Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies
Abstract
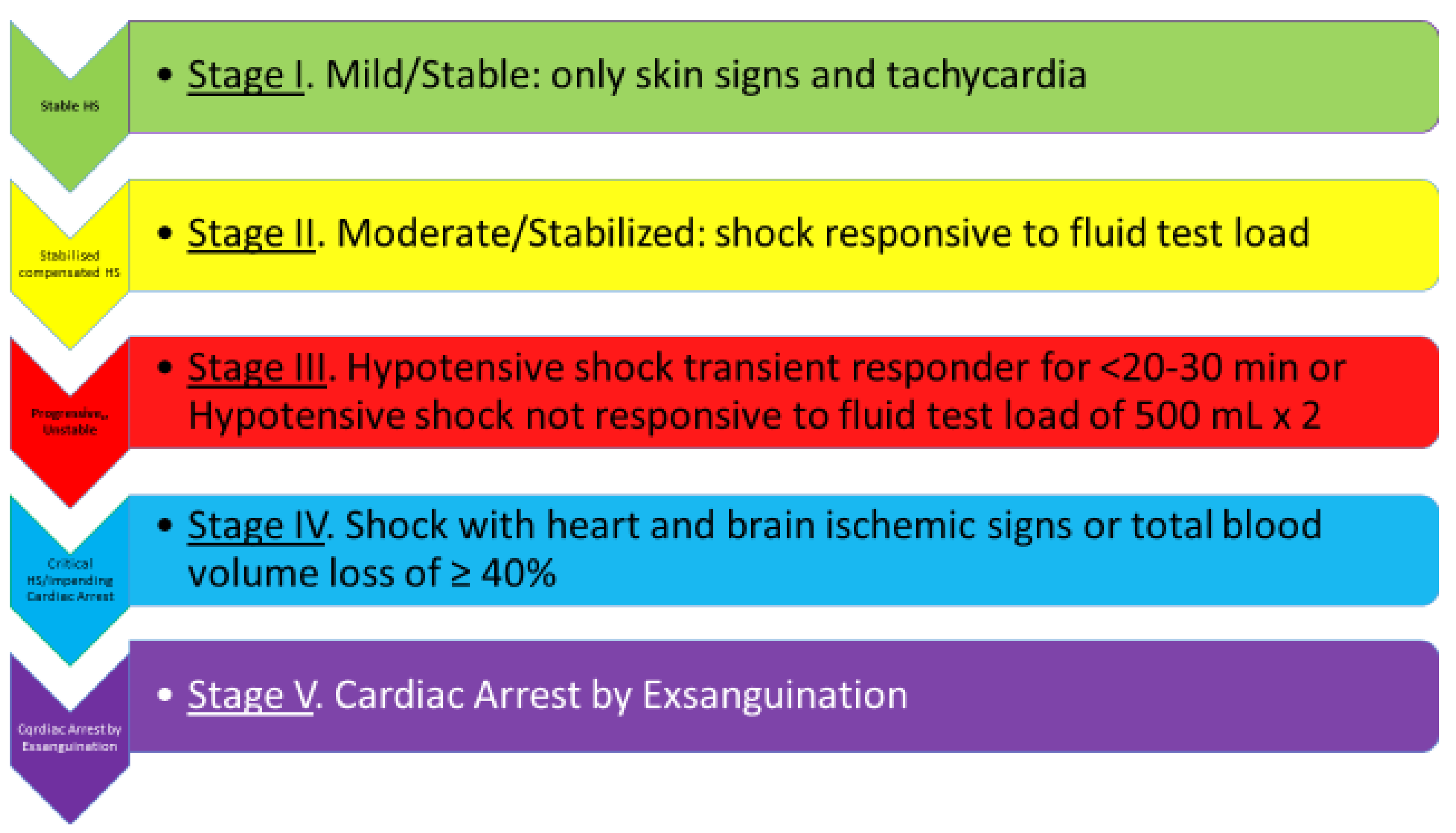
:1. Introduction
1.1. Cardiac Reserve and Fluid Load Test
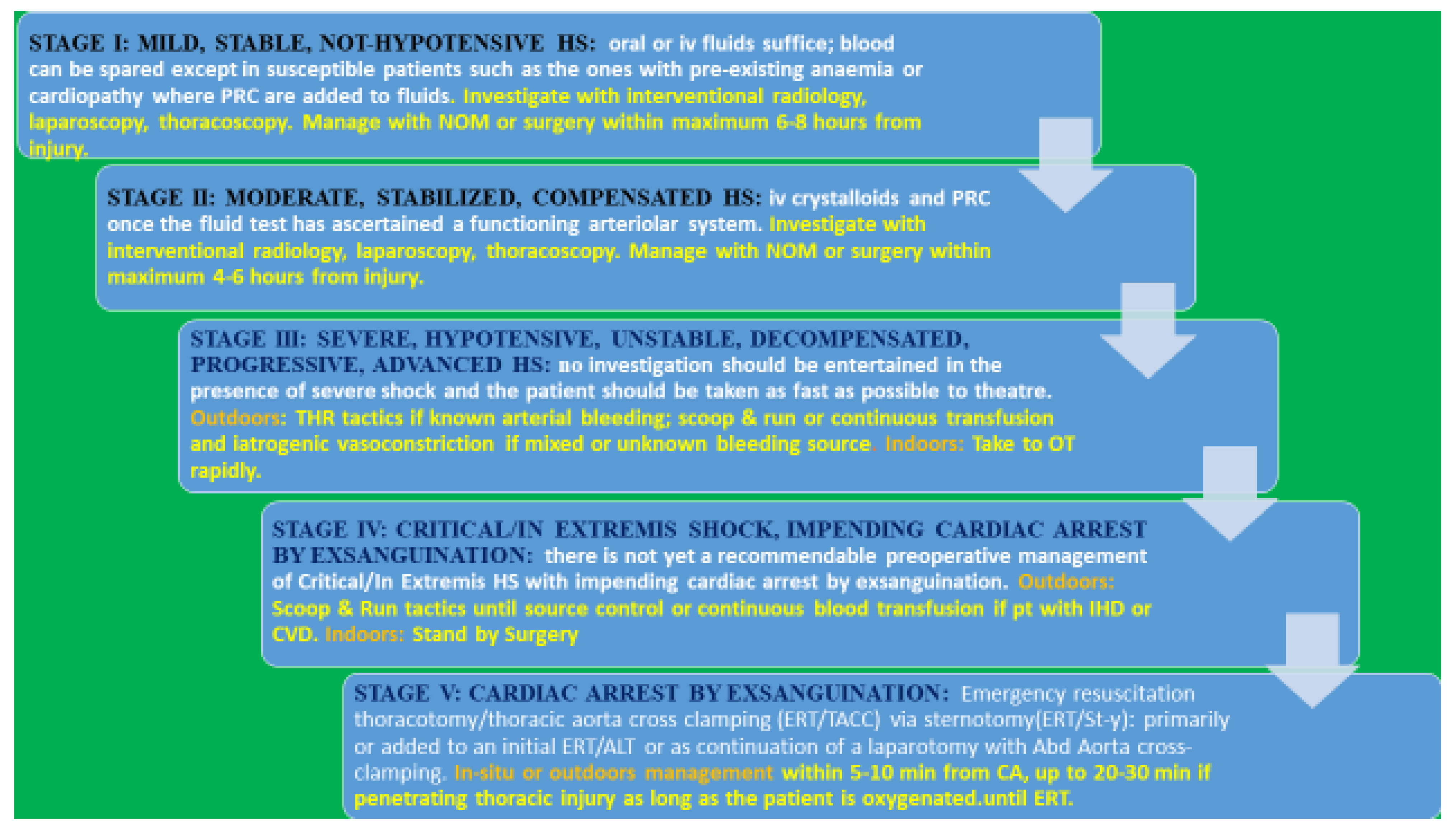
1.2. Management Options of Compensated Stable Shock
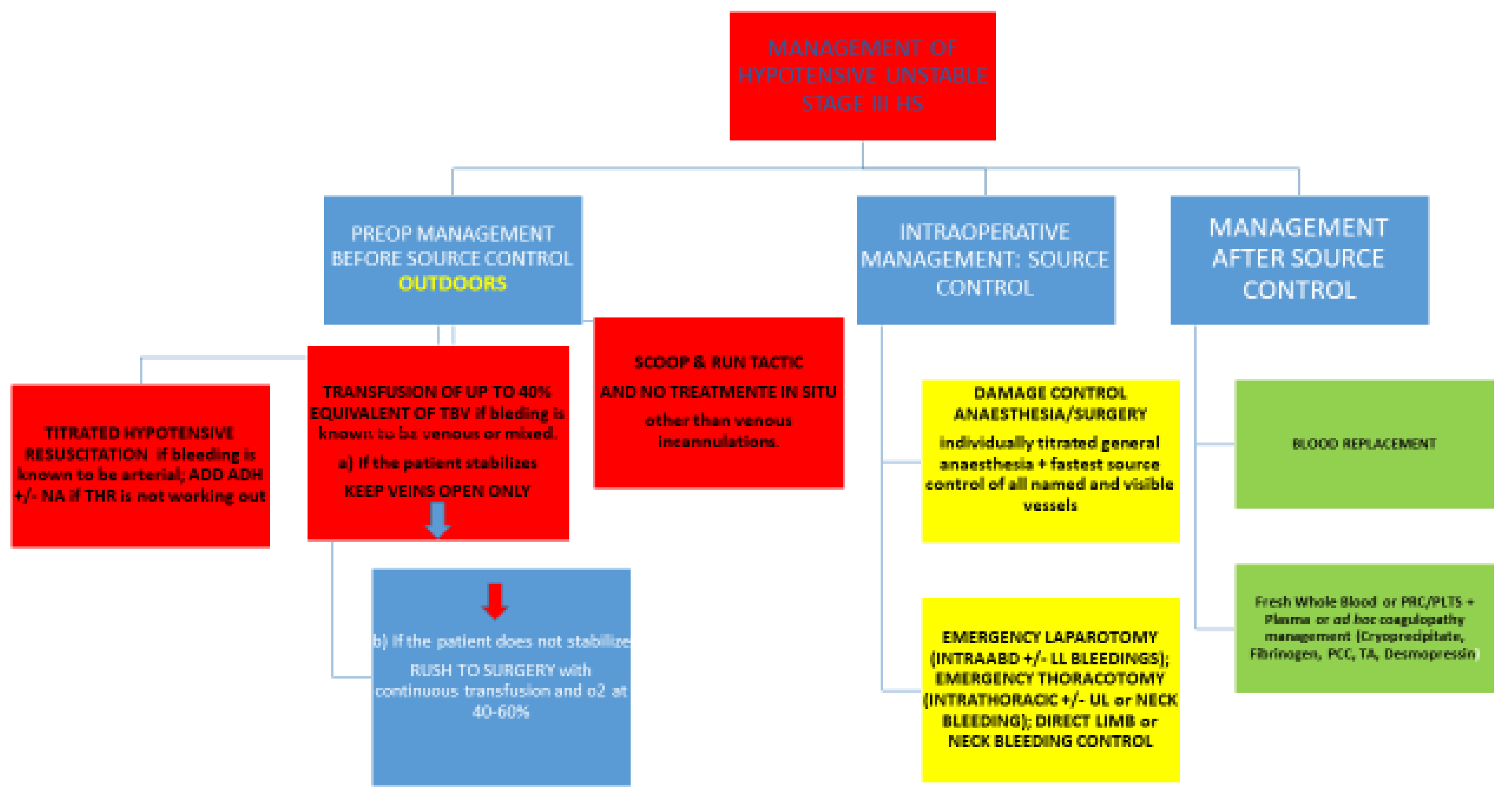
2. Hypotensive, Unstable, Decompensated, Progressing Shock—Stage III
2.1. Management Outdoors
2.1.1. Titrated Hypotensive Resuscitation
2.1.2. Which Amount of Fluid to Use for HR Tactics?
2.1.3. Which Fluid Is Best?
2.1.4. What to do if THR Fails?
2.1.5. What to Do If the Bleeding Outdoors Is Known to Be Venous?
2.1.6. What Fluid to Give in Prehospital Settings If Continuous Transfusion Is the Choice?
2.1.7. Resumé of Fluid Strategy in Progressing HS Stage III
2.2. Management Indoors
2.3. Where the Bleeding Is Coming From?
3. Critical/In Extremis Shock, Impending Cardiac Arrest/Stroke—Stage IV
3.1. How to Manage Impending CA
3.2. Recent Tactics Used to Prevent Stage V of HS, i.e., Cardiac Arrest by Exsanguination
4. Cardiac Arrest by Exsanguination—Stage V
5. The Essential Damage Control Resuscitation (Damage Control Anesthesia and Damage Control Surgery)
5.1. Titrated-to-Response Anesthesia
5.2. Damage Control Surgery
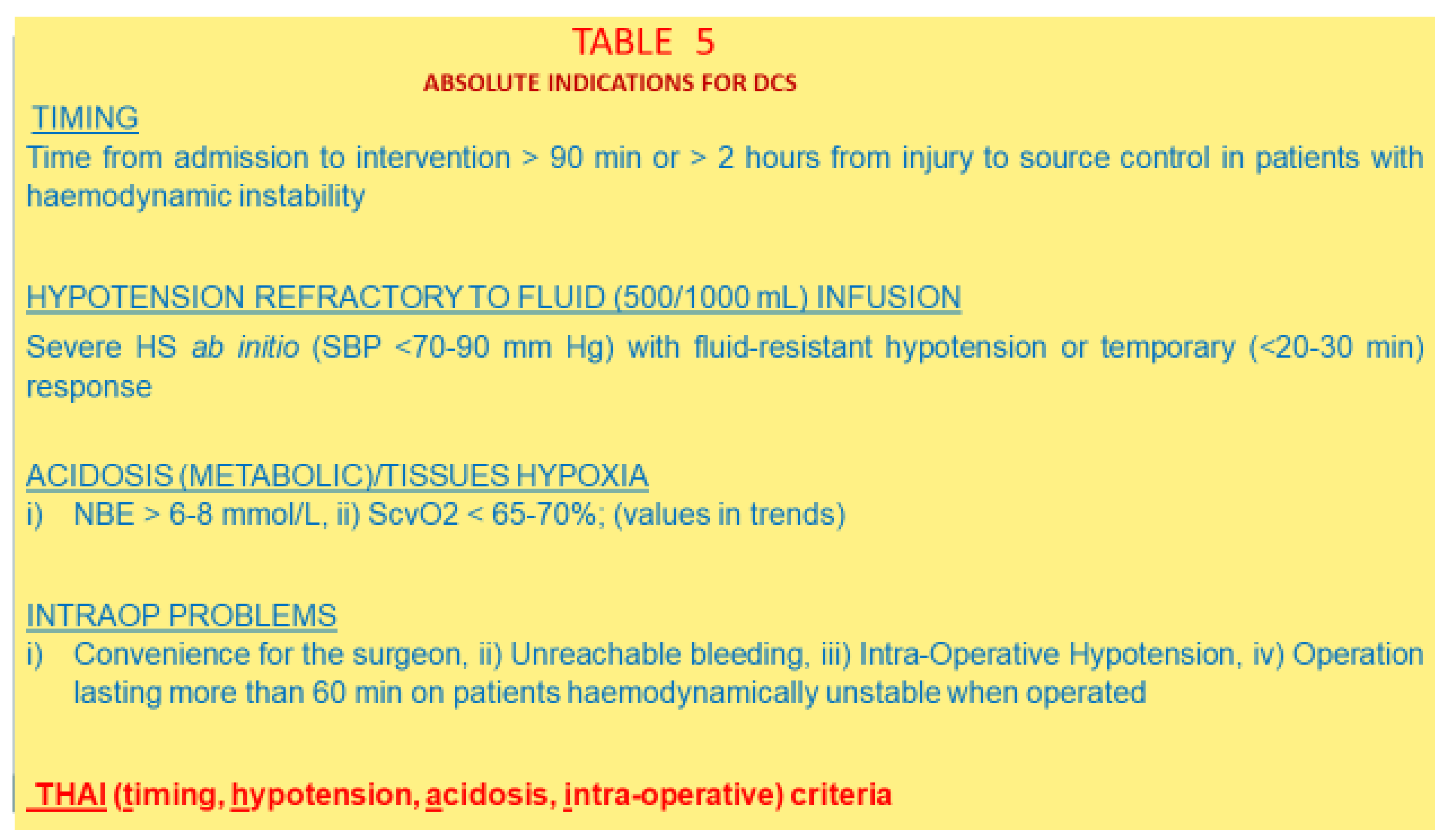
5.2.1. Metabolic Acidosis
5.2.2. Criteria for DCS
5.2.3. The Real Rationale for Performing DCS
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonanno, F.G. Hemorrhagic shock: The “physiology approach”. J. Emerg. Trauma Shock 2012, 5, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, F.G. The need for a physiological classification of haemorrhagic shock. J Emerg. Trauma Shock. 2020, 13, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Cardin, S. Advanced medical monitoring for the battlefield: A review on clinical applicability of compensatory reserve measurements for early and accurate hemorrhage detection. J. Trauma Acute Care Surg. 2022, 93, S147–S154. [Google Scholar] [CrossRef]
- Tiba, M.H.; Awad, A.B.; Pennington, A.; Fung, C.M.; Napolitano, L.M.; Park, P.K.; Machado-Aranda, D.A.; Gunnerson, K.J.; Romfh, P.; Ward, K.R. Resonance Raman Spectroscopy Derived Tissue Hemoglobin Oxygen Saturation in Critically Ill and Injured Patients. Shock 2020, 56, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, F.G. Physiopathology of shock. J. Emerg. Trauma Shock 2011, 4, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, U.; Bobrovsky, B.-Z.; Ben-Dov, I.; Durst, R.; Gabbay, I.E.; Segel, M.J. From a cardio-vascular reserve hypothesis to a proposed measurable index: A pilot empirical validation. Clin. Trials Regul. Sci. Cardiol. 2015, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Convertino, V.A.; Wirt, M.D.; Glenn, J.F.; Lein, B.C. The compensatory reserve for early and accurate prediction of hemodynamic compromise: A review of the underlying physiology. Shock 2015, 45, 580–590. [Google Scholar] [CrossRef]
- Cecconi, M.; Aya, H.D.; Geisen, M.; Ebm, C.; Fletcher, N.; Grounds, R.M.; Rhodes, A. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensiv. Care Med. 2013, 39, 1299–1305. [Google Scholar] [CrossRef]
- Bennett, V.A.; Vidouris, A.; Cecconi, M. Effects of Fluids on the Macro- and Microcirculations. Crit. Care 2018, 22, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, P.; Patel, R.; Kolb, S.; Fatima, S.; Galvagno, S.M.; Haase, D.J.; Ramani, G.V.; Ludmir, J.; Alkhatib, H.; Herr, D.; et al. Echo is a good, not perfect, measure of cardiac output in critically ill surgical patients. J. Trauma Acute Care Surg. 2019, 87, 379–385. [Google Scholar] [CrossRef]
- Jozwiak, M.; Mercado, P.; Teboul, J.L.; Benmalek, A.; Gimenez, J.; Dépret, F.; Rochard, C.; Monnet, X. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit. Care 2019, 23, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aya, H.D.; Ster, I.C.; Fletcher, N.; Grounds, R.M.; Rhodes, A.; Cecconi, M. Pharmacodynamic Analysis of a Fluid Challenge. Crit. Care Med. 2016, 44, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Aya, H.D.; Rhodes, A.; Chis Ster, I.; Fletcher, N.; Grounds, R.M.; Cecconi, M. Hemodynamic effect of different doses of fluids for a fluid challenge: A quasi randomized controlled study. Crit. Care Med. 2017, 45, e161–e168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.B.; Cohen, M.J.; Minei, J.P.; Maier, R.V.; West, M.A.; Billiar, T.R.; Peitzman, A.B.; Moore, E.E.; Cuschieri, J.; Sperry, J.L. Goal-directed resuscitation in the prehospital setting: A propensity-adjusted analysis. J. Trauma Acute Care Surg. 2013, 74, 1207–1214. [Google Scholar] [CrossRef] [Green Version]
- Holcroft, J.W.; Vassar, M.J.; Turner, J.E.; Derlet, R.W.; Kramer, G.C. 3% NaCl and 7.5% NaCl/Dextran 70 in the Resuscitation of Severely Injured Patients. Ann. Surg. 1987, 206, 279–288. [Google Scholar] [CrossRef]
- Wade, C.E.; Kramer, G.C.; Grady, J.J.; Fabian, T.C.; Younes, R.N. Efficacy of hypertonic 7.5% saline and 6% dextran 70 in treating trauma: A meta-analysis of controlled clinical trials. Surgery 1997, 122, 609–616. [Google Scholar] [CrossRef]
- Feldheiser, A.; Pavlova, V.; Bonomo, T.; Jones, A.; Fotopoulou, C.; Sehouli, J.; Wernecke, K.-D.; Spies, C. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br. J. Anaesth. 2012, 110, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Lira, A.; Pinsky, M.R. Choices in fluid type and volume during resuscitation: Impact on patient outcomes. Ann. Intensiv. Care 2014, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Dutton, R.P.; Carson, J.L. Indications for Early Red Blood Cell Transfusion. J. Trauma: Inj. Infect. Crit. Care 2006, 60, S35–S40. [Google Scholar] [CrossRef]
- Regel, G.; Grotz, M.; Weltner, T.; Sturm, J.A.; Tscherne, H. Pattern of organ failure following severe trauma. World J. Surg. 1996, 20, 422–429. [Google Scholar] [CrossRef]
