Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pre- and Postoperative Clinical and Radiological Evaluation
2.3. Surgical Protocol and Intraoperative Fluorescence Characterization
2.4. Histological Analysis and MGMT/IDH Determination
2.5. Endopoints and Study Aims
2.6. Statistical Analysis
3. Results
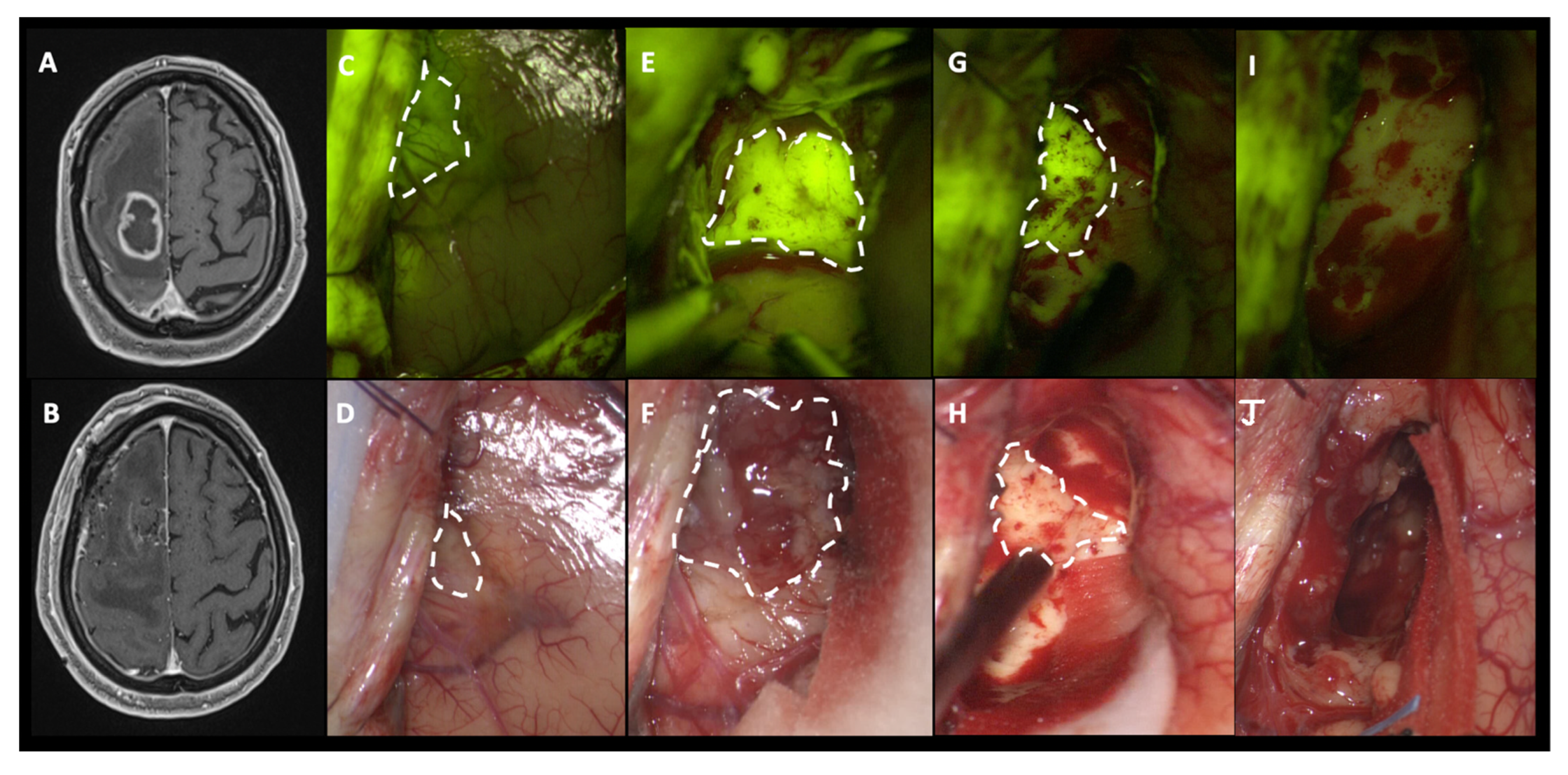
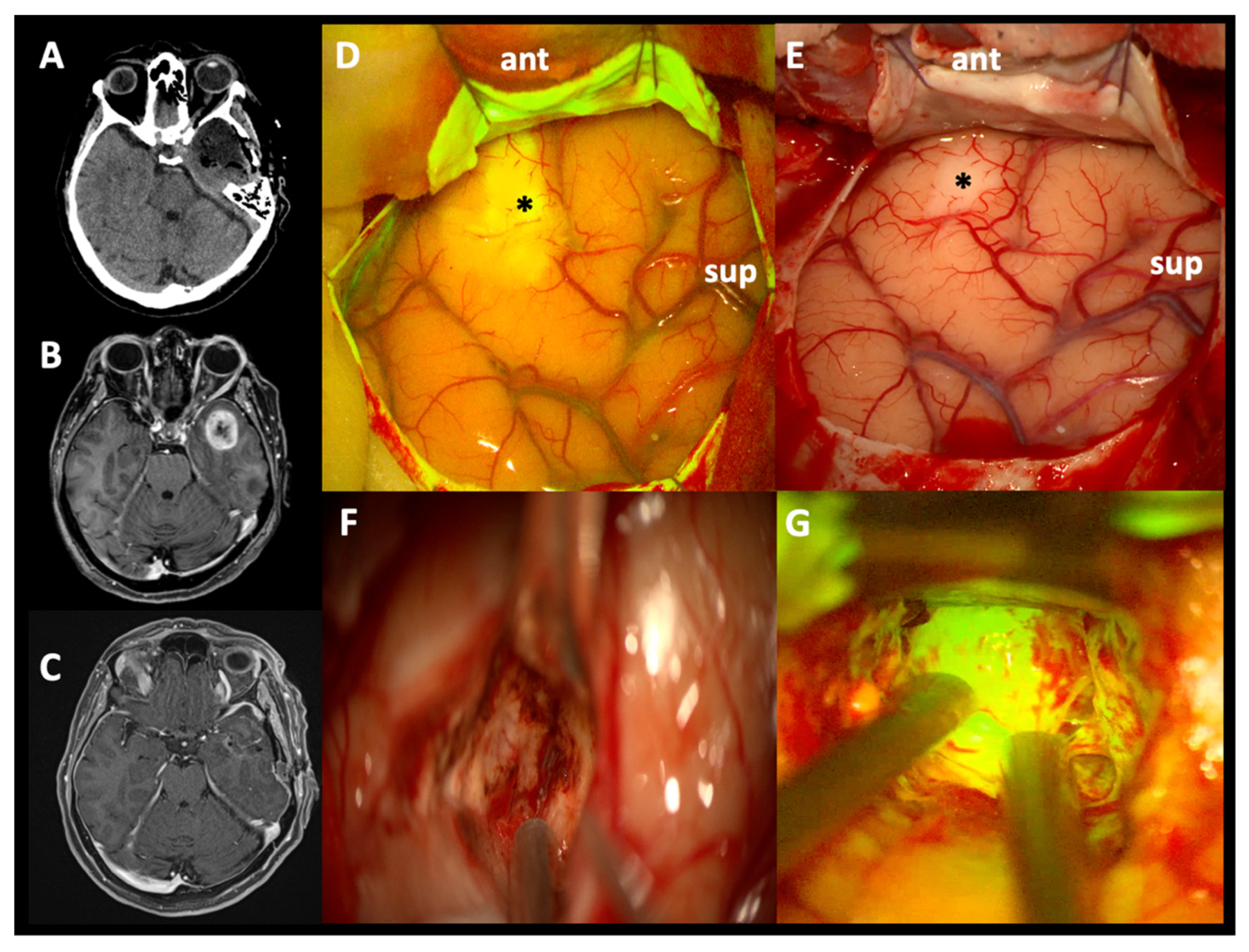
3.1. Intraoperative Fluorescence Characteristics and SF Effects in EOR
3.2. Clinical Outcome, PFS, and OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-Oncology 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Gritsch, S.; Batchelor, T.T.; Castro, L.N.G. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Weller, M. Work in progress: Changes to who brain tumor classification. J. Neurol. Sci. 2021, 429, 343–349. [Google Scholar] [CrossRef]
- Whitfield, B.T.; Huse, J.T. Classification of adult-type diffuse gliomas: Impact of the World Health Organization 2021 update. Brain Pathol. 2022, 32, e13062. [Google Scholar] [CrossRef]
- Clarke, J.L.; Chang, S.M. Neuroimaging: Diagnosis and response assessment in glioblastoma. Cancer J. 2012, 18, 26–31. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.; van den Bent, M.J.; Weller, M.; Fisher, B.M.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Somasundaram, K. Glioblastoma vs temozolomide: Can the red queen race be won? Cancer Biol. Ther. 2019, 20, 1083–1090. [Google Scholar] [CrossRef]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marko, N.F.; Weil, R.J.; Schroeder, J.L.; Lang, F.F.; Suki, D.; Sawaya, R.E. Extent of Resection of Glioblastoma Revisited: Personalized Survival Modeling Facilitates More Accurate Survival Prediction and Supports a Maximum-Safe-Resection Approach to Surgery. J. Clin. Oncol. 2014, 32, 774–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revilla-Pacheco, F.; Rodríguez-Salgado, P.; Barrera-Ramírez, M.; Morales-Ruiz, M.P.; Loyo-Varela, M.; Rubalcava-Ortega, J.; Herrada-Pineda, T. Extent of resection and survival in patients with glioblastoma multiforme. Medicine 2021, 100, e26432. [Google Scholar] [CrossRef]
- Cavallo, C.; De Laurentis, C.; Vetrano, I.G.; Falco, J.; Broggi, M.; Schiariti, M.; Ferroli, P.; Acerbi, F. The utilization of fluorescein in brain tumor surgery: A systematic review. J. Neurosurg. Sci. 2018, 62, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Tonn, J.-C.; Stummer, W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: Practical use, risks, and pitfalls. Clin. Neurosurg. 2008, 55, 20–26. [Google Scholar]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Wirtz, W.S.R.; Albert, F.; Schwaderer, M.; Heuer, C.; Staubert, A.; Tronnier, V.; Knauth, M.; Kunze, S. The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol. Res. 2000, 22, 354–360. [Google Scholar] [CrossRef]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef]
- Prada, F.; Mattei, L.; Del Bene, M.; Aiani, L.; Saini, M.; Casali, C.; Filippini, A.; Legnani, F.G.; Perin, A.; Saladino, A.; et al. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. BioMed Res. Int. 2014, 2014, 484261. [Google Scholar] [CrossRef] [Green Version]
