Differential Diagnosis of Hyperferritinemia in Critically Ill Patients
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Diagnosis of Sepsis, Septic Shock and Other Diagnoses
2.3. Data Collection of Diagnostic Markers
2.4. Statistical Analysis
3. Results
3.1. Study Population and Characteristics
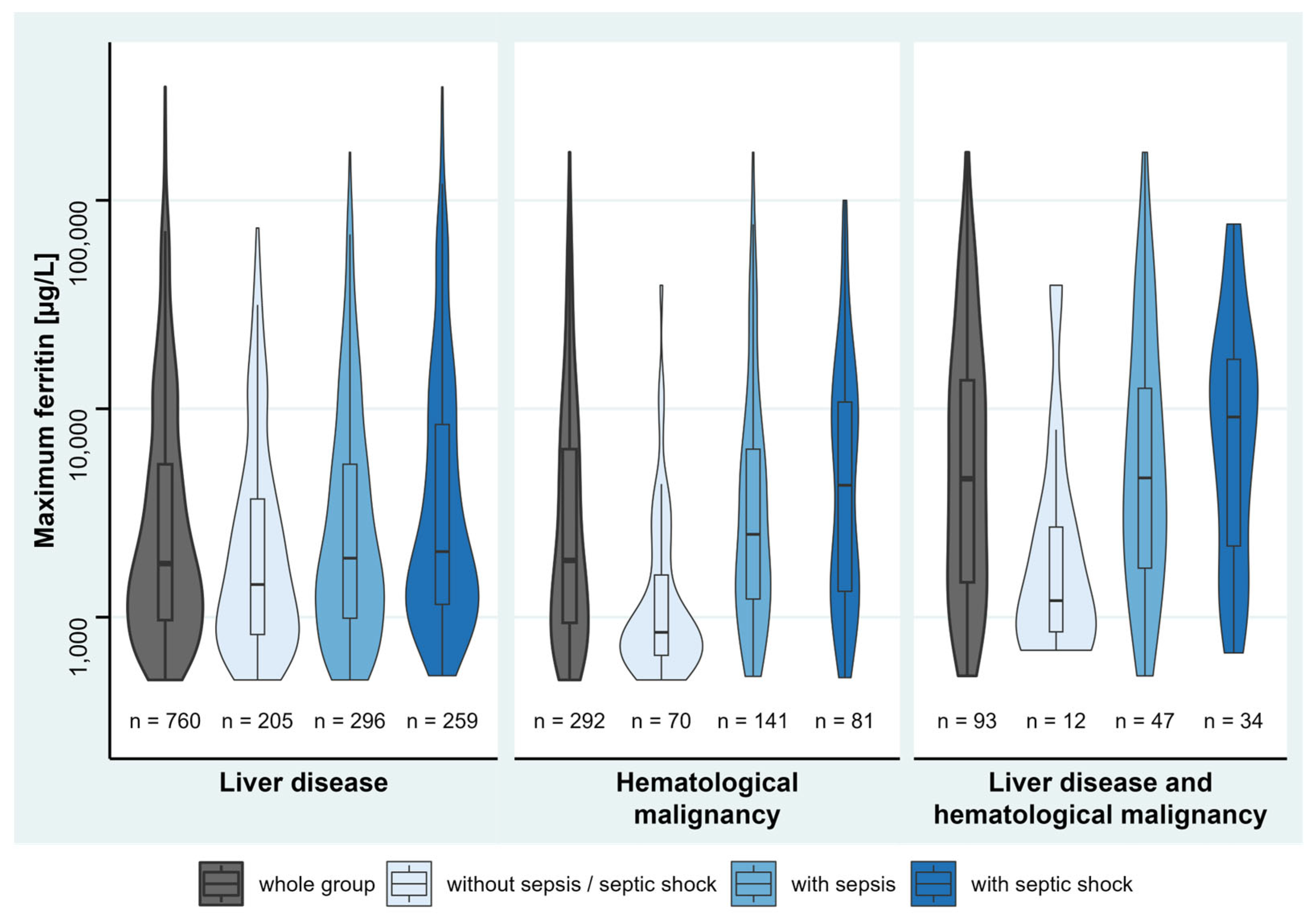
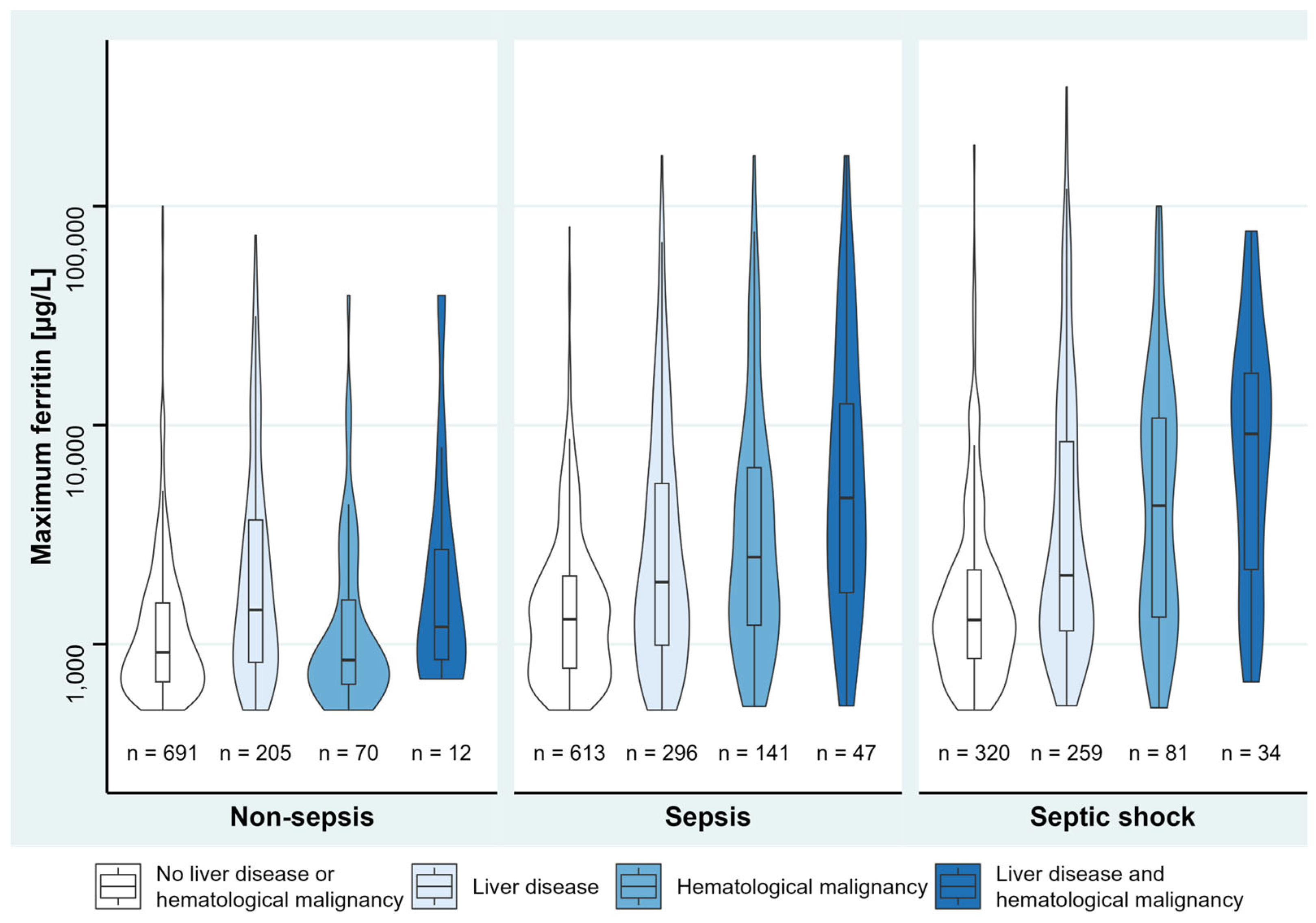
3.2. Underlying Diseases Associated with Hyperferritinemia
3.3. Description of Disease Severity and Hyperferritinemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| AP | alkaline phosphatase |
| aPTT | activated partial thromboplastin time |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CMV | cytomegalovirus |
| COVID-19 | corona virus disease 2019 |
| CRP | c reactive protein |
| EBV | Epstein-Barr virus |
| ECLA | extracorporeal lung assist |
| ECMO | extracorporeal membrane oxygenation |
| ɣGT | gamma glutamyl transferase |
| HLH | hemophagocytic lymphohistiocytosis |
| HIV | human immunodeficiency virus |
| HSV | herpes simplex virus |
| ICD-10 | International Statistical Classification of Diseases and Related Health Problems, 10th Revision |
| ICU | intensive care unit |
| INR | international normalized ratio |
| IL | interleukin |
| LDH | lactate dehydrogenase |
| MAS | macrophage activation syndrome |
| NK | natural killer |
| PCT | procalcitonin |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus type 2 |
| SOFA | sequential organ failure assessment |
| VZV | varicella-zoster virus |
References
- Tsuji, Y.; Ayaki, H.; Whitman, S.P.; Morrow, C.S.; Torti, S.V.; Torti, F.M. Coordinate transcriptional and translational regulation of ferritin in response to oxidative stress. Mol. Cell Biol. 2000, 20, 5818–5827. [Google Scholar] [CrossRef]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An Inflammatory Player Keeping Iron at the Core of Pathogen-Host Interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Goddard, A.F.; James, M.W.; McIntyre, A.S.; Scott, B.B. Guidelines for the management of iron deficiency anaemia. Gut 2011, 60, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C. Epidemiology and diagnostic testing for hemochromatosis and iron overload. Int. J. Lab. Hematol. 2015, 37 (Suppl. S1), 25–30. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, N.; Aljaser, A.; Almutairi, A.; Alshaikh, M.; Eldali, A.; Al-Mayouf, S.M. Utility of serum ferritin and soluble interleukin-2 receptor as markers of disease activity in childhood systemic lupus erythematosus. Int. J. Pediatr. Adolesc. Med. 2020, 7, 112–115. [Google Scholar] [CrossRef]