- Moore, F.A.; Sauaia, A.; Moore, E.E.; Haenel, J.B.; Burch, J.M.; Lezotte, D.C. Post-injury multiple organ failure: A bi-modal phenomenon. J. Trauma 1996, 40, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Garrison, R.N.; Spain, D.A.; Wilson, M.A.; Keelen, P.A.; Harris, P.D. Microvascular Changes Explain the “Two-Hit” Theory of Multiple Organ Failure. Ann. Surg. 1998, 227, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Douzinas, E.E.; Andrianakis, I.; Livaditi, O.; Paneris, P.; Tasoulis, M.; Pelekanou, A.; Betrosian, A.; Giamarellos-Bourboulis, E.J. The level of hypotension during hemorrhagic shock is a major determinant of the post-resuscitation systemic inflammatory response: An experimental study. BMC Physiol. 2008, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, F.G. Clinical pathology of the shock syndromes. J. Emerg. Trauma Shock 2011, 4, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Douzinas, E.E. Hemorrhagic shock resuscitation: A critical issue on the development of post-traumatic multiple organ failure. Crit. Care Med. 2012, 40, 1348–1349. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.L.; Thomson, S.R.; Madiba, T.E.; Muckart, D.J.J. Selective conservativism in trauma management: A South African contri-bution. World J. Surg. 2005, 29, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Omoshoro-Jones, J.A.O.; Nicol, A.J.; Navsaria, P.H.; Zellweger, R.; Krige, J.E.J.; Kahn, D.H. Selective non-operative management of liver gunshot injuries. Br. J. Surg. 2005, 92, 890–895. [Google Scholar] [CrossRef]
- Hershkovitz, Y.; Bodas, M.; Givon, A.; Kessel, B. Time to surgery: Is it truly crucial in initially stable patients with penetrating injury? Injury 2020, 52, 195–199. [Google Scholar] [CrossRef]
- Kumar, V.; Mishra, B.; Joshi, M.K.; Purushothaman, V.; Agarwal, H.; Anwer, M.; Sagar, S.; Kumar, S.; Gupta, A.; Bagaria, D.; et al. Early hospital discharge following non-operative management of blunt liver and splenic trauma: A pilot randomized controlled trial. Injury 2020, 52, 260–265. [Google Scholar] [CrossRef]
- The Brain Trauma foundation; The American Association of Neurological Surgeons; The Joint Section on Neurotrauma and Critical Care. Resuscitation of blood pressure and oxygenation. J. Neurotrauma 2000, 17, 471–482. [Google Scholar] [CrossRef]
- Eastridge, B.J.; Salinas, J.; McManus, J.G.; Blackburn, L.; Bugler, E.M.; Cooke, W.H.; Concertino, V.A.; Wade, C.E.; Holcomb, J.B. Hypotension begins at 110 mm Hg: Redefining “hypotension” with data. J. Trauma 2007, 63, 291–297, discussion 297–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, C.; Ley, E.J.; Bukur, M.; Malinoski, D.; Margulies, D.R.; Mirocha, J.; Salim, A. Redefining hypotension in traumatic brain injury. Injury 2012, 43, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Stein, D.M.; Hu, P.F.; Aarabi, B.; Sheth, K.; Scalea, T.M. Traditional systolic blood pressure targets underestimate hypo-tension-induced secondary brain injury. J. Trauma Acute Care Surg. 2012, 72, 1135–1139. [Google Scholar] [CrossRef]
- Ushida, T.; Kotani, T.; Imai, K.; Nakano-Kobayashi, T.; Nakamura, N.; Moriyama, Y.; Yoshida, S.; Yamashita, M.; Kajiyama, H.; Kikkawa, F. Shock index and postpartum haemorrhage in vaginal deliveries: A multicenter retrospective study. Shock 2021, 55, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Cannon, W.B.; Frasen, J.; Cowel, E.M. The preventive treatment of wound shock. J. Am. Med. Assoc. 1918, 70, 618–621. [Google Scholar]
- Shaftan, G.W.; Chiu, C.J.; Dennis, C.; Harris, B. Fundamentals of physiologic control of arterial hemorrhage. Surgery 1965, 58, 851–856. [Google Scholar]
- Wangensteen, S.L.; Ludewig, R.M. Bleeding and blood pressure. Am. J. Surg. 1969, 118, 413–414. [Google Scholar] [CrossRef]
- Assalia, A.; Schein, M. Resuscitation for haemorrhagic shock. Br. J. Surg. 1993, 80, 213. [Google Scholar] [CrossRef]
- Krausz, M.M. Fluid Resuscitation Strategies in the Israeli Army. J. Trauma: Inj. Infect. Crit. Care 2003, 54, S39–S42. [Google Scholar] [CrossRef]
- Blumenfeld, A.; Melamed, E.; Kalmovich, B. Pre-hospital fluid resuscitation in trauma: The IDF-MC panel Summary [Abstract]. J. Israeli Mil. Med. 2004, 1, 6–10. [Google Scholar]
- Crawford, E.S. Ruptured abdominal aneurysms. J. Vasc. Surg. 1991, 13, 348–350. [Google Scholar] [CrossRef]
- Blair, S.D.; Janvrin, S.B.; McCollum, C.N.; Greenhalgh, R.M. Effect of early blood transfusion on gastrointestinal haemorrhage. Br. J. Surg. 1986, 73, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Kudo, D.; Yoshida, Y.; Kushimoto, S. Permissive hypotension/hypotensive resuscitation and restricted/controlled resuscitation in patients with severe trauma. J. Intensiv. Care 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Zheng, D.; Huang, X.; Zhang, J.; Liang, W.; Huang, Z.; Zhu, S.G. Comparison of Permissive Hypotension vs. Conventional Re-suscitation. Strategies in Adult Trauma Patients with Hemorrhagic Shock: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Research Square, 13 April 2021. [Google Scholar]
- Woodward, L.; Alsabri, M. Permissive Hypotension vs. Conventional Resuscitation in Patients with Trauma or Hemorrhagic Shock: A Review. Cureus 2021, 13, e16487. [Google Scholar] [CrossRef]
- Kappen, T.; Beattie, W.S. Perioperative hypotension 2021: A contrarian view. Br. J. Anaesth. 2021, 127, 167–170. [Google Scholar] [CrossRef]
- Kentner, R.; Safar, P.; Prueckner, S.; Behringer, W.; Wu, X.; Henchir, J.; Ruemelin, A.; Tisherman, S.A. Titrated hypertonic/hyperoncotic solution for hypotensive fluid resuscitation during uncontrolled hemorrhagic shock in rats. Resuscitation 2005, 65, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Alginate plasma expander maintains perfusion and plasma viscosity during extreme he-modilution. Am. J. Physiol. 2005, 288, H1708–H1716. [Google Scholar]
- Martini, J.; Cabrales, P.; Ananda, A.; Acharya, S.A.; Intaglietta, M.; Tsai, A.G. Survival time in severe hemorrhagic shock after perioperative hemodilution is longer with PEG-conjugated human serum albumin than with HES 130/0.4: A microvascular perspective. Crit. Care 2008, 12, R54. [Google Scholar] [CrossRef] [Green Version]
- Villela, N.; Tsai, A.; Cabrales, P.; Intaglietta, M. Improved Resuscitation From Hemorrhagic Shock With Ringer’s Lactate With Increased Viscosity in the Hamster Window Chamber Model. J. Trauma 2011, 71, 418–424. [Google Scholar] [CrossRef]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. IS resuscitation from hemorrhagic shock limited by blood oxygen-carrying capacity or blood viscosity? Shock 2007, 27, 380–389. [Google Scholar] [CrossRef]