- Acerbi, F.; Restelli, F.; De Laurentis, C.; Falco, J.; Cavallo, C.; Broggi, M.; Höhne, J.; Schebesch, K.-M.; Schiariti, M.; Ferroli, P. Fluorescent tracers in neurosurgical procedures: An European survey. J. Neurosurg. Sci. 2018, 65, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Valle, R.D.; Hadjipanayis, C.G.; Stummer, W. Established and emerging uses of 5-ALA in the brain: An overview. J. Neuro-Oncol. 2019, 141, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Tonn, J.C.; Goetz, C.; Ullrich, W.; Stepp, H.; Bink, A.; Pietsch, T.; Pichlmeier, U. 5-Aminolevulinic acid-derived tumor fluorescence: The diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 2014, 74, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stummer, W.; Novotny, A.; Stepp, H.; Goetz, C.; Bise, K.; Reulen, H.J. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J. Neurosurg. 2000, 93, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Katsevman, G.A.; Turner, R.C.; Urhie, O.; Voelker, J.L.; Bhatia, S. Utility of sodium fluorescein for achieving resection targets in glioblastoma: Increased gross- or near-total resections and prolonged survival. J. Neurosurg. 2020, 132, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Schebesch, K.-M.; Höhne, J.; Cavallo, C.; De Laurentis, C.; Eoli, M.; Anghileri, E.; Servida, M.; Boffano, C.; et al. Fluorescein-Guided Surgery for Resection of High-Grade Gliomas: A Multicentric Prospective Phase II Study (FLUOGLIO). Clin. Cancer Res. 2018, 24, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falco, J.; Cavallo, C.; Vetrano, I.G.; de Laurentis, C.; Siozos, L.; Schiariti, M.; Broggi, M.; Ferroli, P.; Acerbi, F. Fluorescein Application in Cranial and Spinal Tumors Enhancing at Preoperative MRI and Operated With a Dedicated Filter on the Surgical Microscope: Preliminary Results in 279 Patients Enrolled in the FLUOCERTUM Prospective Study. Front. Surg. 2019, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Vetrano, I.G.; Acerbi, F.; Falco, J.; Devigili, G.; Rinaldo, S.; Messina, G.; Prada, F.; D’Ammando, A.; Nazzi, V. Fluorescein-guided removal of peripheral nerve sheath tumors: A preliminary analysis of 20 cases. J. Neurosurg. 2021, 134, 260–269. [Google Scholar] [CrossRef]
- Acerbi, F. Fluorescein assistance in neuro-oncological surgery: A trend of the moment or a real technical adjunt? Clin. Neurol. Neurosurg. 2016, 100, 119–120. [Google Scholar] [CrossRef]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Höhne, J.; Schebesch, K.M.; de Laurentis, C.; Akçakaya, M.O.; Pedersen, C.B.; Brawanski, A.; Poulsen, F.R.; Kiris, T.; Cavallo, C.; Broggi, M.; et al. Fluorescein Sodium in the Surgical Treatment of Recurrent Glioblastoma Multiforme. World Neurosurg. 2019, 125, e158–e164. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Banu, M.A.; Canoll, P.; Bruce, J.N. Rationale and Clinical Implications of Fluorescein-Guided Supramarginal Resection in Newly Diagnosed High-Grade Glioma. Front. Oncol. 2021, 11, 666734. [Google Scholar] [CrossRef] [PubMed]
- Höhne, J.; Acerbi, F.; Falco, J.; Akçakaya, M.O.; Schmidt, N.O.; Kiris, T.; De Laurentis, C.; Ferroli, P.; Broggi, M.; Schebesch, K.-M. Lighting Up the Tumor—Fluorescein-Guided Resection of Gangliogliomas. J. Clin. Med. 2020, 9, 2405. [Google Scholar] [CrossRef]