- Kucuk, H.; Varan, O.; Goker, B.; Bitik, B.; Ozturk, M.A.; Haznedaroglu, S.; Tufan, A. Serum ferritin as an activity marker for granulamotosis with polyangiitis. Ren. Fail. 2017, 39, 566–569. [Google Scholar] [CrossRef]
- Bhargava, J.; Panginikkod, S. Still Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Agmon-Levin, N.; Rosário, C.; Katz, B.-S.P.; Zandman-Goddard, G.; Meroni, P.L.; Cervera, R.; Stojanovich, L.; Blank, M.; Pierangeli, S.; Praprotnik, S.; et al. Ferritin in the antiphospholipid syndrome and its catastrophic variant (cAPS). Lupus 2013, 22, 1327–1335. [Google Scholar] [CrossRef]
- Tofano, R.J.; Pescinni-Salzedas, L.M.; Chagas, E.F.B.; Detregiachi, C.R.P.; Guiguer, E.L.; Araujo, A.C.; Bechara, .M.D.; Rubira, C.J.; Barbalho, S.M. Association of Metabolic Syndrome and Hyperferritinemia in Patients at Cardiovascular Risk. Diabetes Metab. Syndr. Obes. 2020, 13, 3239–3248. [Google Scholar] [CrossRef]
- Castiella, A.; Zapata, E.; Urreta, I.; Zubiaurre, L.; Alústiza, J.M.; Otazua, P.; Emparanza, J.I.; Iribarren, A.; Mendarte, U.; Bujanda, L.; et al. Body mass index and alcohol consumption are directly related with liver steatosis. Results from a prospective study of patients referred for hyperferritinemia. Ann. Hepatol. 2020, 19, 697. [Google Scholar] [CrossRef] [PubMed]
- Senjo, H.; Higuchi, T.; Okada, S.; Takahashi, O. Hyperferritinemia: Causes and significance in a general hospital. Hematology 2018, 23, 817–822. [Google Scholar] [CrossRef]
- Schram, A.; Campigotto, F.; Mullally, A.; Fogerty, A.; Massarotti, E.; Neuberg, D.; Berliner, N. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015, 125, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, H.; Li, L.; Liu, C.; Yan, S.; Chen, H.; Li, Y. Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. J. Clin. Lab. Anal. 2020, 34, e23618. [Google Scholar] [CrossRef]