- Cabrales, P.; Intaglietta, M.; Tsai, A.G. Transfusion restores blood viscosity and reinstates microvascular conditions from hemor-rhagic shock independent of oxygen carrying capacity. Resuscitation 2007, 75, 124–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar Vázquez, B.Y.; Wettstein, R.; Cabrales, P.; Tsai, A.G.; Intaglietta, M. Microvascular experimental evidence on the relative significance of restoring oxygen carrying capacity vs blood viscosity in shock resuscitation. Biochem. Biophys. Acta 2008, 1784, 1421–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krismer, A.C.; Dünser, M.W.; Lindner, K.H.; Stadlbauer, K.H.; Mayr, V.D.; Lienhart, H.G.; Arntz, R.H.; Wenzel, V. Vasopressin during cardiopulmonary resuscitation and different shock states: A review of the literature. Biochem. Biophys. Acta 2006, 6, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Voelckel, W.G.; Convertino, V.A.; Lurie, K.G.; Karlbauer, A.; Schöchl, H.; Lindner, K.-H.; Trimmel, H. Vasopressin for Hemorrhagic Shock Management: Revisiting the Potential Value in Civilian and Combat Casualty Care. J. Trauma: Inj. Infect. Crit. Care 2010, 69, S69–S74. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.; Voelckel, W.G.; Wiedermann, F.J.; Wenzel, V.; Lindner, K.H. Successful Resuscitation of a Traumatic Cardiac Arrest Victim in Hemorrhagic Shock with Vasopressin: A Case Report and Brief Review of the Literature. J. Trauma: Inj. Infect. Crit. Care 2004, 57, 177–179. [Google Scholar] [CrossRef]
- Sharma, R.M.; Setlur, R. Vasopressin in haemorrhagic shock. Anesth. Analg. 2005, 101, 833–834. [Google Scholar] [CrossRef] [Green Version]
- Stadlbauer, K.H.; Wenzel, V.; Krismer, A.C.; Voelckel, W.G.; Lindner, K.H. Vasopressin During Uncontrolled Hemorrhagic Shock: Less Bleeding Below the Diaphragm, More Perfusion Above. Anesthesia Analg. 2005, 101, 830–832. [Google Scholar] [CrossRef]
- Tsuneyoshi, I.; Onomoto, M.; Yonetani Kanmura, Y. Low dose vasopressin infusion in patients with severe vasodilatory hypo-tension after prolonged hemorrhage during general anesthesia. J. Anest. 2005, 19, 170–173. [Google Scholar] [CrossRef]
- Roth, J.V. Bolus vasopressin during hemorrhagic shock? Anesth. Analg. 2006, 102, 1098. [Google Scholar] [CrossRef]
- Raab, H.; Lindner, K.H.; Wenzel, V. Preventing cardiac arrest during hemorrhagic shock with vasopressin. Crit. Care Med. 2008, 36, S474–S480. [Google Scholar] [CrossRef]
- Bauer, S.R.; Aloi, J.J.; Ahrens, C.L.; Yeh, J.Y.; Culver, D.A.; Reddy, A.J. Discontinuation of vasopressin before norepinephrine increases the incidence of hypotension in patients recovering from septic shock: A retrospective cohort study. J. Crit. Care 2010, 25, 362. [Google Scholar] [CrossRef] [PubMed]
- Sims, C.A.; Holena, D.; Kim, P.; Pascual, J.; Smith, B.; Martin, N.; Seamon, M.; Shiroff, A.; Raza, S.; Kaplan, L.; et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients With Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019, 154, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Cossu, A.P.; Mura, P.; De Giudici, L.M.; Puddu, D.; Pasin, L.; Evangelista, M.; Xanthos, T.; Musu, M.; Finco, G. Vasopressin in Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Randomized Animal Trials. BioMed Res. Int. 2014, 2014, 421291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickell, W.H.; Wall, M.J.; Pepe, P.E.; Martin, R.R.; Ginger, V.F.; Allen, M.K.; Mattox, K.L. Immediate versus Delayed Fluid Resuscitation for Hypotensive Patients with Penetrating Torso Injuries. N. Engl. J. Med. 1994, 331, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, M.; Straus, D.C.; Schermer, C.R.; Agubuzu, O.; Esposito, T.J.; Crandall, M.L. Pre-hospital transport times and survival for Hypotensive patients with penetrating thoracic trauma. J. Emerg. Trauma Shock 2013, 6, 16–20. [Google Scholar] [CrossRef]
- Waalwijk, J.F.; van der Sluijs, R.; Lokerman, R.D.; Fiddelers, A.A.; Hietbrink, F.; Leenen, L.P.; Poeze, M.; van Heijl, M. Pre-hospital Trauma Triage Re-search Collaborative (PTTRC). The impact of prehospital time intervals on mortality in moderately and severely injured pa-tients. J. Trauma Acute Care Surg. 2022, 92, 520–527. [Google Scholar] [CrossRef]
- Bernard, C. Lecons sur les Phenomenes de la vie Communs aux Animaux and aux Vegetaux; Bailliere: Paris, France, 1878. [Google Scholar]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Sheldon, G.F.; Lim, R.C.; Blaisdell, F.W. The use of fresh blood in the treatment of critically injured patients. J. Trauma: Inj. Infect. Crit. Care 1975, 15, 670–677. [Google Scholar] [CrossRef]
- Modell, H.; Cliff, W.; Michael, J.; McFarland, J.; Wenderoth, M.P.; Wright, A. A physiologist’s view of homeostasis. Adv. Physiol. Educ. 2015, 39, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Spinella, P.C.; Cap, A.P. Whole blood: Back to the future. Curr. Opin. Hematol. 2016, 23, 536–542. [Google Scholar] [CrossRef]
- Tsai, A.G.; Hofmann, A.; Cabrales, P.; Intaglietta, M. Perfusion vs. oxygen delivery in transfusion with “fresh” and “old” red blood cells: The experimental evidence. Transfus. Apher. Sci. 2010, 43, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlman, R.; Callum, J.; Laflamme, C.; Tien, H.; Nascimento, B.; Beckett, A.; Alam, A. Comparison of treatment modalities for hemorrhagic shock. Artif. Cells Blood Substit. Immobil. Biotechnol. 2007, 35, 173–190. [Google Scholar]
- Jacob, M.; Chappell, D.; Becker, B.F. Regulation of blood flow and volume exchange across the microcirculation. Crit. Care 2016, 20, 319. [Google Scholar] [CrossRef] [Green Version]
- Holley, A.D.; Dulhunty, J.; Udy, A.; Midwinter, M.; Lukin, B.; Stuart, J.; Boots, R.; Lassig-Smith, M.; Holley, R.B.; Paratz, J.; et al. Early Sequential Microcirculation Assessment in Shocked Patients as a Predictor of Outcome: A Prospective Observational Cohort Study. Shock 2021, 55, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.L.; Forrester, T.; Ellis, C.G.; Dietrich, H.H. The erithrocyte as a regulator of vascular tone. Am. J. Physiol. 1995, 269, H2155–H2161. [Google Scholar]
- Dietrich, H.H.; Ellsworth, M.L.; Sprague, R.S.; Dacey, R.G. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am. J. Physiol. Circ. Physiol. 2000, 278, H1294–H1298. [Google Scholar] [CrossRef]
- Jagger, J.E.; Bateman, R.M.; Ellsworth, M.L.; Ellis, C.G. Role of erythrocyte in regulating local O2 delivery mediated by haemoglobin oxygenation. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2833–H2839. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, M.L.; Ellis, C.G.; Goldman, D.; Stephenson, A.H.; Dietrich, H.H.; Sprague, R.S. Erythrocytes: Oxygen Sensors and Modulators of Vascular Tone. Physiology 2009, 24, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Morel, N.; Moisan, M. Blood components are essential to regulate microcirculatory blood flow. Crit. Care 2017, 21, 49. [Google Scholar] [CrossRef] [Green Version]
