Neutrophil-to-Lymphocyte Ratio Adds Valuable Information Regarding the Presence of DKA in Children with New-Onset T1DM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Biochemical Assays
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics Stratified by DKA Grade
3.2. Differential WBC Counts
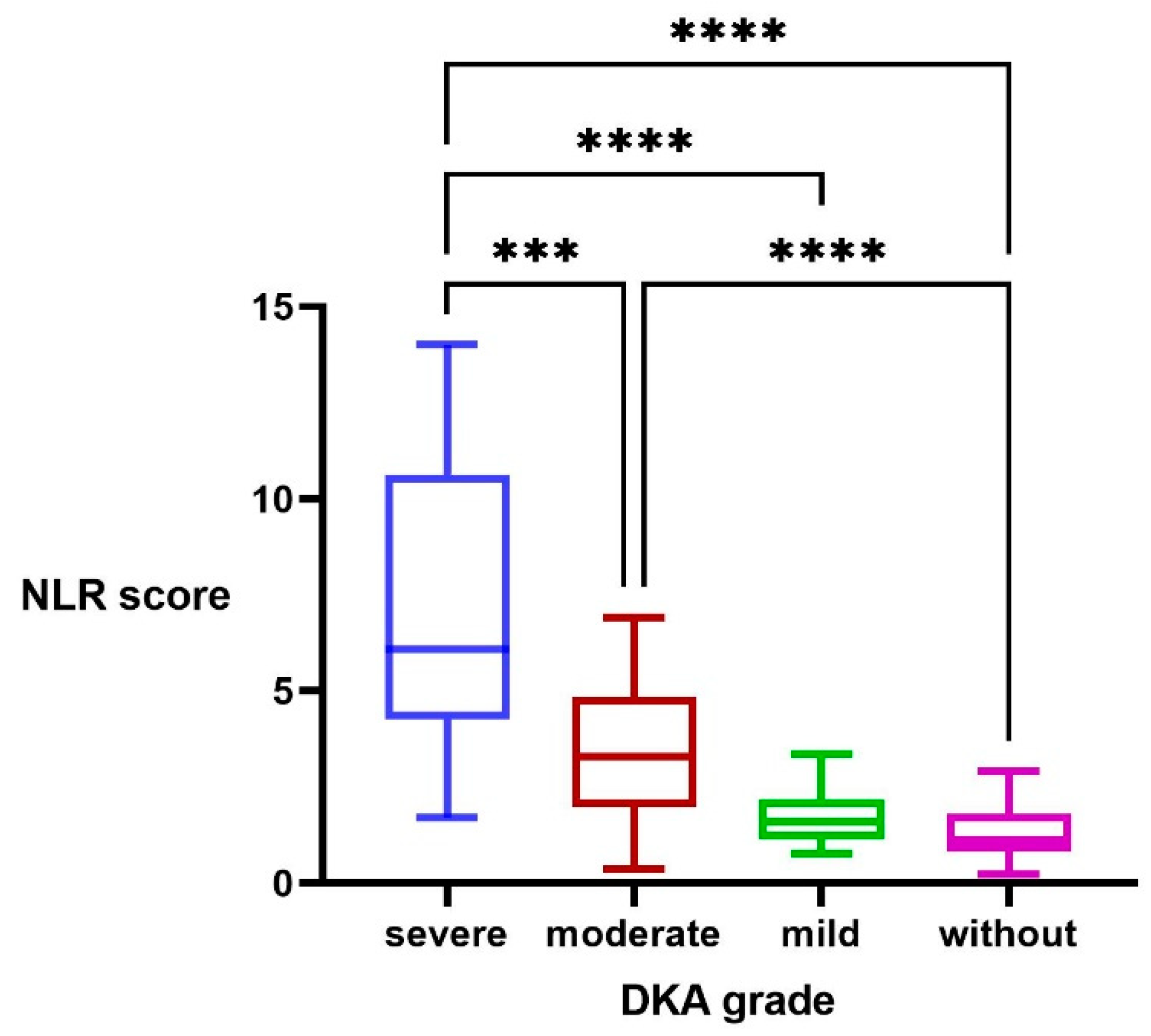
3.3. NLR Score
3.4. Correlation and Regression Analyses
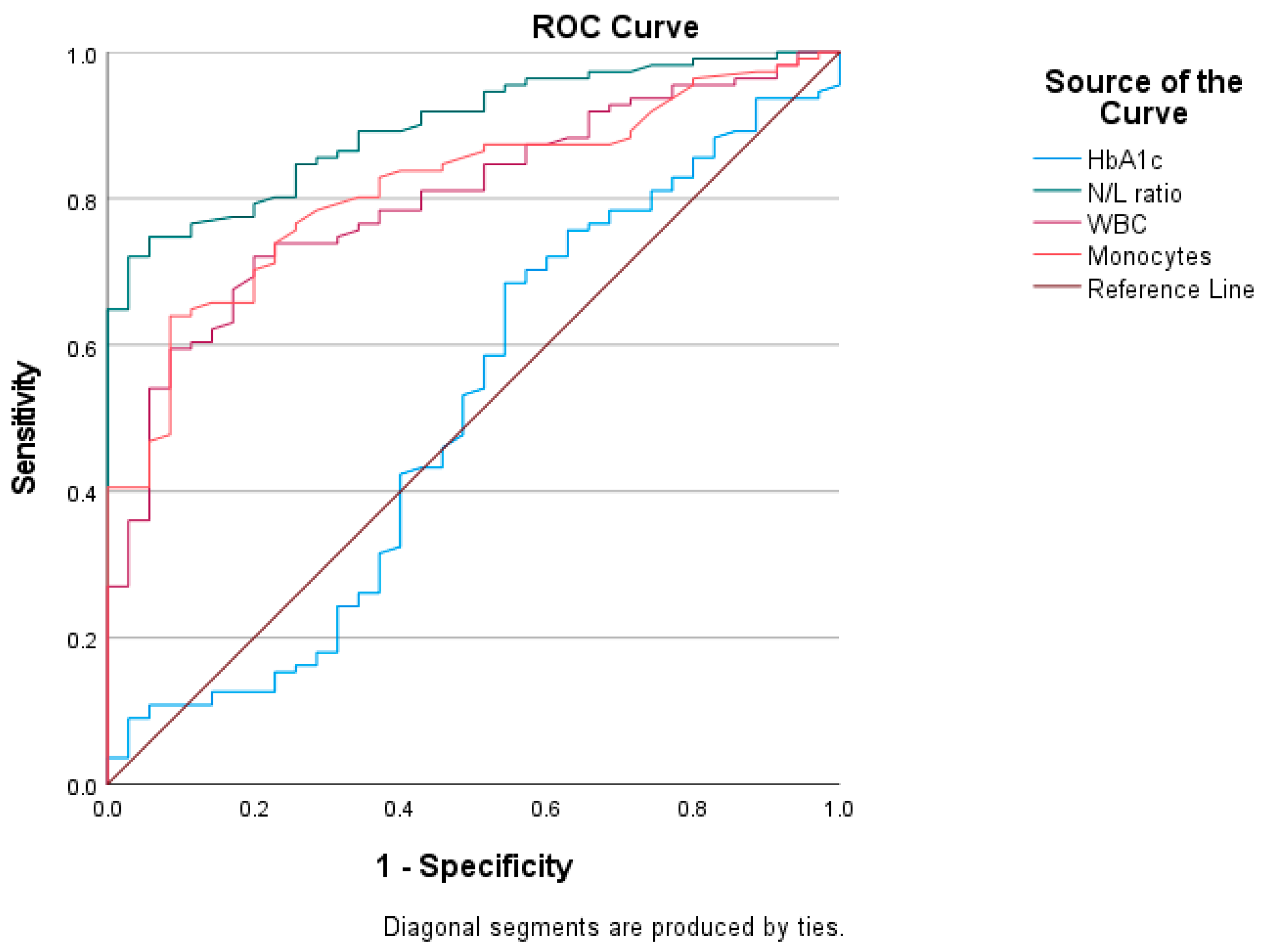
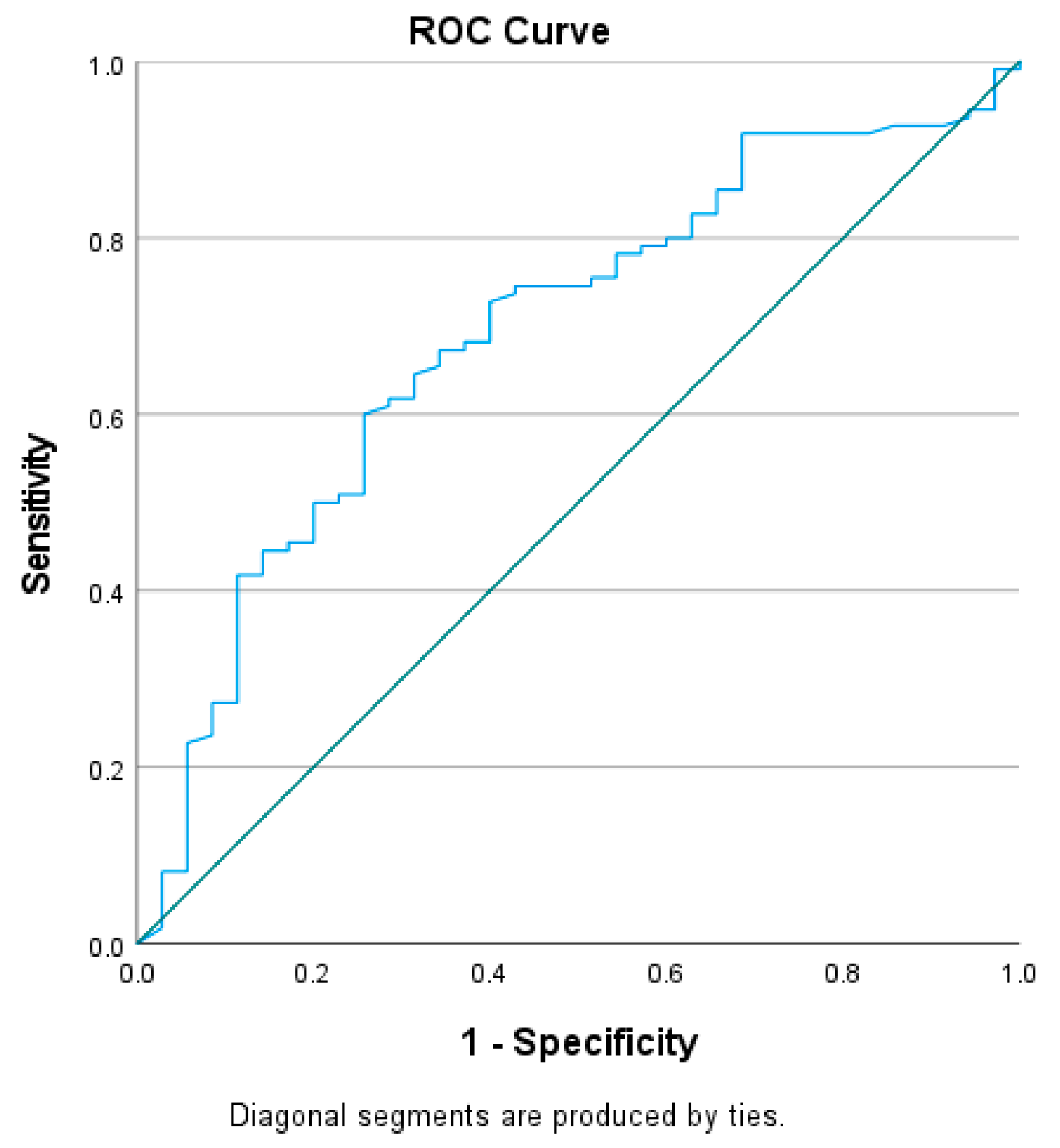
3.5. Receiver Operating Characteristics (ROC) Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfayez, O.M.; Aldmasi, K.S.; Alruwais, N.H.; Bin Awad, N.M.; Al Yami, M.S.; Almohammed, O.A.; Almutairi, A.R. Incidence of Diabetic Ketoacidosis Among Pediatrics with Type 1 Diabetes Prior to and During COVID-19 Pandemic: A Meta-Analysis of Observational Studies. Front. Endocrinol. 