- Karaboyas, A.; Morgenstern, H.; Pisoni, R.L.; Zee, J.; Vanholder, R.; Jacobson, S.H.; Inaba, M.; Loram, L.C.; Port, F.K.; Robinson, B.M. Association between serum ferritin and mortality: Findings from the USA, Japan and European Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2018, 33, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Knaak, C.; Nyvlt, P.; Schuster, F.S.; Spies, C.; Heeren, P.; Schenk, T.; Balzer, F.; La Rosée, P.; Janka, G.; Brunkhorst, F.M.; et al. Hemophagocytic Lymphohistiocytosis in Critically Ill Patients. Shock 2020, 53, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Eo, W.; Kim, S.; Shim, B.; Lee, S. Significance of serum ferritin as a prognostic factor in advanced hepatobiliary cancer patients treated with Korean medicine: A retrospective cohort study. BMC Complement. Altern. Med. 2018, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Bartoloni, E.; Bursi, R.; Cafaro, G.; Guidelli, G.M.; Shoenfeld, Y.; Gerli, R. COVID-19 as part of the hyperferritinemic syndromes: The role of iron depletion therapy. Immunol. Res. 2020, 68, 213–224. [Google Scholar] [CrossRef]
- Vassilopoulos, A.M.W.; Lakhani, A. Update in Hyperferritinemic Syndromes: Recognition and Management-A Scoping Review. Brown J. Hosp. Med. 2022, 1. [Google Scholar] [CrossRef]
- Mahroum, N.; Elsalti, A.; Alwani, A.; Seida, I.; Alrais, M.; Seida, R.; Esirgun, S.N.; Abali, T.; Kiyak, Z.; Zoubi, M.; et al. The mosaic of autoimmunity-Finally discussing in person. The 13(th) international congress on autoimmunity 2022 (AUTO13) Athens. Autoimmun Rev. 2022, 21, 103166. [Google Scholar] [CrossRef]
- Lachmann, G.; Knaak, C.; Vorderwülbecke, G.; La Rosée, P.; Balzer, F.; Schenk, T.; Schuster, F.S.; Nyvlt, P.; Janka, G.; Brunkhorst, F.M.; et al. Hyperferritinemia in Critically Ill Patients. Crit. Care Med. 2020, 48, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Sackett, K.; Cunderlik, M.; Sahni, N.; Killeen, A.A.; Olson, A.P. Extreme Hyperferritinemia: Causes and Impact on Diagnostic Reasoning. Am. J. Clin. Pathol. 2016, 145, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Fauter, M.; Mainbourg, S.; El Jammal, T.; Guerber, A.; Zaepfel, S.; Henry, T.; Gerfaud-Valentin, M.; Sève, P.; Jamilloux, Y. Extreme Hyperferritinemia: Causes and Prognosis. J. Clin. Med. 2022, 11, 5438. [Google Scholar] [CrossRef]