- Spoerke, N.J.; Van, P.Y.; Differding, J.A.; Zink, K.A.; Cho, S.D.; Muller, P.J.; Karahan, Z.A.; Sondeen, J.L.; Holcomb, J.B.; Schreiber, M.A. Red Blood Cells Accelerate the Onset of Clot Formation in Polytrauma and Hemorrhagic Shock. J. Trauma: Inj. Infect. Crit. Care 2010, 69, 1054–1061. [Google Scholar] [CrossRef]
- Leeper, C.M.; Yazer, M.H.; Cladis, F.P.; Saladino, R.; Triulzi, D.J.; Gaines, B.A. Cold-stored whole blood platelet function is preserved in injured children with hemorrhagic shock. J. Trauma Acute Care Surg. 2019, 87, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Gallaher, J.R.; Dixon, A.; Cockcroft, A.; Grey, M.; Dewey, E.; Goodman, A.; Schreiber, M. Large Volume Transfusion with Whole Blood is Safe Compared to Component Therapy. J. Trauma Acute Care Surg. 2020, 89, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Crowe, E.; DeSantis, S.M.; Bonnette, A.; Jansen, J.O.; Yamal, J.M.; Holcomb, J.B.; Pedroza, C.; Harvin, J.A.; Marques, M.B.; Avritscher, E.B.C.; et al. Whole blood transfusion versus component therapy in trauma resuscitation: A systematic review and meta-analysis. J. Am. Coll. Emerg. Physicians Open. 2020, 4, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Naumann, D.N.; Boulton, A.J.; Sandhu, A.; Campbell, K.; Charlton, W.; Gurney, J.M.; Martin, M.J.; Scorer, T.; Doughty, H. Fresh whole blood from walking blood banks for patients with traumatic hemorrhagic shock: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2020, 89, 792–800. [Google Scholar] [CrossRef]
- Siletz, A.E.; Blair, K.J.; Cooper, R.J.; Nguyen, N.C.; Lewis, S.J.; Fang, A.; Ward, D.C.; Jackson, N.J.; Rodriguez, T.; Grotts, J.; et al. A pilot study of stored low titer group O whole blood + component therapy versus component therapy only for civilian trauma patients. J. Trauma Acute Care Surg. 2021, 91, 655–662. [Google Scholar] [CrossRef]
- Lee, J.S.; Khan, A.D.; Wright, F.L.; McIntyre, A.C.; Worlac, W.C.; Cribari, C.; Brockman, V.; Vegan, S.A.; Cofran, J.M.; Scroeppel, T.J. Whole Blood Versus Conventional Blood Component Massive Transfusion Protocol Therapy in Civilian Trauma Patients. Am. Surg. 2021, 5, 880–886. [Google Scholar] [CrossRef]
- Guyette, F.X.; Zenati, M.; Triulzi, D.J.; Yazer, M.H.; Skroczky, H.; Early, J.A.; Strickland, V.P.; Juneja, K.; Holcomb, J.B. Complications of Hemorrhagic Shock and Massive Transfusion—A Comparison before and after the Damage Control Resuscitation ERA. Shock 2020, 56, 42–51. [Google Scholar] [CrossRef]
- Malkin, M.; Nevo, A.; Brundage, S.I.; Schreiber, M. Effectiveness and safety of whole blood compared to balanced blood components in resuscitation of hemorrhaging trauma patients—A systematic review. Injury 2020, 52, 182–188. [Google Scholar] [CrossRef]
- Sperry, J.L.; Martin, M.J.; Moore, E.E.; Sava, J.A.; Ciesla, D.; Rizzo, A.G.; Brown, C.; Brasel, K.; Kozar, R.; Vercruysse, G.; et al. Prehospital resuscitation in adult patients following injury: A Western Trauma Association critical decisions algorithm. J. Trauma Acute Care Surg. 2019, 87, 1228–1231. [Google Scholar] [CrossRef]
- Fecher, A.; Stimpson, A.; Ferrigno, L.; Pohlman, T.H. The pathophysiology and management of hemorrhagic shock in the poly-trauma patient. J. Clin. Med. 2021, 10, 4793. [Google Scholar] [CrossRef]
- Assen, S.; Cardenas, J.; George, M.; Wang, Y.-W.; Wade, C.E.; Meyer, D.; Cotton, B.A. Hemostatic potential of cold-stored non-leukoreduced whole blood over time: An assessment of platelet function and thrombin generation for optimal shelf life. J. Trauma Acute Care Surg. 2020, 89, 429–434. [Google Scholar] [CrossRef]
- Clements, T.; McCoy, C.; Assen, S.; Cardenas, J.; Wade, C.; Meyer, D.; Cotton, B.A. The prehospital use of younger age whole blood is as-sociated with an improved arrival coagulation profile. J. Trauma Acute Care Surg. 2021, 90, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Shand, S.; Curtis, K.; Dinh, M.; Burns, B. What is the impact of prehospital blood product administration for patients with cata-strophic haemorrhage: An integrative review. Injury 2019, 50, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rijnhout, T.W.; Wever, K.E.; Marinus, R.H.; Hoogerwerf, N.; Geeraedts, L.M.; Tan, E.C. Is prehospital blood transfusion effective and safe in haemorrhagic trauma patients? A systematic review and meta-analysis. Injury 2019, 50, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Braverman, M.A.; Smith, A.; Pokorny, D.; Axtman, B.; Shahan, C.P.; Barry, L.; Corral, H.; Jonas, R.B.; Shiels, M.; Schaefer, R.; et al. Prehospital whole blood reduces early mortality in patients with hemorrhagic shock. Transfusion 2021, 61, S15–S21. [Google Scholar] [CrossRef]
- Guerado, E.; Medina, A.; Mata, M.I.; Galvan, J.M.; Bertrand, M.L. Protocols for massive blood transfusion: When and why, and potential complications. Eur. J. Trauma Emerg. Surg. 2015, 42, 283–295. [Google Scholar] [CrossRef]
- Carroll, S.L.; Dye, D.W.; Smedley, W.A.; Stephens, S.W.; Reiff, D.A.; Kerby, J.D.; Holcomb, J.B.; Jansen, J.O. Early and pre-hospital trauma deaths: Who might benefit from advanced resuscitative care? J. Trauma Acute Care Surg. 2020, 88, 776–782. [Google Scholar] [CrossRef]
- Kalkwarf, K.J.; Drake, S.A.; Yang, Y.; Thetford, C.; Myers, L.; Brock, M.; Wolf, D.A.; Persse, D.; Wade, C.E.; Holcomb, J.B. Bleeding to death in a big city: An analysis of all trauma deaths from hemorrhage in a metropolitan area during 1 year. J. Trauma Inj. Infect. Crit. Care 2020, 89, 716–722. [Google Scholar] [CrossRef]
- Fox, E.E.; Holcomb, J.B.; Wade, C.E.; Bulger, E.M.; Tilley, B.C.; Group, P.S. Earlier Endpoints are Required for Haemorrhagic Shock Trials Among Severely Injured Patients. Shock 2017, 47, 567–573. [Google Scholar] [CrossRef]