- Falco, J.; Höhne, J.; Broggi, M.; Rubiu, E.; Restelli, F.; Vetrano, I.G.; Schiariti, M.; Mazzapicchi, E.; Bonomo, G.; Ferroli, P.; et al. Fluorescein-guided surgery for the resection of pilocytic astrocytomas: A multicentric retrospective study. Front. Oncol. 2022, 12, 943085. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Broggi, M.; Broggi, G.; Ferroli, P. What is the best timing for fluorescein injection during surgical removal of high-grade gliomas? Acta Neurochir. 2015, 157, 1377. [Google Scholar] [CrossRef] [PubMed]
- de Laurentis, C.; Höhne, J.; Cavallo, C.; Restelli, F.; Falco, J.; Broggi, M.; Bosio, L.; Vetrano, I.G.; Schiariti, M.; Zattra, C.M.; et al. The impact of fluorescein-guided technique in the surgical removal of CNS tumors in a pediatric population: Results from a multicentric observational study. J. Neurosurg. Sci. 2019, 63, 679–687. [Google Scholar] [CrossRef]
- Acerbi, F.; Cavallo, C.; Schebesch, K.-M.; Akçakaya, M.O.; de Laurentis, C.; Hamamcioglu, M.K.; Broggi, M.; Brawanski, A.; Falco, J.; Cordella, R.; et al. Fluorescein-Guided Resection of Intramedullary Spinal Cord Tumors: Results from a Preliminary, Multicentric, Retrospective Study. World Neurosurg. 2017, 108, 603–609. [Google Scholar] [CrossRef]
- Höhne, J.; Hohenberger, C.; Proescholdt, M.; Riemenschneider, M.J.; Wendl, C.; Brawanski, A.; Schebesch, K.-M. Fluorescein sodium-guided resection of cerebral metastases—An update. Acta Neurochir. 2017, 159, 363–367. [Google Scholar] [CrossRef]
- Mor, V.; Laliberte, L.; Morris, J.N.; Wiemann, M. The Karnofsky performance status scale: An examination of its reliability and validity in a research setting. Cancer 1984, 53, 2002–2007. [Google Scholar] [CrossRef]
- Haak, D.; Page, C.-E.; Deserno, T.M. A Survey of DICOM Viewer Software to Integrate Clinical Research and Medical Imaging. J. Digit. Imaging 2016, 29, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, M.M.; Recinos, P.F.; Nowacki, A.S.; Schroeder, J.L.; Angelov, L.; Barnett, G.H.; Vogelbaum, M.A. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J. Neurosurg. 2014, 121, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.Q.; Bauman, G.S.; Fisher, B.J.; Macdonald, D.R.; Megyesi, J.F.; Watling, C.J. Hypofractionated radiotherapy (XRT) plus concurrent and adjuvant versus salvage temozolomide (TMZ) in elderly patients with glioblastoma multiforme: A review of ten-year single institutional experience. Int. J. Radiat. Oncol. Biol. Phys. 2010, 8, S167. [Google Scholar] [CrossRef]
- Weller, M.; dan der Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Le Rhun, E.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acerbi, F.; Prada, F.; Vetrano, I.G.; Falco, J.; Faragò, G.; Ferroli, P.; DiMeco, F. Indocyanine Green and Contrast-Enhanced Ultrasound Videoangiography: A Synergistic Approach for Real-Time Verification of Distal Revascularization and Aneurysm Occlusion in a Complex Distal Middle Cerebral Artery Aneurysm. World Neurosurg. 2019, 125, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Restelli, F.; Broggi, M.; Schiariti, M.; Ferroli, P. Feasibility of simultaneous sodium fluorescein and indocyanine green injection in neurosurgical procedures. Clin. Neurol. Neurosurg. 2016, 146, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Cordella, R.; Orena, E.; Acerbi, F.; Beretta, E.; Caldiroli, D.; Dimeco, F.; Carozzi, C. Motor evoked potentials and bispectral index-guided anaesthesia in image-guided mini-invasive neurosurgery of supratentorial tumors nearby the cortico-spinal tract. Turk. Neurosurg. 2018, 28, 341–348. [Google Scholar]
