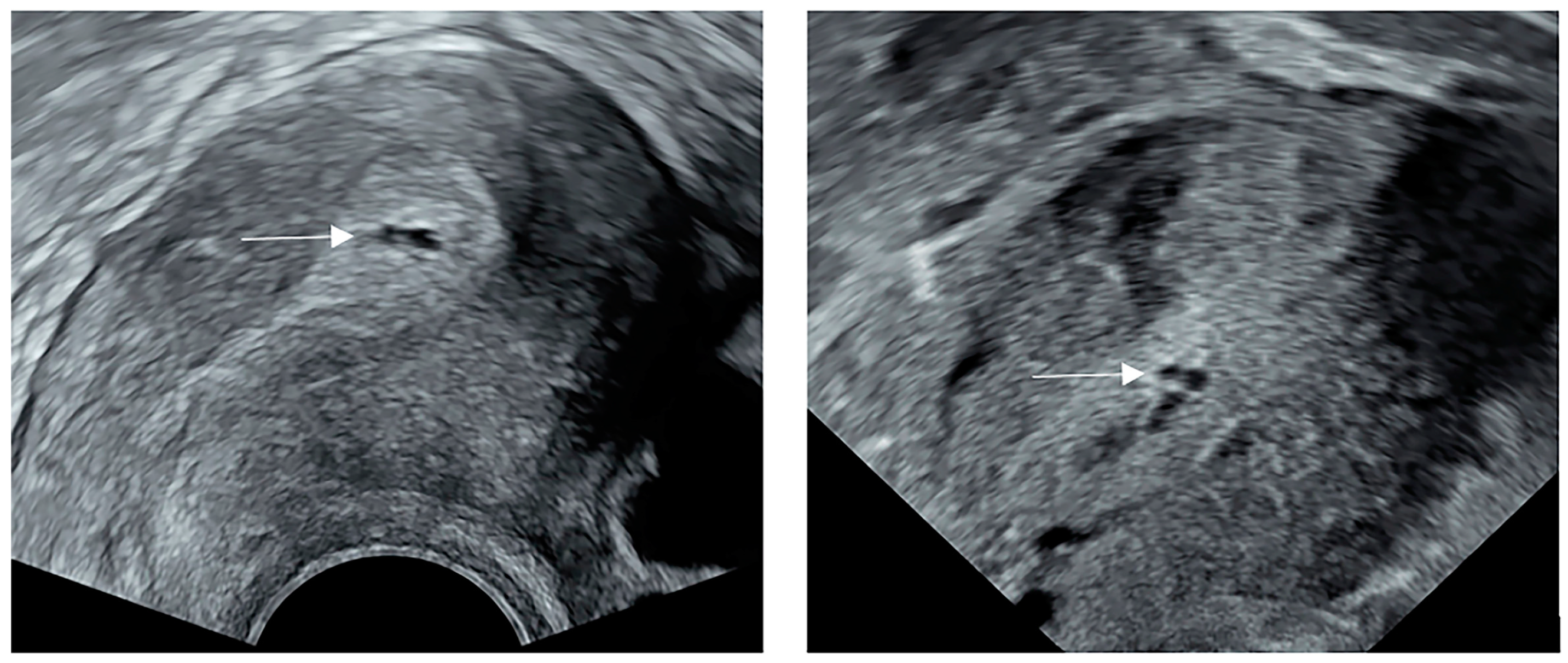
2.3. Ultrasonography Examination
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovacs, P.; Matyas, S.; Kaali, S.G. Embryo selection or uterine environment: Which plays the greater role in blastocyst transfer cycles? J. Obstet. Gynaecol. Res. 2011, 37, 416–421. [Google Scholar] [PubMed]
- Roberts, S.A.; Hirst, W.M.; Brison, D.R.; Vail, A. Embryo and uterine influences on IVF outcomes: An analysis of a UK multi-centre cohort. Hum. Reprod. 2010, 25, 2792–2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindhard, A.; Ravn, V.; Bentin-Ley, U.; Horn, T.; Bangsboell, S.; Rex, S.; Toft, B.; Soerensen, S. Ultrasound characteristics and histological dating of the endometrium in a natural cycle in infertile women compared with fertile controls. Fertil. Steril. 2006, 86, 1344–1355. [Google Scholar] [CrossRef]
- Lass, A.; Williams, G.; Abusheikha, N.; Brinsden, P. The effect of endometrial polyps on outcomes of in vitro fertilization (IVF) cycles. J. Assist. Reprod. Genet. 1999, 16, 410–415. [Google Scholar] [CrossRef]
- Isikoglu, M.; Berkkanoglu, M.; Senturk, Z.; Coetzee, K.; Ozgur, K. Endometrial polyps smaller than 1. 5 cm do not affect ICSI outcome. Reprod. Biomed. Online 2006, 12, 199–204. [Google Scholar] [CrossRef]
- Lane, B.F.; Wong-You-Cheong, J.J. Imaging of endometrial pathology. Clin. Obstet. Gynecol. 2009, 52, 57–72. [Google Scholar] [CrossRef]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am. J. Obstet. Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Casini, M.L.; Rossi, F.; Agostini, R.; Unfer, V. Effects of the position of fibroids on fertility. Gynecol. Endocrinol. 2006, 22, 106–109. [Google Scholar] [CrossRef]
- Somigliana, E.; Vercellini, P.; Daguati, R.; Pasin, R.; De Giorgi, O.; Crosignani, P.G. Fibroids and female reproduction: A critical analysis of the evidence. Hum. Reprod. Update 2007, 13, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Pritts, E.A.; Parker, W.H.; Olive, D.L. Fibroids and infertility: An updated systematic review of the evidence. Fertil. Steril. 2009, 91, 1215–1223. [Google Scholar] [CrossRef]
- Dreisler, E.; Sorensen, S.S.; Ibsen, P.H.; Lose, G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet. Gynecol. 2009, 33, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Al Chami, A.; Saridogan, E. Endometrial Polyps and Subfertility. J. Obstet. Gynaecol. India 2017, 67, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munro, M.G. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil. Steril. 2019, 111, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Leone, F.P.; Timmerman, D.; Bourne, T.; Valentin, L.; Epstein, E.; Goldstein, S.R.; Marret, H.; Parsons, A.K.; Gull, B.; Istre, O.; et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: A consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet. Gynecol. 2010, 35, 103–112. [Google Scholar] [CrossRef]