- Geeraedts, L.M., Jr.; Kaasjager, H.A.; van Vugt, A.B.; Frolke, J.P. Exsanguination in trauma: A review of diagnostics and treatment options. Injury 2009, 40, 11–20. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Chapman, M.P.; McVaney, K.; Bryskiewicz, G.; Blechar, R.; Chin, T.; Burlew, C.C.; Pieracci, F.; West, F.B.; et al. Plasma-first resuscitation to treat haemor-rhagic shock during emergency ground transportation in an urban area: A randomised trial. Lancet 2018, 392, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.L.; Guyette, F.X.; Brown, J.B.; Yazer, M.H.; Triulzi, D.J.; Early-Young, B.J.; Adams, P.W.; Daley, B.J.; Miller, R.S.; Harbrecht, B.G.; et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N. Engl. J. Med. 2018, 379, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.W.; Warren, K.A.; Guyette, F.X.; Yazer, M.H.; Brown, J.B.; Daily, B.J.; Miller, R.S.; Harbrecht, B.G.; Claridge, J.A.; Phelan, H.A.; et al. PAMPer study group. Implementation of a pre-hospital air medical thawed plasma program: Is it even feasible? J. Trauma Acute Care Surg. 2019, 87, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Fenger-Eriksen, C.; Fries, D.; David, J.S.; Bouzat, P.; Lance, M.D.; Grottke, O.; Spahn, D.R.; Schoechl, H.; Maegele, M. Pre-hospital plasma transfusion: A valuable coagulation support or an expensive fluid therapy? Crit. Care 2019, 23, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beattie, G.; Cohan, C.M.; Ng, V.L.; Victorino, G.P. Liquid plasma: A solution to optimizing early and balanced plasma resuscitation in massive transfusion. J. Trauma Acute Care Surg. 2020, 89, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Halmin, M.; Boström, F.; Brattström, O.; Lundahl, J.; Wikman, A.; Östlund, A.; Edgren, G. Effect of plasma-to-RBC ratios in trauma patients: A cohort study with time-dependent data. Crit. Care Med. 2013, 41, 1905–1914. [Google Scholar] [CrossRef]
- Allen, C.J.; Shariatmadar, S.; Meizoso, J.P.; Hanna, M.M.; Mora, J.L.; Ray, J.J.; Namias, N.; Dudaryk, R.; Proctor, K.G. Liquidplasma use during “super” massive transfusion protocol. J. Surg. Res. 2015, 199, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Shlaifer, A.; Siman-Tov, M.; Radomislensky, I.; Peleg, K.; Shina, A.; Baruch, E.N.; Glassberg, E.; Yitzhak, A. Prehospital administration of freeze-dried plasma, is it the solution for trauma casualties? J. Trauma Acute Care Surg. 2017, 83, 675–682. [Google Scholar] [CrossRef]
- Liu, Q.P.; Carney, R.; Sundaram, S.; Fell, M.A. Single-donor spray-dried plasma. Transfusion 2019, 59, 707–713. [Google Scholar] [CrossRef]
- Shuja, F.; Shults, C.; Duggan, M.; Tabbara, M.; Butt, M.U.; Fischer, T.H.; Schreiber, M.A.; Tieu, B.; Holcomb, J.B.; Sondeen, J.L.; et al. Development and testing of freeze-dried plasma for the treatment of trauma-associated coagulopathy. J. Trauma 2008, 65, 975–985. [Google Scholar] [CrossRef] [Green Version]
- Glassberg, E.; Nadler, R.; Gendler, S.; Abramovich, A.; Spinella, P.C.; Gerhardt, R.T.; Holcomb, J.B.; Kreiss, Y. Freeze-dried plasma at the point of injury: From concept to doctrine. Shock 2013, 40, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Huebner, B.R.; Moore, E.E.; Moore, H.B.; Sauaia, A.; Stettler, G.; Dzieciatkowska, M.; Hansen, K.; Banerjee, A.; Silliman, C.C. Freeze-dried plasma enhances clot formation and inhibits fibrinolysis in the presence of tissue plasminogen activator similar to pooled liquid plasma. Transfusion 2017, 57, 2007–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuja, F.; Finkelstein, R.A.; Fukudome, E.; Duggan, M.; Kheirbek, T.; Hamwi, K.; Fischer, T.H.; Fikry, K.; Demoya, M.; Velmahos, G.C.; et al. Development and Testing of Low-Volume Hyperoncotic, Hyperosmotic Spray-Dried Plasma for the Treatment of Trauma-Associated Coagulopathy. J. Trauma: Inj. Infect. Crit. Care 2011, 70, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Reitz, K.M.; Moore, H.B.; Guyette, F.X.; Sauaia, A.; Pusateri, A.E.; Moore, E.E.; Hassoune, A.; Chapman, M.P.; Daley, B.J.; Miller, A.S.; et al. Prehospital plasma in injured patients is associated with survival principally in blunt injury: Results from two randomized prehospital plasma trials. Acute Care Surg. 2020, 88, 33–41. [Google Scholar] [CrossRef]
- Pusateri, A.E.; Moore, E.E.; Moore, H.B.; Le, T.D.; Guyette, F.X.; Chapman, M.P.; Sauaia, A.; Ghasabyan, A.; Chandler, J.; McVaney, K.; et al. Association of Prehospital Plasma Transfusion with Survival in Trauma Patients with Hemorrhagic Shock When Transport Times Are Longer Than 20 Minutes: A Post Hoc Analysis of the PAMPer and COMBAT Clinical Trials. JAMA Surg. 2020, 155, e195085. [Google Scholar] [CrossRef]
- Jackson, B.P.; Sperry, J.L.; Yazer, M.H. Prehospital plasma transfusion: What does literature show? Transf. Med. Hemother. 2021, 48, 358–365. [Google Scholar] [CrossRef]
- Milford, E.M.; Reade, M.C. Resuscitation Fluid Choices to Preserve the Endothelial Glycocalyx. Crit. Care 2019, 23, 77. [Google Scholar] [CrossRef] [Green Version]
- Naumann, D.N.; Hazeldine, J.; Midwinter, M.J.; Hutchings, S.D.; Harrison, P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J. Trauma Acute Care Surg. 2018, 84, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Keel, M.; Trentz, O. Pathophysiology of polytrauma. Injury 2005, 36, 691–709. [Google Scholar] [CrossRef]
- Hardaway, R.M. Traumatic shock. Mil. Med. 2006, 171, 278–279. [Google Scholar] [CrossRef] [Green Version]
- Bonanno, G.F. Shock—A reappraisal: The holistic approach. J. Emerg. Trauma Shock 2012, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kor, D.J.; Stubbs, J.R.; Gajic, O. Perioperative coagulation management--fresh frozen plasma. Best Pract Res Clin Anaesthesiol. 2010, 24, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Inaba, K.; Branco, B.C.; Rhee, P.; Blackbourne, L.H.; Holcomb, J.B.; Teixeira, P.G.; Shulman, I.; Nelson, J.; Demetriades, D. Impact of Plasma Transfusion in Trauma Patients Who Do Not Require Massive Transfusion. J. Am. Coll. Surg. 2010, 210, 957–965. [Google Scholar] [CrossRef] [PubMed]
- McQuilten, Z.K.; Crighton, G.; Brunskill, S.; Morison, J.K.; Richter, T.H.; Waters, N.; Murphy, M.F.; Wood, E.M. Optimal Dose, Timing and Ratio of Blood Products in Massive Transfusion: Results from a Systematic Review. Transfus. Med. Rev. 2018, 32, 6–15. [Google Scholar] [CrossRef]
- Hallet, J.; Lauzier, F.; Mailloux, O.; Trottier, V.; Archambault, P.; Zarychanski, R.; Turgeon, A.F. The use of higher platelet: RBC transfusion ratio in the acute phase of trauma resuscitation: A systematic review. Crit. Care Med. 2013, 41, 2800–2811. [Google Scholar] [CrossRef]