- Acerbi, F.; Vetrano, I.G.; Sattin, T.; Falco, J.; De Laurentis, C.; Zattra, C.; Bosio, L.; Rossini, Z.; Broggi, M.; Schiariti, M.; et al. Use of ICG videoangiography and FLOW 800 analysis to identify the patient-specific venous circulation and predict the effect of venous sacrifice: A retrospective study of 172 patients. Neurosurg. Focus 2018, 45, E7. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Vetrano, I.G.; Falco, J.; Gioppo, A.; Ciuffi, A.; Ziliani, V.; Schiariti, M.; Broggi, M.; Faragò, G.; Ferroli, P. In Situ Side-to-Side Pericallosal-Pericallosal Artery and Callosomarginal-Callosomarginal Artery Bypasses for Complex Distal Anterior Cerebral Artery Aneurysms: A Technical Note. Oper. Neurosurg. 2020, 19, E487–E495. [Google Scholar] [CrossRef]
- Acerbi, F.; Mazzapicchi, E.; Falco, J.; Vetrano, I.G.; Restelli, F.; Faragò, G.; La Corte, E.; Bonomo, G.; Bersano, A.; Canavero, I.; et al. The Role of Bypass Surgery for the Management of Complex Intracranial Aneurysms in the Anterior Circulation in the Flow-Diverter Era: A Single-Center Series. Brain Sci. 2022, 12, 1339. [Google Scholar] [CrossRef]
- Acerbi, F.; Broggi, M.; Eoli, M.; Anghileri, E.; Cuppini, L.; Pollo, B.; Schiariti, M.; Visintini, S.; Orsi, C.; Franzini, A.; et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: Preliminary results in 12 cases. Acta Neurochir. 2013, 155, 1277–1286. [Google Scholar] [CrossRef]
- Eoli, M.; Menghi, F.; Bruzzone, M.G.; De Simone, T.; Valletta, L.; Pollo, B.; Bissola, L.; Silvani, A.; Bianchessi, D.; D’Incerti, L.; et al. Methylation of O6-methylguanine DNA methytransferase and loss of heterozygosity on 19q and/or 17p are overlapping features of secondary glioblastomas with prolonged survival. Clin. Cancer Res. 2007, 13, 2606–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leao, D.; Craig, P.; Godoy, L.; Da La Leite, C.; Policeni, B. Response Assessment in Neuro-Oncology Criteria for Gliomas: Practical Approach Using Conventional and Advanced Techniques. Am. J. Neuroradiol. 2019, 41, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Jusue-Torres, I.; Navarro-Ramirez, R.; Raza, S.M.; Pascual-Gallego, M.; Ibrahim, A.; Hernandez-Hermann, M.; Gomez, L.; Ye, X.; Weingart, J.D.; et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro-Oncology 2014, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, F.; Pollo, B.; De Laurentis, C.; Restelli, F.; Falco, J.; Vetrano, I.G.; Broggi, M.; Schiariti, M.; Tramacere, I.; Ferroli, P.; et al. Ex Vivo Fluorescein-Assisted Confocal Laser Endomicroscopy (CONVIVO® System) in Patients With Glioblastoma: Results From a Prospective Study. Front. Oncol. 2020, 10, 2911. [Google Scholar] [CrossRef]
- Restelli, F.; Pollo, B.; Vetrano, I.; Cabras, S.; Broggi, M.; Schiariti, M.; Falco, J.; de Laurentis, C.; Raccuia, G.; Ferroli, P.; et al. Confocal Laser Microscopy in Neurosurgery: State of the Art of Actual Clinical Applications. J. Clin. Med. 2021, 10, 2035. [Google Scholar] [CrossRef]