- Mary, E.; Lesli, M.; Vickie, A. Callen’s Ultrasonography in Obstetrics and Gynecology, 6th ed.; People Medical Publishing House: Beijing, China, 2019. [Google Scholar]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 523–533. [Google Scholar] [CrossRef]
- Lundin, K.; Ahlström, A. Quality control and standardization of embryo morphology scoring and viability markers. Reprod. Biomed. Online 2015, 31, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Gonen, Y.; Casper, R.F. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J. Vitr. Fertil. Embryo Transf. 1990, 7, 146–152. [Google Scholar] [CrossRef]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage matters: The epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, S.H.; Won, E.; Park, Y.R.; Kim, H.J. Propensity score matching: A conceptual review for radiology researchers. Korean J. Radiol. 2015, 16, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Liu, S.; Shi, L.; Shi, J. Impact of endometrial cavity fluid on assisted reproductive technology outcomes. Int. J. Gynaecol. Obstet. 2016, 132, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Detti, L.; Christiansen, M.E.; Peregrin-Alvarez, I. Endometrial Abnormalities: Correlation Between Different Diagnostic Modalities. J. Ultrasound Med. 2022, 41, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Malpani, A.; Singer, J.; Wolverson, M.K.; Merenda, G. Endometrial hyperplasia: Value of endometrial thickness in ultrasonographic diagnosis and clinical significance. J. Clin. Ultrasound 1990, 18, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, K.; Takeuchi, T.; Mizuta, S.; Matsubayashi, H.; Ishikawa, T. Endometritis: New time, new concepts. Fertil. Steril. 2018, 110, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Amin, T.N.; Saridogan, E.; Jurkovic, D. Ultrasound and intrauterine adhesions: A novel structured approach to diagnosis and management. Ultrasound Obstet. Gynecol. 2015, 46, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Donnez, O.; Dolmans, M.M. Introduction: Uterine adenomyosis, another enigmatic disease of our time. Fertil. Steril. 2018, 109, 369–370. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, A.; Gurunath, S.; Fatima, F.; Bhattacharya, S. Adenomyosis and subfertility: A systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum. Reprod. Update 2012, 18, 374–392. [Google Scholar] [CrossRef]
- Donaghay, M.; Lessey, B.A. Uterine receptivity: Alterations associated with benign gynecological disease. Semin. Reprod. Med. 2007, 25, 461–475. [Google Scholar] [CrossRef]
- Lessey, B.A.; Young, S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef]
- Bhusane, K.; Bhutada, S.; Chaudhari, U.; Savardekar, L.; Katkam, R.; Sachdeva, G. Secrets of Endometrial Receptivity: Some Are Hidden in Uterine Secretome. Am. J. Reprod. Immunol. 2016, 75, 226–236. [Google Scholar] [CrossRef]