- Ponschab, M.; Schochl, H.; Gabriel, C.; Süssner, S.; Cadamuro, J.; Haschke-Becher, E.; Gratz, J.; Zipperle, J.; Redl, H.; Schlimp, C.J.; et al. Haemostatic profile of reconstituted blood in a proposed 1:1:1 ratio of packed red blood cells, platelet concentrate and four different plasma preparations. Anaesthesia 2015, 70, 528–536. [Google Scholar] [CrossRef]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Stanworth, S.J.; Davenport, R.; Curry, N.; Seeney, F.; Eaglestone, S.; Edwards, A.; Martin, K.; Allard, S.; Woodford, M.; Lecky, F.E.; et al. Mortality from trauma haemorrhage and opportunities for improvement in transfusion practice. Br. J. Surg. 2016, 103, 357–365. [Google Scholar] [CrossRef]
- Stansbury, L.G.; Hess, A.S.; Thompson, K.; Kramer, B.; Scalea, T.M.; Hess, J.R. The clinical significance of platelet counts in the first 24 h after severe injury. Transfusion 2012, 53, 783–789. [Google Scholar] [CrossRef]
- Peralta, R.; Vijay, A.; El-Menyar, A.; Consunji, R.; Afifi, I.; Mahmood, I.; Asim, M.; Latifi, R.; Al Thani, H. Early high ratio platelet transfusion in trauma resusci-tation and its outcomes. Int J Crit Illn Inj Sci. 2016, 6, 188–193. [Google Scholar]
- Vulliamy, P.; Gillespie, S.; Gall, L.S.; Green, L.; Brohi, K.; Davenport, R.A. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J. Trauma: Inj. Infect. Crit. Care 2017, 83, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.F.; Cheng, C.T.; Fu, C.Y.; Huang, Y.A.; Hsu, C.P.; OuYang, C.H.; Liao, C.H.; Hsieh, C.H.; Chang, S.H. Aspirin does not increase the need for haemostatic interventions in blunt liver and spleen injuries. Injury 2021, 52, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Askari, R.; Davis, K.A.; Dorfman, J.; Eid, A.I.; Elsharkawy, A.E.; Kasotakis, G.; Mackey, S.; Odom, S.; Okafor, B.U.; et al. The effect of anticoagulation on outcomes after liver and spleen injuries: A research consortium of New England centers for trauma (ReCONECT) study. Injury 2020, 51, 1994–1998. [Google Scholar] [CrossRef] [PubMed]
- van Erp, I.A.; Mokhtari, A.K.; Moheb, M.E.; Bankhead-Kendall, B.K.; Fawley, J.; Parks, J.; Fagenholz, P.J.; King, D.R.; Mendoza, A.E.; Velmahos, G.C.; et al. Comparison of outcomes in non-head injured trauma patients using pre-injury warfarin or direct oral anticoagulant therapy. Injury 2020, 51, 2546–2552. [Google Scholar] [CrossRef]
- Bonanno, F. Early coagulopathy in trauma and major bleeding: Is it time to challenge the dogma? Trauma 2021, 23, 171–174. [Google Scholar] [CrossRef]
- Kuramatsu, J.B.; Sembill, J.A.; Huttner, H.B. Reversal of oral anticoagulation in patients with acute intracerebral haemorrhage. Crit. Care. 2019, 23, 206. [Google Scholar] [CrossRef] [Green Version]
- Collaborators, C.T.; Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef]
- Roberts, I.; Shakur, H.; Coats, T.; Hunt, B.; Balogun, E.; Barnetson, L.; Cook, L.; Kawahara, T.; Perel, P.; Prieto-Merino, D.; et al. The CRASH-2 trial: A randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol. Assess. 2013, 17, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Weng, S.; Wang, W.; Wei, Q.; Lan, H.; Su, J.; Xu, Y. Effect of Tranexamic Acid in Patients with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 123, 128–135. [Google Scholar] [CrossRef]
- Alhelaly, M.M.; Soliman, A.M.; Khaled, A.; Ellotf, H.; Attia, M.M.; Elmaraezy, A. Efficacy of tranexamic acid in traumatic brain injury: Updated systematic review and meta-analysis. Trauma 2018, 21, 167–175. [Google Scholar] [CrossRef]
- Undurraga Perl, V.J.; Leroux, B.; Cook, M.R.; Watson, J.; Fair, K.; Martin, D.T.; Kerby, J.D.; Williams, C.; Inaba, K.; Wade, C.E.; et al. Damage-control resuscitation and emergency lap-arotomy: Findings from the PROPPR study. J. Trauma Acute Care Surg. 2016, 80, 568–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heckbert, S.R.; Vedder, N.B.; Hoffman, W.; Winn, R.K.; Hudson, L.D.; Jurkovich, G.J.; Copass, M.K.; Harlan, J.M.; Rice, C.L.; Maier, R.V.; et al. Outcome after Hemorrhagic Shock in Trauma Patients. J. Trauma 1998, 45, 545–549. [Google Scholar]
- Cotton, B.A.; Guy, J.S.; Morris, J.A.; Abumrad, N.N. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock 2006, 26, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Hußmann, B.; Lefering, R.; Taeger, G.; Waydhas, C.; Ruchholtz, S.; Lendemans, S.; the DGU Trauma Registry. Influence of pre-hospital fluid resuscitation on patients with multiple injuries in hemorrhagic shock, in patients from the DGU trauma registry. J. Emerg. Trauma & Shock 2011, 4, 465–471. [Google Scholar]
- ATLS Subcommittee, American College of Surgeons’ Committee on Trauma, and International ATLS Working Group. Advanced trauma life support (ATLS): The ninth edition. J. Trauma Acute Care Surg. 2013, 74, 1363–1366. [Google Scholar]
- Harada, M.Y.; Ko, A.; Barmparas, G.; Smith, E.J.; Patel, B.K.; Dhillon, N.K.; Thomsen, G.M.; Ley, E.J. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int. J. Surg. 2016, 38, 78–82. [Google Scholar] [CrossRef]
- Ravi, P.R.; Puri, B. Fluid resuscitation in haemorrhagic shock in combat casualties. Disaster Mil. Med. 2017, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Schoemaker, W.C.; Peitzmann, A.B.; Bellamy, R.; Bellomo, R.; Bruttig, S.P.; Capone, A.; Dubick, M.; Kramer, G.C.; McKenzie, J.E.; Pepe, P.E.; et al. resuscitation from severe haemorrhage. Crit. Care Med. 1996, 24, S12–S23. [Google Scholar] [CrossRef]
- Brunauer, A.; Koköfer, A.; Bataar, O.; Gradwohl-Matis, I.; Dankl, D.; Dünser, M.W. The arterial blood pressure associated with terminal cardiovascular collapse in critically ill patients: A retrospective cohort study. Crit. Care 2014, 18, 719. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Uchida, M.; Nagata, I.; Saitoh, D.; Tamiya, N. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: A nationwide cohort study in Japan. Crit. Care 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Wasicek, P.J.; Li, Y.; Yang, S.; Teeter, W.A.; Scalea, T.M.; Hu, P.; Brenner, M.L. Examination of hemodynamics in patients in hemorrhagic shock undergoing Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Injury 2018, 50, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, C.A.; Rodríguez, F.; Parra, M.; Herrera, J.P.; Guzmán-Rodríguez, M.; Orlas, C.; Caicedo, E.Y.; Serna, J.J.; Salcedo, A.; Del Valle, A.M.; et al. Resuscitative endovascular balloon of the aorta is feasible in penetrating chest trauma with major hemorrhage: Proposal of a new institutional deployment algorithm. J. Trauma Acute Care Surg. 2020, 89, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.E.; Rasmussen, T.E.; Tisherman, S.A.; Cannon, J.W. Emerging hemorrhage control and resuscitation strategies in trauma: Endovascular to extracorporeal. J. Trauma Acute Care Surg. 2020, 89, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Stokes, S.C.; Theodorou, C.M.; Zakaluzny, S.A.; DuBose, J.J.; Russo, R.M. Resuscitative endovascular balloon occlusion of the aorta in combat casualties: The past, present, and future. J. Trauma Acute Care Surg. 2021, 91, S56–S64. [Google Scholar] [CrossRef]