- Falco, J.; Agosti, A.; Vetrano, I.; Bizzi, A.; Restelli, F.; Broggi, M.; Schiariti, M.; DiMeco, F.; Ferroli, P.; Ciarletta, P.; et al. In Silico Mathematical Modelling for Glioblastoma: A Critical Review and a Patient-Specific Case. J. Clin. Med. 2021, 10, 2169. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nakamura, H.; Makino, K.; Hide, T.; Muta, D.; Kamada, H.; Kuratsu, J.-I. Prognostic value of isocitrate dehydrogenase 1, O6-methylguanine-DNA methyltransferase promoter methylation, and 1p19q co-deletion in Japanese malignant glioma patients. World J. Surg. Oncol. 2013, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-Methylguanine Methyltransferase (MGMT) Promoter Methylation With Clinical Outcomes in Glioblastoma and Clinical Strategies to Modulate MGMT Activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Facchino, S.; Abdouh, M.; Bernier, G. Brain Cancer Stem Cells: Current Status on Glioblastoma Multiforme. Cancers 2011, 3, 1777–1797. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low- and high-grade glioma. J. Neuro-Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, A.; Singh, S.K.; Agnihotri, S.; Jalali, S.; Burrell, K.; Aldape, K.D.; Zadeh, G. GBM’s multifaceted landscape: Highlighting regional and microenvironmental heterogeneity. Neuro-Oncology 2014, 16, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M.; Stummer, W.; Molina, E.S.; Wölfer, J. Simultaneous fluorescein sodium and 5-ALA in fluorescence-guided glioma surgery. Acta Neurochir. 2015, 157, 877–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eljamel, M.S.; Mahboob, S.O. The effectiveness and cost-effectiveness of intraoperative imaging in high-grade glioma resection; a comparative review of intraoperative ALA, fluorescein, ultrasound and MRI. Photodiagnosis Photodyn. Ther. 2016, 16, 35–43. [Google Scholar] [CrossRef]
- Moore, G.E. Fluorescein as an agent in the differentiation of normal and malignant tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.S.; Barry, C.; McAllister, I.L.; Constable, I. Fluorescein angiography and adverse drug reactions revisited: The Lions Eye experience. Clin. Exp. Ophthalmol. 2006, 34, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Smith, E.J.; Barreau, A.; Nyaeme, M.; Cramer, S.W.; Najafali, D.; Krist, D.T.; Arnold, P.M.; Hassaneen, W. Comparison of fluorescein sodium, 5-ALA, and intraoperative MRI for resection of high-grade gliomas: A systematic review and network meta-analysis. J. Clin. Neurosci. 2022, 98, 240–247. [Google Scholar] [CrossRef]
- Palmieri, G.; Cofano, F.; Salvati, L.F.; Monticelli, M.; Zeppa, P.; Di Perna, G.; Melcarne, A.; Altieri, R.; La Rocca, G.; Sabatino, G.; et al. Fluorescence-Guided Surgery for High-Grade Gliomas: State of the Art and New Perspectives. Technol. Cancer Res. Treat. 2021, 20, 15330338211021605. [Google Scholar] [CrossRef]
- Koc, K.; Anik, I.; Cabuk, B.; Ceylan, S. Fluorescein sodium-guided surgery in glioblastoma multiforme: A prospective evaluation. Br. J. Neurosurg. 2008, 22, 99–103. [Google Scholar] [CrossRef]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.-M.; WiIdrick, D.M. Neurosurgical Outcomes in a Modern Series of 400 Craniotomies for Treatment of Parenchymal Tumors. Neurosurgery 1998, 42, 1044–1055. [Google Scholar] [CrossRef]
- Carrabba, G.; Fava, E.; Giussani, C.; Acerbi, F.; Portaluri, F.; Songa, V.; Stocchetti, N.; Branca, V.; Gaini, S.M.; Bello, L. Cortical and subcortical motor mapping in rolandic and perirolandic glioma surgery: Impact on postoperative morbidity and extent of resection. J. Neurosurg. Sci. 2007, 51, 45–51. [Google Scholar] [PubMed]