- Gray, C.A.; Burghardt, R.C.; Johnson, G.A.; Bazer, F.W.; Spencer, T.E. Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 2002, 124, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Wu, G.; Johnson, G.A.; Wang, X. Environmental factors affecting pregnancy: Endocrine disrupters, nutrients and metabolic pathways. Mol. Cell. Endocrinol. 2014, 398, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Filant, J.; Spencer, T.E. Uterine glands: Biological roles in conceptus implantation, uterine receptivity and decidualization. Int. J. Dev. Biol. 2014, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E. Biological roles of uterine glands in pregnancy. Semin. Reprod. Med. 2014, 32, 346–357. [Google Scholar] [CrossRef] [Green Version]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human placental development and function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef]
- Mahutte, N.; Hartman, M.; Meng, L.; Lanes, A.; Luo, Z.C.; Liu, K.E. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: An analysis of live birth rates from 96,000 autologous embryo transfers. Fertil. Steril. 2022, 117, 792–800. [Google Scholar] [CrossRef]
- Espinós, J.J.; Fabregues, F.; Fontes, J.; García-Velasco, J.A.; Llácer, J.; Requena, A.; Checa, M.; Bellver, J. Impact of chronic endometritis in infertility: A SWOT analysis. Reprod. Biomed. Online 2021, 42, 939–951. [Google Scholar] [CrossRef]
- Kitaya, K.; Ishikawa, T. Chronic endometritis: Simple can be harder than complex? Fertil. Steril. 2021, 115, 1443–1444. [Google Scholar] [CrossRef]
- McQueen, D.B.; Bernardi, L.A.; Stephenson, M.D. Chronic endometritis in women with recurrent early pregnancy loss and/or fetal demise. Fertil. Steril. 2014, 101, 1026–1030. [Google Scholar] [CrossRef]
- Evans-Hoeker, E.A.; Young, S.L. Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 2014, 32, 392–401. [Google Scholar]
- Kakkos, S.K.; Christeas, N.; Lampropoulos, G.; Papadoulas, S.; Makri, R.; Zampakis, P.; Siablis, D.; Tsolakis, I.A. Endovascular management of aortoenteric fistulas with aortic cuff extenders: Report of two cases. Int. Angiol. 2011, 30, 290–294. [Google Scholar] [PubMed]
- Savasi, V.; Leone, F.P.; Fusè, F.; Parisi, F.; Cetin, I. Assisted reproductive technologies and uterine factors influencing their success. Minerva Ginecol. 2013, 65, 505–524. [Google Scholar] [PubMed]
- Wang, Y.; Yao, Z.; Zhao, H.; Yue, C.; Yu, Q.; Zhang, Y.; Guo, Z.; Xu, Z.; Zhang, L.; Yan, L. Reproductive Outcomes of In Vitro Fertilization-Intracytoplasmic Sperm Injection after Transcervical Resection of Adhesions: A Retrospective Cohort Study. J. Minim. Invasive Gynecol. 2021, 28, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Hanstede, M.M.F.; van der Meij, E.; Veersema, S.; Emanuel, M.H. Live births after Asherman syndrome treatment. Fertil. Steril. 2021, 116, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Deans, R.; Vancaillie, T.; Ledger, W.; Liu, J.; Abbott, J.A. Live birth rate and obstetric complications following the hysteroscopic management of intrauterine adhesions including Asherman syndrome. Hum. Reprod. 2018, 33, 1847–1853. [Google Scholar] [CrossRef]