2022, 13, 856958. [Google Scholar] [CrossRef] [PubMed]
- Duca, L.M.; Wang, B.; Rewers, M.; Rewers, A. Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes Predicts Poor Long-term Glycemic Control. Diabetes Care 2017, 40, 1249–1255. [Google Scholar] [CrossRef] [Green Version]
- Dhatariya, K.K.; Glaser, N.S.; Codner, E.; Umpierrez, G.E. Diabetic Ketoacidosis. Nat. Rev. Dis. Prim. 2020, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Umpierrez, G.E.; Kitabchi, A.E. Diabetic Ketoacidosis: Risk Factors and Management Strategies. Treat. Endocrinol. 2003, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Saydah, S.H.; Shrestha, S.S.; Zhang, P.; Zhou, X.; Imperatore, G. Medical Costs among Youth Younger than 20 Years of Age with and without Diabetic Ketoacidosis at the Time of Diabetes Diagnosis. Diabetes Care 2019, 42, 2256–2261. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, W.; Zhou, Y.; Zhang, T.; Chi, H.; Xu, C. Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells. Open Life Sci. 2021, 16, 1365–1376. [Google Scholar] [CrossRef]
- Dalton, R.R.; Hoffman, W.H.; Passmore, G.G.; Martin, S.L. Plasma C-reactive protein levels in severe diabetic ketoacidosis. Ann. Clin. Lab. Sci. 2003, 33, 435–442. [Google Scholar]
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. [Updated 2021 May 9]. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK279052 (accessed on 15 October 2022).