| Nr. | Sex | Age (years) | Location 1 | KPS in | Vol. (cm3) | IONM | Histology | MGMT | Resection | RV cm3 | KPS Out | OS (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 61 | R parietal | 90 | 5.38 | yes | GBM | methylated | 100% | 0 | 90 | 26 |
| 2 | M | 82 | R parietal | 90 | 0.74 | yes | GBM | methylated | 100% | 0 | 90 | 5 |
| 3 | M | 53 | R frontal | 90 | 43.01 | no | GBM | methylated | 100% | 0 | 90 | 14 |
| 4 | M | 66 | R parietal | 90 | 89.65 | no | GBM | methylated | 100% | 0 | 90 | 45 |
| 5 | M | 66 | L temporal | 90 | 21.57 | yes | GBM | methylated | 100% | 0 | 90 | 15 |
| 6 | M | 51 | R parietal | 100 | 19.09 | no | GBM | unmethylated | 100% | 0 | 100 | 42 |
| 7 | M | 71 | R temporal | 80 | 54.36 | no | GBM | methylated | 100% | 0 | 90 | 78 |
| 8 | M | 81 | L frontal | 90 | 7.61 | no | GBM | methylated | 100% | 0 | 90 | 10 |
| 9 | M | 62 | R fronto-temporal | 90 | 0.88 | no | GBM | methylated | 100% | 0 | 90 | 22 |
| 10 | F | 70 | L temporal | 90 | 3.41 | yes | GBM | methylated | 100% | 0 | 90 | 28 |
| 11 | M | 53 | R parietal | 90 | 92.53 | yes | GBM | methylated | 100% | 0 | 70 | 34 |
| 12 | M | 49 | L parietal | 90 | 120.24 | no | GBM | methylated | 100% | 0 | 90 | 23 |
| 13 | M | 66 | L temporal | 80 | 15.69 | no | GBM | methylated | 100% | 0 | 70 | 4 |
| 14 | M | 62 | L frontal | 100 | 25.04 | no | GBM | unmethylated | 100% | 0 | 100 | 29 |
| 15 | M | 65 | L temporal | 90 | 219.53 | no | GBM | unmethylated | 100% | 0 | 90 | 10 |
| 16 | F | 59 | L parietal | 90 | 44.25 | yes | GBM | methylated | 100% | 0 | 90 | 16 |
| 17 | F | 54 | L temporal | 60 | 39.79 | yes | GBM | methylated | 100% | 0 | 40 | 15 |
| 18 | F | 51 | L parietal | 100 | 1.74 | no | GBM | unmethylated | 100% | 0 | 100 | 11 |
| 19 | M | 48 | R parietal | 90 | 15.83 | yes | GBM | unmethylated | 100% | 0 | 60 | 4 |
| 20 | M | 72 | R temporo-parietal | 90 | 117.64 | yes | GBM | methylated | 100% | 0 | 90 | 12 |
| 21 | M | 45 | L parietal | 90 | 6.28 | awake | GBM | unmethylated | 100% | 0 | 80 | 45 |
| 22 | M | 69 | L temporal | 90 | 16.84 | no | GBM | unmethylated | 100% | 0 | 90 | 19 |
| 23 | F | 75 | L parietal | 80 | 31.69 | yes | GBM | methylated | 100% | 0 | 60 | 12 |
| 24 | F | 47 | R parietal | 90 | 21.49 | awake | GBM | methylated | 100% | 0 | 90 | 23 |
| 25 | F | 74 | L fronto-parietal | 90 | 7.74 | no | GBM | methylated | 100% | 0 | 70 | 6 |
| 26 | F | 73 | R parietal | 80 | 16.89 | yes | GBM | unmethylated | 100% | 0 | 80 | 4 |
| 27 | M | 73 | R frontal | 90 | 122.76 | awake | GBM | unmethylated | 99.4% | 0.71 | 40 | 9 |
| 28 | F | 69 | L parietal | 90 | 4.95 | no | GBM | methylated | 100% | 0 | 90 | 23 |
| 29 | M | 57 | R frontal | 90 | 48.89 | yes | GBM | methylated | 100% | 0 | 90 | 37 |
| 30 | F | 60 | R parieto-occipital | 90 | 21.16 | no | GBM | methylated | 90.4% | 2.04 | 80 | 17 |
| 31 | M | 45 | R frontal | 90 | 37.25 | no | GBM | unmethylated | 98.4% | 0.59 | 100 | 8 |
| 32 | M | 75 | L frontal | 60 | 99.21 | no | GBM | methylated | 100% | 0 | 50 | 13 |
| 33 | F | 64 | L temporal | 90 | 26.71 | no | GBM | unmethylated | 100% | 0 | 90 | 20 |
| 34 | M | 76 | L frontal | 90 | 82.97 | no | GBM | unmethylated | 100% | 0 | 90 | 5 |