- Henter, J.-I.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Knaak, C.; Nyvlt, P.; Schuster, F.S.; Spies, C.; Heeren, P.; Schenk, T.; Balzer, F.; La Rosée, P.; Janka, G.; Brunkhorst, F.M.; et al. Hemophagocytic lymphohistiocytosis in critically ill patients: Diagnostic reliability of HLH-2004 criteria and HScore. Crit. Care 2020, 24, 244. [Google Scholar] [CrossRef]
- Lachmann, G.; Spies, C.; Schenk, T.; Brunkhorst, F.M.; Balzer, F.; La Rosée, P. Hemophagocytic Lymphohistiocytosis: Potentially Underdiagnosed in Intensive Care Units. Shock 2018, 50, 149–155. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Connor, J.R. The significance of ferritin in cancer: Anti-oxidation, inflammation and tumorigenesis. Biochim. Biophys. Acta 2013, 1836, 245–254. [Google Scholar] [CrossRef]
- Yamazaki, E.; Tomita, N.; Koyama, S.; Ogusa, E.; Ishii, Y.; Takahashi, H.; Miyashita, K.; Matsuura, S.; Tachibana, T.; Takasaki, H.; et al. Serum ferritin level is prognostic of patient outcome in extranodal NK/T cell lymphoma, nasal type. Med. Oncol. 2014, 31, 149. [Google Scholar] [CrossRef]
- Jones, P.A.; Miller, F.M.; Worwood, M.; Jacobs, A. Ferritinaemia in leukaemia and Hodgkin’s disease. Br. J. Cancer 1973, 27, 212–217. [Google Scholar] [CrossRef]
- Anupama, K.V.; Rao, P.S.; Adappa, S.; Balanthimogru, P.; Mahabala, C. Correlation between serum ferritin and bone marrow iron stores. Trop. Doct. 2017, 47, 217–221. [Google Scholar]
- Beer, T.; Vadakara, J. Etiologies and Short-Term Mortality in Patients with Ultraelevated Serum Ferritin. South Med. J. 2015, 108, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Wang, H.Y.; Wang, W. Measurement and clinical significance of serum TPO and LDH levels in patients with myelodysplastic syndrome and acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2010, 18, 671–674. [Google Scholar] [PubMed]
- Erez, A.; Shental, O.; Tchebiner, J.Z.; Laufer-Perl, M.; Wasserman, A.; Sella, T.; Guzner-Gur, H. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr. Med. Assoc. J. 2014, 16, 439–443. [Google Scholar]
- Anderson, E.R.; Shah, Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013, 3, 315–330. [Google Scholar]
- Buzzetti, E.; Petta, S.; Manuguerra, R.; Luong, T.V.; Cabibi, D.; Corradini, E.; Craxì, A.; Pinzani, M.; Tsochatzis, E.; Pietrangelo, A. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease. Liver Int. 2019, 39, 1325–1334. [Google Scholar] [CrossRef]