- Russell, C.D.; Parajuli, A.; Gale, H.J.; Bulteel, N.S.; Schuetz, P.; de Jager, C.P.C.; Loonen, A.J.M.; Merekoulias, G.I.; Baillie, J.K. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J. Infect. 2019, 78, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wu, H.F.; Ma, S.G.; Bai, F.; Hu, W.; Jin, Y.; Liu, H. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int. J. Med. Sci. 2013, 10, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, A.M.; Araby, E. Neutrophil-Lymphocyte and Platelet-Lymphocyte ratios as a marker for diabetes control and complications. Benha Med. J. 2021, 38, 984–995. [Google Scholar] [CrossRef]
- Durmus, E.; Kivrak, T.; Gerin, F.; Sunbul, M.; Sari, I.; Erdogan, O. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio are Predictors of Heart Failure. Arq. Bras. Cardiol. 2015, 105, 606–613. [Google Scholar] [CrossRef]
- Zhou, B.; Zhan, C.; Wu, J.; Liu, J.; Zhou, J.; Zheng, S. Prognostic Significance of Preoperative Neutrophil-to-Lymphocyte Ratio in Surgically Resectable Pancreatic Neuroendocrine Tumors. Med. Sci. Monit. 2017, 23, 5574–5588. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, E.; López-Sobaler, A.M.; Ortega, R.M.; Delgado-Losada, M.L.; López-Parra, A.M.; Aparicio, A. Association between Neutrophil-to-Lymphocyte Ratio with Abdominal Obesity and Healthy Eating Index in a Representative Older Spanish Population. Nutrients 2020, 12, 855. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. 1), S111–S124. [Google Scholar] [CrossRef]
- Ho, J.; Rosolowsky, E.; Pacaud, D.; Huang, C.; Lemay, J.A.; Brockman, N.; Rath, M.; Doulla, M. Diabetic Ketoacidosis at Type 1 Diabetes Diagnosis in Children During the COVID-19 Pandemic. Pediatr. Diabetes 2021, 22, 552–557. [Google Scholar] [CrossRef]
- Boboc, A.A.; Novac, C.N.; Ilie, M.T.; Ieșanu, M.I.; Galoș, F.; Bălgrădean, M.; Berghea, E.C.; Ionescu, M.D. The Impact of SARS-CoV-2 Pandemic on the New Cases of T1DM in Children. A Single-Centre Cohort Study. J. Pers. Med. 2021, 11, 551. [Google Scholar] [CrossRef]
- Kamrath, C.; Mönkemöller, K.; Biester, T.; Rohrer, T.R.; Warncke, K.; Hammersen, J.; Holl, R.W. Ketoacidosis in Children and Adolescents with Newly Diagnosed Type 1 Diabetes During the COVID-19 Pandemic in Germany. JAMA J. Am. Med. Assoc. 2020, 324, 801–804. [Google Scholar] [CrossRef]
- Dilek, S.Ö.; Gürbüz, F.; Turan, I.; Celiloǧlu, C.; Yüksel, B. Changes in the Presentation of Newly Diagnosed Type 1 Diabetes in Children During the COVID-19 Pandemic in a Tertiary Center in Southern Turkey. J. Pediatr. Endocrinol. Metab. 2021, 34, 1303–1309. [Google Scholar] [CrossRef]
- Dżygało, K.; Nowaczyk, J.; Szwilling, A.; Kowalska, A. Increased Frequency of Severe Diabetic Ketoacidosis at Type 1 Diabetes Onset Among Children During COVID-19 Pandemic Lockdown: An Observational Cohort Study. Pediatr. Endocrinol. Diabetes Metab. 2020, 26, 167–175. [Google Scholar] [CrossRef]
- Jacob, R.; Weiser, G.; Krupik, D.; Takagi, D.; Peled, S.; Pines, N.; Hashavya, S.; Gur-Soferman, H.; Gamsu, S.; Kaplan, O.; et al. Diabetic Ketoacidosis at Emergency Department Presentation During the First Months of the SARS-CoV-2 Pandemic in Israel: A Multicenter Cross-Sectional Study. Diabetes Ther. 2021, 12, 1569–1574. [Google Scholar] [CrossRef]