- Pabuçcu, R.; Atay, V.; Orhon, E.; Urman, B.; Ergün, A. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil. Steril. 1997, 68, 1141–1143. [Google Scholar] [CrossRef]
- Zhang, W.X.; Cao, L.B.; Zhao, Y.; Li, J.; Li, B.F.; Lv, J.N.; Yan, L.; Ma, J.L. Endometrial cavity fluid is associated with deleterious pregnancy outcomes in patients undergoing in vitro fertilization/intracytoplasmic sperm injection: A retrospective cohort study. Ann. Transl. Med. 2021, 9, 9. [Google Scholar] [CrossRef]
- He, R.H.; Zhu, X.M. How to deal with fluid in the endometrial cavity during assisted reproductive techniques. Curr. Opin. Obstet. Gynecol. 2011, 23, 190–194. [Google Scholar] [CrossRef]
- He, R.H.; Gao, H.J.; Li, Y.Q.; Zhu, X.M. The associated factors to endometrial cavity fluid and the relevant impact on the IVF-ET outcome. Reprod. Biol. Endocrinol. 2010, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Harb, H.; Al-Rshoud, F.; Karunakaran, B.; Gallos, I.D.; Coomarasamy, A. Hydrosalpinx and pregnancy loss: A systematic review and meta-analysis. Reprod. Biomed. Online 2019, 38, 427–441. [Google Scholar] [CrossRef] [Green Version]
- Nikolettos, N.; Asimakopoulos, B.; Vakalopoulos, I.; Simopoulou, M. Endometrial fluid accumulation during controlled ovarian stimulation for ICSI treatment. A report of three cases. Clin. Exp. Obstet. Gynecol. 2002, 29, 290–292. [Google Scholar] [PubMed]
- Ahmadi, F.; Akhbari, F.; Zamani, M.; Ramezanali, F.; Cheraghi, R. Value of Endometrial Echopattern at HCG Administration Day in Predicting IVF Outcome. Arch. Iran. Med. 2017, 20, 101–104. [Google Scholar] [PubMed]
- Zhao, J.; Zhang, Q.; Li, Y. The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reprod. Biol. Endocrinol. 2012, 10, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.L.; Wu, F.R.; Luo, C.; Chen, X.; Shi, X.Y.; Zheng, H.Y.; Ni, Y.P. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: A retrospective cohort study. Reprod. Biol. Endocrinol. 2010, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Järvelä, I.Y.; Sladkevicius, P.; Kelly, S.; Ojha, K.; Campbell, S.; Nargund, G. Evaluation of endometrial receptivity during in-vitro fertilization using three-dimensional power Doppler ultrasound. Ultrasound Obstet. Gynecol. 2005, 26, 765–769. [Google Scholar] [CrossRef]
- Check, J.H.; Lurie, D.; Dietterich, C.; Callan, C.; Baker, A. Adverse effect of a homogeneous hyperechogenic endometrial sonographic pattern, despite adequate endometrial thickness on pregnancy rates following in-vitro fertilization. Hum. Reprod. 1993, 8, 1293–1296. [Google Scholar] [CrossRef]
- Lee, R.K.; Yu, S.L.; Chih, Y.F.; Tsai, Y.C.; Lin, M.H.; Hwu, Y.M.; Huang, W.Y.; Su, J.T. Effect of endometrial cavity fluid on clinical pregnancy rate in tubal embryo transfer (TET). J. Assist. Reprod. Genet. 2006, 23, 229–234. [Google Scholar] [CrossRef]