- Wong, K.; Adams, P.C. The diversity of liver diseases among outpatient referrals for an elevated serum ferritin. Can. J. Gastroenterol. 2006, 20, 467–470. [Google Scholar] [CrossRef]
- Batsaikhan, B.; Gantumur, G.; Huang, C.-I.; Yeh, M.-L.; Huang, C.-F.; Lin, Z.-Y.; Chen, S.-C.; Huang, J.-F.; Yu, M.-L.; Lee, J.-C.; et al. Elevated serum ferritin level associated with hepatic steatosis and fibrosis in hepatitis C virus-infected patients. J. Chin. Med. Assoc. 2019, 82, 99–104. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Bai, G.; Han, W.; Guo, R.; Cui, N. mTOR Modulates the Endoplasmic Reticulum Stress-Induced CD4(+) T Cell Apoptosis Mediated by ROS in Septic Immunosuppression. Mediat. Inflamm. 2022, 2022, 6077570. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; on behalf of the Hellenic Sepsis Study Group; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Rosário, C.; Zandman-Goddard, G.; Meyron-Holtz, E.G.; D’Cruz, D.P.; Shoenfeld, Y. The hyperferritinemic syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013, 11, 185. [Google Scholar] [CrossRef]
- Moore, C., Jr.; Ormseth, M.; Fuchs, H. Causes and significance of markedly elevated serum ferritin levels in an academic medical center. J. Clin. Rheumatol. 2013, 19, 324–328. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 2583) | Ferritin <9083 µg/L (n = 2373) | Ferritin ≥9083 µg/L (n = 210) | p Value | |
|---|---|---|---|---|
| Age [years] | 62 (49–73) | 63 (50–73) | 52 (37–64) | <0.001 ‡ |
| Male sex [n] (%) | 1588 (61.5%) | 1474 (62.1%) | 114 (54.3%) | 0.025 † |
| Body Mass Index [kg/m2] | 25.0 (22.0–29.0) | 25.0 (22.0–29.0) | 24.1 (21.0–29.0) | 0.121 ‡ |
| Maximum ferritin [µg/L] | 1289 (784–2480) | 1190 (757–1997) | 16,964 (12,127–37,406) | <0.001 ‡ |
| Sepsis without shock [n] (%) | 1003 (38.8%) | 928 (39.1%) | 75 (35.7%) | 0.334 † |
| Septic shock [n] (%) | 626 (24.2%) | 538 (22.7%) | 88 (41.9%) | <0.001 † |
| Tuberculosis [n] (%) | 20 (0.8%) | 18 (0.8%) | 2 (1.0%) | 0.759 † |
| Hepatitis [n] (%) | 159 (6.2%) | 139 (5.9%) | 20 (9.5%) | 0.034 † |
| VZV [n] (%) | 31 (1.2%) | 29 (1.2%) | 2 (1.0%) | 0.731 † |
| Malaria [n] (%) | 4 (0.2%) | 3 (0.1%) | 1 (0.5%) | 0.217 † |