- Lawrence, C.; Seckold, R.; Smart, C.; King, B.R.; Howley, P.; Feltrin, R.; Smith, T.A.; Roy, R.; Lopez, P. Increased Paediatric Presentations of Severe Diabetic Ketoacidosis in an Australian Tertiary Centre During the COVID-19 Pandemic. Diabetes Med. 2021, 38, e14417. [Google Scholar] [CrossRef] [PubMed]
- McGlacken-Byrne, S.M.; Drew, S.E.V.; Turner, K.; Peters, C.; Amin, R. The SARS-CoV-2 Pandemic Is Associated with Increased Severity of Presentation of Childhood Onset Type 1 Diabetes Mellitus: A Multi-Centre Study of the First COVID-19 Wave. Diabetes Med. 2021, 38, e1464. [Google Scholar] [CrossRef] [PubMed]
- Jhuang, Y.H.; Kao, T.W.; Peng, T.C.; Chen, W.L.; Li, Y.W.; Chang, P.K.; Wu, L.W. Neutrophil to lymphocyte ratio as predictor for incident hypertension: A 9-year cohort study in Taiwan. Hypertens. Res. 2019, 42, 1209–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, K.W.; Kwon, H.M.; Jeong, H.Y.; Park, J.H.; Kim, S.H.; Jeong, S.M. High neutrophil to lymphocyte ratios predict intracranial atherosclerosis in a healthy population. Atherosclerosis 2018, 269, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br. J. Cancer 2012, 107, 695–699. [Google Scholar] [CrossRef] [Green Version]
- Mertoglu, C.; Gunay, M. Neutrophil-Lymphocyte ratio and Platelet-Lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S127–S131. [Google Scholar] [CrossRef]
- Imtiaz, F.; Shafique, K.; Mirza, S.S.; Ayoob, Z.; Vart, P.; Rao, S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int. Arch. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Wheelock, K.M.; Saulnier, P.J.; Tanamas, S.K.; Vijayakumar, P.; Weil, E.J.; Looker, H.C.; Hanson, R.L.; Lemley, K.V.; Yee, B.; Knowler, W.C.; et al. White blood cell fractions correlate with lesions of diabetic kidney disease and predict loss of kidney function in Type 2 diabetes. Nephrol. Dial. Transplant. 2018, 33, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.A.; Abeer, M.; El Baky, N.E.D.A.; Kandil, M.E.; Rasshed, I.A.; Ahmed, A.N.; Ibrahim, M.H. Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio in Well Controlled and Uncontrolled Children and Adolescents with Type 1 Diabetes Mellitus. Res. J. Pharm. Biol. Chem. 2021, 12, 13. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, Y.; Wang, J.; Wang, G.; Wu, Y. The Predictive Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio Levels of Diabetic Peripheral Neuropathy. J. Pain Res. 2021, 14, 2049–2058. [Google Scholar] [CrossRef]
- Jambrik, Z.; Monti, S.; Coppola, V.; Agricola, E.; Mottola, G.; Miniati, M.; Picano, E. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 2010, 105, 186–191. [Google Scholar] [CrossRef]
- Tamhane, U.U.; Aneja, S.; Montgomery, D.; Rogers, E.K.; Eagle, K.A.; Gurm, H.S. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am. J. Cardiol. 2008, 102, 653–657. [Google Scholar] [CrossRef]
- Wang, J.R.; Chen, Z.; Yang, K.; Yang, H.J.; Tao, W.Y.; Li, Y.P.; Jiang, Z.J.; Bai, C.F.; Yin, Y.C.; Duan, J.M.; et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol. Metab. Syndr. 2020, 12, 55. [Google Scholar] [CrossRef]