- Abdou, H.; Madurska, M.J.; Edwards, J.; Patel, N.; Richmond, M.J.; Galvagno, S.; Kundi, R.; DuBose, J.J.; Scalea, T.M.; Morrison, J.J. A technique for open chest selective aortic arch perfusion. J. Trauma Acute Care Surg. 2021, 90, e158–e162. [Google Scholar] [CrossRef]
- Gattinoni, L.; Vassalli, F.; Romitti, F.; Vasques, E.; Pasticci, L.; Duscio, E.; Quintel, M. Extracorporeal gas exchange: When to start and how to end? Crit. Care. 2019, 23, 203. [Google Scholar] [CrossRef] [Green Version]
- Huh, U.; Song, S.; Chung, S.W.; Kim, S.-P.; Lee, C.W.; Ahn, H.Y.; Bae, M.; Kim, S.H. Is extracorporeal cardiopulmonary resuscitation practical in severe chest trauma? A systematic review in single center of developing country. J. Trauma Acute Care Surg. 2017, 83, 903–907. [Google Scholar] [CrossRef]
- Amos, T.; Bannon-Murphy, H.; Yeung, M.; Gooi, J.; Marasco, S.; Udy, A.; Fitzgerald, M. ECMO (extra corporeal membrane oxygenation) in major trauma: A 10 year single centre experience. Injury 2021, 52, 2515–2521. [Google Scholar] [CrossRef]
- Reul, G.J.; Mattox, K.L.; Beall, A.C., Jr.; Jordan, G.L., Jr. Recent advances in the operative management of massive chest trauma. Ann. Thorac. Surg. 1973, 16, 52–66. [Google Scholar] [CrossRef]
- Durham, L.A., 3rd; Richardson, R.J.; Wall, M.J.; Jr Pepe, P.E.; Mattox, K.L. Emergency center thoracotomy: Impact of prehospital re-suscitation. J. Trauma 1992, 32, 775–779. [Google Scholar] [CrossRef]
- Rhee, P.M.; Acosta, J.; Bridgeman, A.; Wang, D.; Jordan, M.; Rich, N. Survival after emergency department thoracotomy: Review of published data from the past 25 years. J. Am. Coll. Surg. 2000, 190, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Degiannis, E.; Loogna, P.; Doll, D.; Bonanno, F.; Bowley, D.M.; Smith, M.D. Penetrating Cardiac Injuries: Recent Experience in South Africa. World J. Surg. 2006, 30, 1258–1264. [Google Scholar] [CrossRef]
- Seamon, M.J.; Haut, E.R.; Van Arendonk, K.; Barbosa, R.R.; Chiu, W.C.; Dente, C.J.; Fox, N.; Jawa, R.S.; Khwaja, K.; Lee, J.K.; et al. An evidence-based approach to patient selection for emergency department thoracotomy: A practice management guideline from the eastern association for the surgery of trauma. J. Trauma Acute Care Surg. 2015, 79, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Corday, E. Four-minute limit for cardiac resuscitation. J. Am. Med. Assoc. 1956, 161, 1454–1458. [Google Scholar] [CrossRef] [PubMed]
- Safar, P. Cerebral resuscitation after cardiac arrest: A review. Circulation 1986, 74, 138–153. [Google Scholar]
- Safar, P. Resuscitation from clinical death: Pathophysiologic limits and therapeutic potentials. Crit. Care Med. 1988, 16, 923–941. [Google Scholar] [CrossRef] [PubMed]
- Frezza, E.E.; Mezghebe, H. Is 30 min the golden period to perform emergency room thoracotomy (ERT) in penetrating chest injuries? J. Cardiovasc. Surg. 1999, 40, 147–151. [Google Scholar]
- Doll, D.; Bonanno, F.; Degiannis, S.E. Emergency Department Thoracotomy (EDT). Trauma 2005, 7, 105–108. [Google Scholar] [CrossRef]
- Chinn, M.; Colella, M.R. Trauma Resuscitation: An evidence-based review of prehospital traumatic cardiac arrest. JEMS J. Emerg. Med. Serv. 2017, 42, 26–32. [Google Scholar]
- Boffard, K.D. The chest. In Manual of Definitive Surgical Trauma Care; Boffard, K.D., Ed.; Taylor & Francis: Abingdon, UK, 2019; pp. 88–114. [Google Scholar]
- Yanagawa, Y.; Tanaka, T.; Kaneda, H.; Omae, T. Complete Maternal Recovery after Prolonged Cardiac Arrest Due to Atonic Postpartum Hemorrhaging. J. Emerg. Trauma Shock 2021, 14, 249–250. [Google Scholar] [CrossRef]
- Boffard, K.D. Trauma anaesthesia. In Manual of Definitive Surgical Trauma Care, V Ed, Boffard KD, Editor; Taylor & Francis Group: Abingdon, UK, 2019; pp. 305–314. [Google Scholar]
- Perbet, S.; De Jong, A.; Delmas, J.; Futier, E.; Pereira, B.; Jaber, S.; Constantin, J.-M. Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: A multicenter observational study. Crit. Care 2015, 19, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabre, P.; Combes, X.; Lapostolle, F.; Dhaouadi, M.; Ricard-Hibon, A.; Vivien, B.; Bertrand, L.; Beltramini, A.; Gamand, P.; Albizzati, S.; et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: A multicentre randomised controlled trial. Lancet 2009, 374, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.; Leibovici, D.; Shemer, J.; Henig, A.; Shapira, S. Ketamine in the field: The use of ketamine for induction of anaesthesia before intubation in injured patients in the field. Injury 1997, 28, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Perris, A.; Klein, J.; Mahoney, P. Anaesthesia in haemodynamically compromised emergency patients: Does ketamine represent the best choice of induction agent? Anaesthesia 2009, 64, 532–539. [Google Scholar] [CrossRef]
- Bonanno, G.F. Ketamine in war/tropical surgery (a final tribute to the racemic mixture). Injury 2002, 33, 323–327. [Google Scholar] [CrossRef]
- Cuthbertson, B.H.; Sprung, C.L.; Annane DChevret, S.; Garfield, M.; Goodman, S. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009, 35, 1868–1876. [Google Scholar] [CrossRef]
- Adams, H.A.; Werner, C. Vom Razemat zum Eutomer: (S)-Ketamin—Renaissance einer Substanz? Anaesthesist 1997, 46, 1026–1042. [Google Scholar] [CrossRef]
- Adams, H.A. Endokrine Reaktionen nach S-( + )-Ketamin. Der Anaesthesist 1997, 46, S30–S37. [Google Scholar] [CrossRef]
- Kienbaum, P.; Heuter, T.; Pavlakovic, G.; Michel, M.C.; Peters, J.S. ( + )-Ketamine Increases Muscle Sympathetic Activity and Maintains the Neural Response to Hypotensive Challenges in Humans. Anesthesiology 2001, 94, 252–258. [Google Scholar] [CrossRef]