| 35 | M | 62 | L occipital | 90 | 126.91 | no | GBM | unmethylated | 100% | 0 | 70 | 24 |
| 36 | M | 47 | R temporal | 100 | 73.98 | no | GBM | unmethylated | 100% | 0 | 100 | 24 |
| 37 | F | 72 | R temporal | 80 | 18.14 | no | GBM | methylated | 100% | 0 | 100 | 11 |
| 38 | M | 55 | R frontal | 70 | 189.29 | no | GBM | unmethylated | 90.3% | 18.44 | 70 | 5 |
| 39 | M | 69 | R temporal | 80 | 88.71 | no | GBM | methylated | 100% | 0 | 90 | 29 |
| 40 | F | 61 | R thalamus | 80 | 81.39 | yes | GBM | unmethylated | 95.7% | 3.51 | 50 | 9 |
| 41 | M | 65 | L fronto-insular | 80 | 52.59 | awake | GBM | unmethylated | 100% | 0 | 70 | 23 |
| 42 | F | 30 | R fronto-temporo-insular | 60 | 113.51 | no | GBM | methylated | 100% | 0 | 50 | 10 |
| 43 | M | 80 | R parietal | 90 | 15.08 | no | GBM | unmethylated | 100% | 0 | 90 | 14 |
| 44 | F | 48 | L frontal | 90 | 73.19 | yes | GBM | methylated | 94.4% | 4.07 | 80 | 4 |
| 45 | F | 61 | R parietal | 90 | 12.22 | no | GBM | methylated | 100% | 0 | 90 | 27 |
| 46 | M | 56 | R frontal | 70 | 176.21 | yes | GBM | methylated | 100% | 0 | 80 | 13 |
| 47 | M | 58 | L temporo-insular | 80 | 137.65 | yes | GBM | unmethylated | 100% | 0 | 30 | 16 |
| 48 | M | 36 | R temporal | 90 | 8.39 | no | GBM | unmethylated | 100% | 0 | 90 | 14 |
| 49 | F | 70 | L parieto-occipital | 80 | 83.05 | no | GBM | unmethylated | 100% | 0 | 80 | 9 |
| 50 | F | 72 | L fronto-temporal | 80 | 18.42 | no | GBM | methylated | 100% | 0 | 90 | 16 |
| 51 | F | 68 | L fronto-parietal | 60 | 42.86 | yes | GBM | methylated | 100% | 0 | 40 | alive (39) |
| 52 | M | 63 | L parieto-occipital | 90 | 66.57 | no | GBM | unmethylated | 100% | 0 | 80 | 11 |
| 53 | M | 61 | R temporal | 80 | 235.64 | no | GBM | methylated | 99.4% | 1.49 | 90 | alive (38) |
| 54 | F | 64 | L parietal | 90 | 27.79 | no | GBM | unmethylated | 100% | 0 | 90 | 29 |
| 55 | M | 66 | L parietal | 80 | 86.27 | yes | GBM | methylated | 100% | 0 | 70 | alive (37) |
| 56 | M | 68 | L frontal | 90 | 29.51 | yes | GBM | unmethylated | 100% | 0 | 80 | 18 |
| 57 | M | 57 | R temporal | 100 | 44.57 | no | GBM | unmethylated | 100% | 0 | 90 | 16 |
| 58 | M | 54 | R fronto-temporal | 90 | 9.65 | no | GBM | unmethylated | 98.2% | 0.17 | 90 | 8 |
| 59 | M | 49 | R frontal | 90 | 39.19 | no | GBM | methylated | 100% | 0 | 90 | alive (33) |
| 60 | M | 38 | L fronto-parietal | 80 | 61.28 | yes | GBM | methylated | 100% | 0 | 90 | alive (32) |
| 61 | M | 56 | R parieto-occipital | 80 | 21.44 | no | GBM | methylated | 96.1% | 0.83 | 80 | 20 |
| 62 | M | 49 | L temporo-occipital | 50 | 31.62 | no | GBM | unmethylated | 100% | 0 | 70 | alive (30) |
| 63 | F | 71 | L temporal | 80 | 80.39 | no | GBM | methylated | 100% | 0 | 80 | alive (29) |
| 64 | M | 51 | R thalamic | 80 | 42.17 | yes | GBM | methylated | 100% | 0 | 50 | 15 |
| 65 | F | 65 | L temporo-occipital | 90 | 2.58 | awake | GBM | methylated | 100% | 0 | 90 | 25 |
| 66 | M | 52 | R temporal | 70 | 186.45 | no | GBM | unmethylated | 100% | 0 | 80 | 19 |
| 67 | M | 66 | L temporo-parietal | 80 | 65.53 | no | GBM | unmethylated | 100% | 0 | 90 | 13 |
| 68 | M | 60 | L temporal | 90 | 1.98 | awake | GBM | methylated | 100% | 0 | 90 | alive (25) |
| 69 | F | 73 | R occipital | 70 | 91.39 | no | GBM | methylated | 98.7% | 1.17 | 80 | 15 |