- Sawant, A.C.; Adhikari, P.; Narra, S.R.; Srivatsa, S.S.; Mills, P.K.; Srivatsa, S.S. Neutrophil to lymphocyte ratio predicts short- and long-term mortality following revascularization therapy for ST elevation myocardial infarction. Cardiol. J. 2014, 21, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Rucker, A.J.; Rudemiller, N.P.; Crowley, S.D. Salt, Hypertension, and Immunity. Annu. Rev. Physiol. 2018, 80, 283–307. [Google Scholar] [CrossRef]
- Prats-Puig, A.; Gispert-Saüch, M.; Díaz-Roldán, F.; Carreras-Badosa, G.; Osiniri, I.; Planella-Colomer, M.; Mayol, L.; de Zegher, F.; Ibánez, L.; Bassols, J.; et al. Neutrophil-to-lymphocyte ratio: An inflammation marker related to cardiovascular risk in children. Thromb. Haemost. 2015, 114, 727–734. [Google Scholar] [CrossRef]
- Alamri, B.N.; Ferris, J.; Matheson, K.; De Tugwell, B. MON-638 The WBC Differential in Relation to DKA Severity. J. Endocr. Soc. 2020, 4 (Suppl. 1), 638. [Google Scholar] [CrossRef]
- Sefil, F.; Ulutas, K.T.; Dokuyucu, R.; Sumbul, A.T.; Yengil, E.; Yagiz, A.E.; Yula, E.; Ustun, I.; Gokce, C. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J. Int. Med. Res. 2014, 42, 581–588. [Google Scholar] [CrossRef]
- Lee, H.J.; Yu, H.W.; Jung, H.W.; Lee, Y.A.; Kim, J.H.; Chung, H.R.; Yoo, J.; Kim, E.; Yu, J.; Shin, C.H.; et al. Factors Associated with the Presence and Severity of Diabetic Ketoacidosis at Diagnosis of Type 1 Diabetes in Korean Children and Adolescents. J. Korean Med. Sci. 2017, 32, 303–309. [Google Scholar] [CrossRef]
- Khanolkar, A.R.; Amin, R.; Taylor-Robinson, D.; Viner, R.M.; Warner, J.; Gevers, E.F.; Stephenson, T. Diabetic Ketoacidosis Severity at Diagnosis and Glycaemic Control in the First Year of Childhood Onset Type 1 Diabetes-A Longitudinal Cohort Study. Int. J. Environ. Res. Public Health 2017, 15, 26. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.; Yuan, J.; Chiavaroli, V.; Dong, G.; Huang, K.; Wu, W.; Ullah, R.; Jin, B.; Lin, H.; Derraik, J.G.B.; et al. 10-Year Incidence of Diabetic Ketoacidosis at Type 1 Diabetes Diagnosis in Children Aged Less Than 16 Years from a Large Regional Center (Hangzhou, China). Front. Endocrinol. 2021, 12, 653519. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.; Yang, W.; Ren, J.; Ge, W.; Yang, P. Apoptosis as an underlying mechanism in lymphocytes induced by riboflavin and ultraviolet light. Transfus. Apher. Sci. 2020, 59, 102899. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Hadadi, Z.; Attar, F.; Mousavi, S.E.; Zargar, S.S.; Tajik, A.; Saboury, A.A.; Rezayat, S.M.; Falahati, M. ROS-mediated heme degradation and cytotoxicity induced by iron nanoparticles: Hemoglobin and lymphocyte cells as targets. J. Biomol. Struct. Dyn. 2018, 36, 4235–4245. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.G.; Jin, Y.; Xu, W.; Hu, W.; Bai, F.; Wu, X.J. Increased serum levels of ischemia-modified albumin and C-reactive protein in type 1 diabetes patients with ketoacidosis. Endocrine 2012, 42, 570–576. [Google Scholar] [CrossRef]