| HSV [n] (%) | 107 (4.1%) | 98 (4.1%) | 9 (4.3%) | 0.913 † |
| Influenza [n] (%) | 58 (2.2% | 52 (2.2%) | 6 (2.9%) | 0.532 † |
| Liver disease (except hepatitis) [n] (%) | 760 (29.4%) | 619 (26.1%) | 141 (67.1%) | <0.001 † |
| History of stem cell/organ transplantation [n] (%) | 316 (12.2%) | 272 (11.5%) | 44 (21.0%) | <0.001 † |
| CMV [n] (%) | 139 (5.4%) | 118 (5.0%) | 21 (10.0%) | 0.002 † |
| HIV [n] (%) | 70 (2.7%) | 62 (2.6%) | 8 (3.8%) | 0.306 |
| EBV [n] (%) | 40 (1.5%) | 34 (1.4%) | 6 (2.9%) | 0.109 † |
| Renal disease [n] (%) | 1820 (70.5%) | 1674 (70.5%) | 146 (69.5%) | 0.756 † |
| (Bacterial/viral/fungal) infection [n] (%) | 2250 (87.1%) | 2067 (87.1%) | 183 (87.1%) | 0.987 † |
| Inflammation without infection [n] (%) | 843 (32.6%) | 790 (33.3%) | 53 (25.2%) | 0.017 † |
| Autoimmune disease [n] (%) | 185 (7.2%) | 172 (7.2%) | 13 (6.2%) | 0.569 † |
| Solid malignancy [n] (%) | 428 (16.6%) | 401 (16.9%) | 27 (12.9%) | 0.131 † |
| Hematological malignancy [n] (%) | 292 (11.3%) | 239 (10.1%) | 53 (25.2%) | <0.001 † |
| Pre-existing immunosuppression [n] (%) | 728 (28.2%) | 638 (26.9%) | 90 (42.9%) | <0.001 ‡ |
| Hemoglobin [g/dL] | 8.8 (7.9–9.9) | 8.8 (7.9–9.9) | 8.9 (7.8–10.1) | 0.807 ‡ |
| Platelet count [/nL] | 170 (88–270) | 179 (101–278) | 56 (25–136) | <0.001 ‡ |
| Leukocyte count [/nL] | 9.0 (6.1–13.3) | 9.1 (6.2–13.2) | 8.5 (3.6–15.9) | 0.057 ‡ |
| Triglycerides [mg/dL], n = 765|90 | 158 (104–247) | 155 (103–233) | 218 (121–420) | <0.001 ‡ |
| Fibrinogen [mg/dL], n = 991|153 | 3.8 (2.5–5.3) | 4.0 (2.7–5.3) | 2.9 (1.8–4.5) | <0.001 ‡ |
| INR, n = 2341|209 | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | 1.6 (1.3–2.3) | <0.001 ‡ |
| aPTT [sec], n = 2347|209 | 45.7 (38.8–55.9) | 45.1 (38.5–55.1) | 51.1 (43.7–64.0) | <0.001 ‡ |
| AST [U/L], n = 2120|207 | 47 (26–108) | 43 (25–89) | 350 (91–2955) | <0.001 ‡ |
| ALT [U/L], n = 2107|208 | 34 (18–79) | 32 (17–66) | 207 (47–1478) | <0.001 ‡ |
| Bilirubin [mg/dl], n = 2050|198 | 0.8 (0.4–2.4) | 0.7 (0.4–2.0) | 3.3 (1.3–9.2) | <0.001 ‡ |
| ɣGT [U/l], n = 2082|208 | 124 (55–272) | 121 (54–266) | 157 (76–375) | <0.001 ‡ |
| AP [U/l], n = 1758|205 | 138 (86–245) | 134 (84–230) | 212 (114–455) | <0.001 ‡ |
| Albumin [g/L], n = 1779|195 | 26 (22.3–30.2) | 26.0 (22.4–30.3) | 25.4 (21.6–29.8) | 0.060 ‡ |