| 70 | M | 69 | R parieto-occipital | 80 | 45.65 | no | GBM | methylated | 100% | 0 | 80 | alive (23) |
| 71 | M | 55 | CC (L splenium) | 90 | 1.75 | no | GBM | methylated | 100% | 0 | 90 | 14 |
| 72 | F | 77 | R parietal | 90 | 53.01 | no | GBM | methylated | 100% | 0 | 90 | alive (22) |
| 73 | M | 52 | R temporo-parietal | 80 | 334.69 | yes | GBM | unmethylated | 93.2% | 22.76 | 70 | 10 |
| 74 | M | 62 | L parietal | 90 | 9.05 | no | GBM | methylated | 100% | 0 | 90 | alive (19) |
| 75 | F | 61 | R temporal | 60 | 145.09 | no | GBM | methylated | 100% | 0 | 90 | 13 |
| 76 | M | 82 | R temporal | 80 | 150.05 | no | GBM | unmethylated | 100% | 0 | 80 | 10 |
| 77 | F | 73 | L temporal | 80 | 77.09 | no | GBM | methylated | 98.4% | 1.25 | 80 | 15 |
| 78 | M | 54 | R temporo-parietal | 100 | 70.05 | no | GBM | unmethylated | 100% | 0 | 90 | 14 |
| 79 | F | 44 | R cerebellar | 90 | 6.38 | no | GBM | methylated | 100% | 0 | 100 | alive (16) |
| 80 | F | 60 | L frontal | 100 | 41.37 | no | GBM | methylated | 100% | 0 | 100 | 15 |
| 81 | M | 73 | R frontal | 90 | 18.71 | yes | GBM | methylated | 100% | 0 | 80 | alive (13) |
| 82 | F | 47 | L temporo-occipital | 80 | 32.71 | no | GBM | unmethylated | 100% | 0 | 90 | 6 |
| 83 | M | 63 | L temporal | 70 | 30.54 | no | GBM | unmethylated | 93.9% | 1.84 | 70 | alive (12) |
| 84 | M | 74 | L frontal | 80 | 11.83 | yes | GBM | unmethylated | 100% | 0 | 60 | alive (11) |
| 85 | M | 45 | R frontal | 100 | 145.89 | yes | GBM | methylated | 100% | 0 | 60 | alive (9) |
| 86 | M | 73 | L temporal | 90 | 18.39 | no | GBM | methylated | 95.9% | 0.74 | 90 | alive (8) |
| 87 | M | 53 | L fronto-parietal | 80 | 88.41 | yes | GBM | unmethylated | 97.5% | 2.23 | 80 | alive (7) |
| 88 | M | 66 | R frontal | 60 | 18.09 | yes | GBM | unmethylated | 100% | 0 | 40 | alive (6) |
| 89 | M | 60 | L temporo-occipital | 80 | 91.19 | no | GBM | methylated | 100% | 0 | 70 | alive (6) |
| 90 | M | 43 | R temporal | 90 | 63.62 | yes | GBM | unmethylated | 100% | 0 | 90 | alive (6) |
| 91 | F | 58 | L frontal | 80 | 96.64 | yes | GBM | unmethylated | 100% | 0 | 80 | alive (5) |
| 92 | F | 61 | L frontal | 90 | 47.74 | no | GBM | methylated | 100% | 0 | 90 | alive (4) |
| 93 | F | 70 | R temporal | 90 | 89.13 | yes | GBM | unmethylated | 96.7% | 2.93 | 90 | alive (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falco, J.; Rubiu, E.; Broggi, M.; Farinotti, M.; Vetrano, I.G.; Schiariti, M.; Anghileri, E.; Eoli, M.; Pollo, B.; Moscatelli, M.; et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. J. Clin. Med. 2023, 12, 178. https://doi.org/10.3390/jcm12010178
Falco J, Rubiu E, Broggi M, Farinotti M, Vetrano IG, Schiariti M, Anghileri E, Eoli M, Pollo B, Moscatelli M, et al. Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients. Journal of Clinical Medicine. 2023; 12(1):178. https://doi.org/10.3390/jcm12010178
Chicago/Turabian StyleFalco, Jacopo, Emanuele Rubiu, Morgan Broggi, Mariangela Farinotti, Ignazio G. Vetrano, Marco Schiariti, Elena Anghileri, Marica Eoli, Bianca Pollo, Marco Moscatelli, and et al. 2023. "Towards an Established Intraoperative Oncological Favorable Tool: Results of Fluorescein-Guided Resection from a Monocentric, Prospective Series of 93 Primary Glioblastoma Patients" Journal of Clinical Medicine 12, no. 1: 178. https://doi.org/10.3390/jcm12010178