- Sharara, F.I.; Prough, S.G. Endometrial fluid collection in women with PCOS undergoing ovarian stimulation for IVF. A report of four cases. J. Reprod. Med. 1999, 44, 299–302. [Google Scholar]

| Characteristic | Before PSM (n = 30,713) | After PSM (n = 537) | ||||
|---|---|---|---|---|---|---|
| Cystic Anechoic Zone | No Cystic Anechoic Zone | *D | Cystic Anechoic Zone | No Cystic Anechoic Zone | *D | |
| (n = 182) | (n = 30,531) | (n = 179) | (n = 358) | |||
| Age (years) | 32.65 ± 4.67 | 32.29 ± 4.97 | 0.077 | 32.93 ± 4.66 | 33.21 ± 5.70 | −0.030 |
| BMI (kg/m2) | 24.43 ± 3.69 | 23.89 ± 3.63 | 0.146 | 24.45 ± 3.63 | 24.68 ± 4.10 | −0.024 |
| Baseline sex hormone | ||||||
| Basal FSH (IU/L) | 7.03 ± 3.31 | 7.33 ± 6.42 | −0.090 | 7.13 ± 3.38 | 7.09 ± 3.15 | 0.062 |
| Basal LH (IU/L) | 5.19 ± 5.41 | 5.64 ± 8.21 | −0.083 | 5.12 ± 5.14 | 5.42 ± 2.43 | 0.074 |
| Basal E2 (pg/mL) | 52.21 ± 131.31 | 78.41 ± 216.43 | −0.200 | 50.73 ± 123.99 | 57.32 ± 134.99 | −0.030 |
| AFC, no. | ||||||
| Left ovary | 6.42 ± 3.93 | 6.07 ± 4.17 | 0.081 | 6.36 ± 3.87 | 6.55 ± 4.16 | −0.049 |
| Right ovary | 6.53 ± 3.75 | 6.25 ± 4.20 | 0.075 | 6.42 ± 3.66 | 6.28 ± 4.67 | 0.039 |
| Type of infertility, no. (%) | 0.100 | −0.011 | ||||
| Primary infertility | 74 (40.7) | 14,281 (45.6) | 75 (36.6) | 60 (29.3) | ||
| Secondary infertility | 108 (59.3) | 17,060 (54.4) | 130 (63.4) | 145 (70.7) | ||
| Indications for IVF/ICSI, no. (%) | 0.049 | 0.031 | ||||
| Male factor | 25 (13.7) | 7530 (24.0) | 26 (12.7) | 37 (18.0) | ||
| Tubal factor | 139 (76.4) | 19,985 (63.8) | 157 (76.6) | 142 (69.3) | ||
| Combined factors | 9 (4.9) | 740 (2.4) | 9 (4.4) | 6 (2.9) | ||
| Others | 9 (4.9) | 3086 (9.8) | 13 (6.3) | 20 (9.8) | ||
| Protocol of COH, no. (%) | 0.248 | −0.011 | ||||
| Short agonist | 36 (19.8) | 8214 (26.2) | 43 (21.0) | 42 (20.5) | ||
| Long agonist | 66 (36.3) | 13,255 (42.3) | 73 (35.6) | 86 (42.0) | ||
| Others | 80 (44.0) | 9872 (31.5) | 89(43.4) | 77 (37.6) | ||
| Duration of ovarian stimulation (days) | 11.23 ± 2.88 | 10.37 ± 3.16 | 0.299 | 11.14 ± 2.83 | 10.97 ± 2.55 | 0.049 |
| Starting dosage of GnRH (IU) | 179.90 ± 54.22 | 187.26 ± 64.09 | −0.136 | 183.62 ± 57.29 | 184.76 ± 64.89 | −0.020 |
| Total dosage of GnRH (IU) | 2412.43 ± 1213.42 | 2105.71 ± 1011.26 | 0.253 | 2418.48 ± 1200.73 | 2343.05 ± 1188.46 | 0.065 |
| E2 level on HCG trigger day (pg/mL) | 2702.25 ± 1546.78 | 2743.31 ± 1494.77 | −0.027 | 2570.42 ± 1653.43 | 2560.72 ± 1715.50 | −0.025 |
| EMT on hCG trigger day (cm) | 1.14 ± 0.24 | 1.07 ± 0.21 | 0.279 | 1.12 ± 0.23 | 1.09 ± 0.20 | −0.066 |
| Number of oocytes retrieved, no. | 8.76 ± 4.53 | 8.88 ± 4.51 | −0.026 | 8.76 ± 4.58 | 8.57 ± 4.53 | −0.003 |
| Number of embryos transferred, no. | 1.63 ± 0.52 | 1.71 ± 0.51 | −0.161 | 1.59 ± 0.52 | 1.56 ± 0.50 | −0.011 |
| Outcomes | Before Matching (n = 31,523) | After Matching (n = 537) | ||||
|---|---|---|---|---|---|---|
| Cystic Anechoic Zone (n = 182) | No Cystic Anechoic Zone (n = 31,341) | p | Cystic Anechoic Zone (n = 179) | No Cystic Anechoic Zone (n = 358) | p | |