| Creatinine [mg/dl], n = 2254|170 | 1.3 (0.7–2.6) | 1.3 (0.7–2.7) | 1.7 (0.8–2.6) | 0.112 ‡ |
| CRP [mg/L], n = 2306|208 | 81.5 (38.4–155.8) | 79 (37–150) | 119 (56–222) | <0.001 ‡ |
| PCT [µg/L], n = 1446|165 | 1.0 (0.4–3.6) | 0.8 (0.3–2.7) | 3.6 (1.5–12.4) | <0.001 ‡ |
| Lactate [mg/dl], n = 2347|207 | 13 (9–20) | 13 (9–19) | 25 (12–60) | <0.001 ‡ |
| LDH [U/L], n = 1142|155 | 361 (246–593) | 339 (237–450) | 1175 (489–2880) | <0.001 ‡ |
| Max. core body temperature [°C], n = 2250|202 | 38.2 (37.5–38.9) | 38.2 (37.5–38.9) | 38.3 (37.6–39.1) | 0.033 ‡ |
| Hemodialysis [n] (%) | 1357 (52.5%) | 1219 (51.4%) | 138 (65.7%) | <0.001 † |
| ECLA/ECMO [n] (%) | 188 (7.3%) | 168 (7.1%) | 20 (9.5%) | 0.191 † |
| ICU admission SOFA score | 6 (3–9) | 6 (3–9) | 8 (4–12) | <0.001 ‡ |
| Maximum SOFA score | 11 (7–15) | 11 (7–15) | 15 (9–19) | <0.001 ‡ |
| ICU duration [d] | 19 (6–47) | 20 (6–47) | 15 (5–43) | 0.047 ‡ |
| Inpatient duration [d] | 38 (18–76) | 39 (19–77) | 31 (12–74) | 0.009 ‡ |
| Mortality [n] (%) | 741 (28.7%) | 626 (26.4%) | 115 (54.8%) | <0.001 † |
| Covariates | Regression Coefficient | 95% CI | p Value |
|---|---|---|---|
| Age | −38.6 | −70.7, −6.4 | 0.019 |
| Sex (male) | 62.0 | −994.6, 1118.5 | 0.908 |
| Body Mass Index | −28.1 | −104.0, 47.8 | 0.468 |
| Maximum SOFA score | 190.9 | 82.0, 299.8 | <0.001 |
| Sepsis or septic shock * | 903.6 | 129.9, 1677.3 | 0.022 |
| Pre-existing immunosuppression | 965.8 | −352.8, 2284.4 | 0.151 |
| Liver disease | 4068.3 | 2861.8, 5274.8 | <0.001 |
| History of stem cell/organ transplantation | 134.6 | −1656.7, 1925.9 | 0.883 |
| Hematological malignancy | 3148.0 | 1504.0, 4792.0 | <0.001 |
| Covariates | Regression Coefficient | 95% CI | p Value |
|---|---|---|---|
| Age | −30.6 | −62.3, 1.1 | 0.059 |
| Sex (male) | −157.9 | −1200.9, 885.0 | 0.767 |
| Body Mass Index | −22.2 | −97.0, 52.6 | 0.560 |
| Maximum SOFA score | 135.4 | 26.7, 244.0 | 0.015 |
| Sepsis or septic shock * | 935.2 | 168.0, 1702.4 | 0.017 |
| Pre-existing immunosuppression | 893.3 | −398.9, 2185.6 | 0.175 |
| Registration for high-urgency liver transplantation (Z75.77) | −71.6 | −5719.9, 5576.6 | 0.980 |
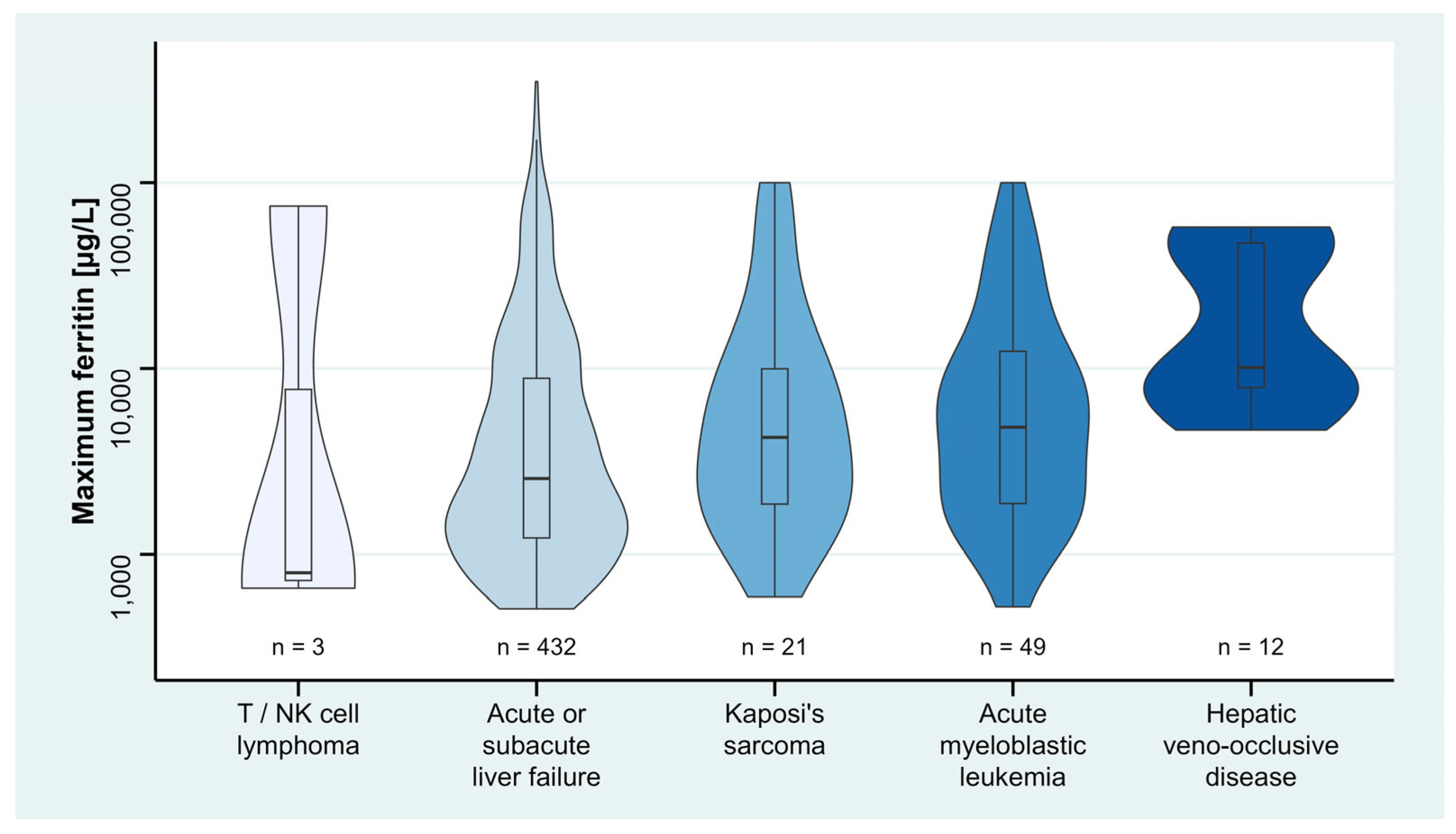
| Acute or subacute liver failure (K72.0) | 7539.9 | 5538.9, 9541.0 | <0.001 |