| Parameters | Non-DKA (n = 35) | Mild DKA (n = 25) | Moderate DKA (n = 33) | Severe DKA (n = 62) | p |
|---|---|---|---|---|---|
| Age (years) | 10 (5–13) | 9 (6.5–13) | 7.00 (3.50–11) | 9.00 (5–12) | 0.381 |
| Males% (n) | 42 (15) | 76 (19) | 48 (16) | 41 (26) b | 0.028 |
| HbA1c (%) | 11.37 ± 1.95 | 11.68 ± 1.94 | 11.37 ± 2.16 | 11.52 ± 2.04 | 0.931 |
| C-peptide (ng/mL) | 0.639 (0.41–0.94) | 0.481 (0.35–0.67) | 0.533 (0.29–0.77) | 0.330 (0.18–0.47) a, c | <0.001 |
| Blood pH | 7.36 (7.34–7.37) | 7.28 (7.23–7.29) | 7.17 (7.13–7.20) a | 6.97 (6.89–7.03) a, b, c | 0.000 |
| WBCs (×103/mm3) | 8.53 (6.64–10.13) | 8.12 (6.68–8.90) | 12.27 (9.92–15.47) a, b | 18.78 (14.06–24.52) a, b, c | <0.001 |
| Neutrophils (×103/mm3) | 3.79 (2.99–5.24) | 4.58 (3.38–5.21) | 8.97 (6.24–12.6) a, b | 14.63 (11.06–18) a, b, c | 0.000 |
| Lymphocytes (×103/mm3) | 2.92 (2.50–4.66) | 2.71 (1.96–3.49) | 2.86 (2.04–3.96) | 2.33 (1.59–3.2) a | 0.003 |
| Thrombocytes (×103/mm3) | 299 (229–327) | 259 (223–344) | 347 (283–405) a | 342 (388–422) a, b | <0.001 |
| Monocytes (×103/mm3) | 0.60 (0.49–0.74) | 0.68 (0.51–0.77) | 0.87 (0.70–1.39) a | 1.71 (1.15–2.40) a, b, c | <0.001 |
| Eosinophiles (×103/mm3) | 0.12 (0.05–0.22) | 0.09 (0.03–0.13) | 0.06 (0.02–0.20) | 0.00 (0–0.02) a, b | <0.001 |
| NLR | 1.11 (0.80–1.80) | 1.58 (1.17–1.93) | 3.71 (1.98–4.85) a, b | 5.77 (4.04–9.63) a, b, c | 0.000 |
| pH | B | 95% CI for B | SE B | ß | R2 | ∆R2 | |
|---|---|---|---|---|---|---|---|
| LL | UL | ||||||
| Model | 0.654 | 0.640 | |||||
| Age | 0.010 *** | 0.004 | 0.015 | 0.003 | 0.239 *** | ||
| Gender | 0.003 | −0.036 | 0.042 | 0.020 | 0.007 | ||
| HbA1c | 0.001 | −0.009 | 0.011 | 0.005 | 0.008 | ||
| C peptide | 0.074 * | −0.014 | 0.135 | 0.030 | 0.145 * | ||
| NLR | −0.038 *** | −0.044 | −0.032 | 0.003 | −0.770 *** | ||
| Variable | AUC | S.E. | 95% CI | Cut-Off | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|
| HbA1c | 0.504 | 0.060 | 0.386–0.622 | 11.38 | 49.5 | 51.4 |
| C peptide | 0.690 | 0.050 | 0.591–0.789 | 0.554 | 68.2 | 60.0 |
| WBC | 0.800 | 0.039 | 0.723–0.877 | 8.860 | 79.2 | 57.1 |
| Monocytes | 0.815 | 0.037 | 0.742–0.887 | 0.675 | 80.2 | 62.9 |
| NLR | 0.903 | 0.051 | 0.854–0.952 | 1.84 | 80.2 | 80.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scutca, A.-C.; Nicoară, D.-M.; Mărăzan, M.; Brad, G.-F.; Mărginean, O. Neutrophil-to-Lymphocyte Ratio Adds Valuable Information Regarding the Presence of DKA in Children with New-Onset T1DM. J. Clin. Med. 2023, 12, 221. https://doi.org/10.3390/jcm12010221
Scutca A-C, Nicoară D-M, Mărăzan M, Brad G-F, Mărginean O. Neutrophil-to-Lymphocyte Ratio Adds Valuable Information Regarding the Presence of DKA in Children with New-Onset T1DM. Journal of Clinical Medicine. 2023; 12(1):221. https://doi.org/10.3390/jcm12010221
Chicago/Turabian StyleScutca, Alexandra-Cristina, Delia-Maria Nicoară, Monica Mărăzan, Giorgiana-Flavia Brad, and Otilia Mărginean. 2023. "Neutrophil-to-Lymphocyte Ratio Adds Valuable Information Regarding the Presence of DKA in Children with New-Onset T1DM" Journal of Clinical Medicine 12, no. 1: 221. https://doi.org/10.3390/jcm12010221