| Biochemical pregnancy rate, % (no.) | 57.7 (105) | 60.7 (19,038) | 0.400 | 57.5 (103) | 63.1 (226) | 0.210 |
| Clinical pregnancy rate, % (no.) | 50.0 (91) | 53.4 (16,726) | 0.364 | 50.3 (90) | 56.4 (202) | 0.188 |
| Live birth rate, % (no.) | 37.9 (69) | 43.5 (13,631) | 0.130 | 38.0 (68) | 48.6 (174) | 0.025 |
| Pregnancy loss, % (no./total no.) | ||||||
| During biochemical pregnancy | 13.3 (14/105) | 12.1 (2312/19,038) | 0.710 | 12.6 (13/103) | 10.6 (24/226) | 0.593 |
| During clinical pregnancy | 22.0 (20/91) | 16.5 (2763/16,726) | 0.162 | 22.2 (20/90) | 12.4 (25/202) | 0.031 |
| Outcomes | Crude RR (95%CI) | p | * Adjusted RR (95%CI) | p |
|---|---|---|---|---|
| Biochemical pregnancy rate | 0.912 (0.786–1.058) | 0.222 | 0.909 (0.786–1.051) | 0.198 |
| Clinical pregnancy rate | 0.891 (0.750–1.058) | 0.188 | 0.887 (0.748–1.051) | 0.166 |
| Live birth rate | 0.782 (0.630–0.969) | 0.025 | 0.788 (0.638–0.974) | 0.027 |
| Pregnancy loss | ||||
| During biochemical pregnancy | 0.841 (0.447–1.585) | 0.593 | 0.839 (0.441–1.597) | 0.593 |
| During clinical pregnancy | 0.557 (0.327–0.949) | 0.031 | 0.557 (0.325–0.953) | 0.033 |
| A Type (n = 400) | B Type (n = 136) | p | |
|---|---|---|---|
| Cystic anechoic zone | 78 (43.8%) | 100 (56.2%) | <0.001 |
| No cystic anechoic zone | 322 (89.9%) | 36 (10.1%) |
| The Diameter of Uterine Cystic Anechoic Zone (cm) | r | p | |||
|---|---|---|---|---|---|
| 0.1 < d< 0.3 (n = 106) | 0.3 ≤ d < 0.6 (n = 49) | 0.6 ≤ d ≤ 1.8 (n = 24) | |||
| Biochemical pregnancy rate, % (no.) | 57.5 (61) | 59.2 (29) | 54.2 (13) | −0.007 | 0.921 |
| Clinical pregnancy rate, % (no.) | 50.9 (54) | 53.1 (26) | 41.7 (10) | −0.032 | 0.670 |
| Live birth rate, % (no.) | 38.7 (41) | 38.8 (19) | 33.3 (8) | −0.025 | 0.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhao, S.; Lv, J.; Lv, H.; Yan, L. The Cystic Anechoic Zone of Uterine Cavity Newly Observed during Controlled Ovarian Hyperstimulation Affects Pregnancy Outcomes of Fresh Embryo Transfer. J. Clin. Med. 2023, 12, 134. https://doi.org/10.3390/jcm12010134
Tian Y, Zhao S, Lv J, Lv H, Yan L. The Cystic Anechoic Zone of Uterine Cavity Newly Observed during Controlled Ovarian Hyperstimulation Affects Pregnancy Outcomes of Fresh Embryo Transfer. Journal of Clinical Medicine. 2023; 12(1):134. https://doi.org/10.3390/jcm12010134
Chicago/Turabian StyleTian, Yizheng, Shengrui Zhao, Jianan Lv, Hong Lv, and Lei Yan. 2023. "The Cystic Anechoic Zone of Uterine Cavity Newly Observed during Controlled Ovarian Hyperstimulation Affects Pregnancy Outcomes of Fresh Embryo Transfer" Journal of Clinical Medicine 12, no. 1: 134. https://doi.org/10.3390/jcm12010134
APA StyleTian, Y., Zhao, S., Lv, J., Lv, H., & Yan, L. (2023). The Cystic Anechoic Zone of Uterine Cavity Newly Observed during Controlled Ovarian Hyperstimulation Affects Pregnancy Outcomes of Fresh Embryo Transfer. Journal of Clinical Medicine, 12(1), 134. https://doi.org/10.3390/jcm12010134