| Chronic liver failure (K72.1) | 4695.8 | −1022.0, 10,413.6 | 0.107 |
| Grade one hepatic encephalopathy (K72.71) | 2890.7 | −1343.2, 7124.6 | 0.181 |
| Grade two hepatic encephalopathy (K72.72) | −1742.8 | −5975.2, 2489.6 | 0.419 |
| Grade three hepatic encephalopathy (K72.73) | −1078.2 | −4925.2, 2768.9 | 0.583 |
| Grade four hepatic encephalopathy (K72.74) | 951.7 | −2413.3, 4316.7 | 0.579 |
| Hepatic encephalopathy, grade unspecified (K72.79) | −3277.5 | −5966.3, −588.7 | 0.017 |
| Hepatic veno-occlusive disease (K76.5) | 15,783.9 | 8237.4, 23,330.4 | <0.001 |
| History of stem cell/ organ transplantation | 70.3 | −1732.5, 1873.1 | 0.939 |
| T/NK cell lymphoma (C84.5) | 22,919.1 | 8103.3, 37,734.9 | 0.002 |
| Acute lymphocytic leukemia (C91.00) | 5093.2 | −2408.3, 12,594.7 | 0.183 |
| Acute myeloblastic leukemia (C92.00) | 5235.3 | 1475.0, 8995.6 | 0.006 |
| Kaposi’s sarcoma (D46.7) | 9118.6 | 3485.2, 14,752.0 | 0.002 |
| Aplastic anemia (D61.9) | 4083.2 | −185.2, 8351.7 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, F.S.; Nyvlt, P.; Heeren, P.; Spies, C.; Adam, M.F.; Schenk, T.; Brunkhorst, F.M.; Janka, G.; La Rosée, P.; Lachmann, C.; et al. Differential Diagnosis of Hyperferritinemia in Critically Ill Patients. J. Clin. Med. 2023, 12, 192. https://doi.org/10.3390/jcm12010192
Schuster FS, Nyvlt P, Heeren P, Spies C, Adam MF, Schenk T, Brunkhorst FM, Janka G, La Rosée P, Lachmann C, et al. Differential Diagnosis of Hyperferritinemia in Critically Ill Patients. Journal of Clinical Medicine. 2023; 12(1):192. https://doi.org/10.3390/jcm12010192
Chicago/Turabian StyleSchuster, Friederike S., Peter Nyvlt, Patrick Heeren, Claudia Spies, Moritz F. Adam, Thomas Schenk, Frank M. Brunkhorst, Gritta Janka, Paul La Rosée, Cornelia Lachmann, and et al. 2023. "Differential Diagnosis of Hyperferritinemia in Critically Ill Patients" Journal of Clinical Medicine 12, no. 1: 192. https://doi.org/10.3390/jcm12010192
APA StyleSchuster, F. S., Nyvlt, P., Heeren, P., Spies, C., Adam, M. F., Schenk, T., Brunkhorst, F. M., Janka, G., La Rosée, P., Lachmann, C., & Lachmann, G. (2023). Differential Diagnosis of Hyperferritinemia in Critically Ill Patients. Journal of Clinical Medicine, 12(1), 192. https://doi.org/10.3390/jcm12010192