- Trimmel, H.; Helbok, R.; Staudinger, T.; Jaksch, W.; Messerer, B.; Schöchl, H.; Likar, R. S ( + )-ketamine: Current trends in emergency and intensive care medicine. Wien Klin Wochensch 2018, 130, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Burch, J.M.; Ortiz, V.B.; Richardson, R.J.; Martin, R.R.; Mattox, K.L.; Jordan, G.L. Abbreviated laparotomy and planned reoperation for critically injured patients. Ann. Surg. 1992, 215, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, M.F.; Schwab, C.W.; McGonigal, M.D.; Philips, G.R., 3rd; Fruchterman, T.M.; Kauder, D.R.; Latenser, B.A.; Angood, P.A. ‘Damage control’: An approach for improved survival in exsanguinating penetrating abdominal injury. J. Trauma 1993, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E. ‘Thomas G. Orr Memorial Lecture’: Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am. J. Surg. 1996, 172, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, F. Extending damage control philosophy to non-haemorrhagic situations: Implications for a reclassification of SHOCK states. ANZ J. Surg. 2008, 78, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, E.H.; Miller, P.R.; Meredith, W.J.; Rahman, N.; Chang, M.C. Elevated Arterial Base Deficit in Trauma Patients: A Marker of Impaired Oxygen Utilization. J. Am. Coll. Surg. 1998, 187, 384–392. [Google Scholar] [CrossRef]
- Paydar, S.; Fazelzadeh, A.; Abbasi, H.; Bolandparvaz, S. Base Deficit: A Better Indicator for Diagnosis and Treatment of Shock in Trauma Patients. J. Trauma Inj. Infect. Crit. Care 2011, 70, 1580–1581. [Google Scholar] [CrossRef]
- Mutschler, M.; Nienaber, U.; Brockamp, T.; Wafaisade, A.; Fabian, T.; Paffrath, T.; Bouillon, B.; Maegele, M. Trauma Register DGU? Renaissance of base deficit for the initial assessment of trauma patients: A base deficit-based classification for hypovolemic shock developed on data from 16,305 patients derived from the Trauma Register DGU? Crit. Care. 2013, 17, R42. [Google Scholar] [CrossRef] [Green Version]
- Mullner, M.; Sterz, F.; Domanovits, H.; Behringer, W.; Binder, M.; Laggner, A.N. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibril-lation. Intensive Care Med. 1997, 23, 1138–1143. [Google Scholar] [CrossRef]
- Shapiro, M.B.; Jenkins, D.H.; Schwab, C.W.; Rotondo, A.M.F. Damage Control: Collective Review. J. Trauma Inj. Infect. Crit. Care 2000, 49, 969–978. [Google Scholar] [CrossRef]
- Ordoñez, C.A.; Badiel, M.; Pino, L.F.; Salamea, J.C.; Loaiza, J.H.; Parra, M.W.; Puyana, J.C. Damage control resuscitation: Early decision strategies in abdominal gunshot wounds using an easy “ABCD” mnemonic. J. Trauma Acute Care Surg. 2012, 73, 1074–1078. [Google Scholar] [CrossRef]
- Roberts, D.J.; Bobrovitz, N.; Zygun, D.A.; Ball, C.G.; Kirkpatrick, A.W.; Faris, P.D.; Brohi, K.; D’Amours, S.; Fabian, T.C.; Inaba, K.; et al. Indications for Use of Damage Control Surgery in Civilian Trauma Patients: A Content Analysis and Expert Appropriateness Rating Study. Ann. Surg. 2016; 263, 1018–1027. [Google Scholar]
- Pimentel, S.K.; Rucinski, T.; Meskau, M.P.A.; Cavassin, G.P.; Kohl, N.H. Damage control surgery: Are we losing control over indications? Rev Col Bras Cir. 2018, 45, e1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weale, R.D.; Kong, V.Y.; Blodgett, J.M.; Buitendag, J.; Ras, A.; Laing, G.; Bruce, J.L.; Bekker, W.; Manchev, V.; Clarke, D. Lessons learnt from the Pietermaritzburg experience with damage control laparotomy for trauma. J. R. Army Med. Corps 2018, 164, 428–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, J.R.; Trooskin, S.Z.; Doshi, P.J. Time to laparotomy for intra-abdominal bleeding from trauma does affect survival for delays up to 90 min. J. Trauma 2002, 52, 420–425. [Google Scholar] [PubMed]
- Shafi, S.; Gentilello, L. Pre-Hospital Endotracheal Intubation and Positive Pressure Ventilation Is Associated with Hypotension and Decreased Survival in Hypovolemic Trauma Patients: An Analysis of the National Trauma Data Bank. J. Trauma: Inj. Infect. Crit. Care 2005, 59, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Bilello, J.F.; Davis, J.W.; Lemaster, D.; Townsend, R.N.; Parks, S.N.; Sue, L.P.; Kaups, K.L.; Groom, T.; Egbalieh, B. Prehospital Hypotension in Blunt Trauma: Identifying the “Crump Factor”. J. Trauma 2011, 70, 1038–1042. [Google Scholar] [CrossRef] [Green Version]
- Damme, C.D.; Luo, J.; Buesing, K.L. Isolated prehospital hypotension correlates with injury severity and outcomes in patients with trauma. Trauma Surg. Acute Care Open 2016, 1, e000013. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, H.; Hara, Y.; Yagi, T.; Saito, N.; Mashiko, K.; Iida, H.; Motomura, T.; Nakayama, F.; Okada, K.; Yasumatsu, H.; et al. Impact of urgent resuscitative surgery for life-threatening torso trauma. Surg. Today 2016, 47, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Bruns, B.; Lindsey, M.; Rowe, K.; Brown, S.; Minei, J.P.; Gentilello, L.M.; Shafi, S. Hemoglobin drops within minutes of injuries and predicts need for an intervention to stop haemorrhage. J. Trauma 2007, 63, 312–315. [Google Scholar]
- Asensio, A.J.; McDuffie, L.; Petrone, P.; Roldán, G.; Forno, W.; Gambaro, E.; Salim, A.; Demetriades, D.; Murray, J.; Velmahos, G.; et al. Reliable variables in the exsanguinated patient which indicate damage control and predict outcome. Am. J. Surg. 2001, 182, 743–751. [Google Scholar] [CrossRef]
- Green, R.S.; Butler, M.B.; Erdogan, M. Increased mortality in trauma patients who develop post-intubation hypotension. J. Trauma 2017, 83, 569–574. [Google Scholar] [CrossRef]
- Karmy-Jones, R.; Jurkovich, G.J.; Nathens, A.B.; Shatz, D.V.; Brundage, S.; Wall, M.J., Jr.; Engelardt, S.; Hoyt, D.B.; Holcroft, J.; Knudson, M.M. Timing of urgent thoracotomy for hem-orrhage after trauma: A multicentre study. Arch. Surg. 2001, 136, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizushima, Y.; Nakao, S.; Watanabe, H.; Matsuoha, T. Thoracotomy for blunt chest trauma: Is chest tube output a useful criterion? Acute Med. Surg. 2016, 3, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, L.B.; Johnson, K.D.; Weigelt, J.; Scheinberg, R. Early versus delayed stabilization of femoral fractures: A prospective randomized study. J. Bone Joint Surg. Am. 1989, 71, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Smith, R.M.; Banks, R.E.; Windsor, A.C.J.; Dickson, R.A.; Guillou, P.J. Stimulation of inflammatory markers after blunt trauma. Br. J. Surg. 1998, 85, 986–990. [Google Scholar] [CrossRef]
- Harwood, P.J.; Giannoudis, P.V.; van Griensven, M.; Krettek, C.; Pape, H.-C. Alterations in the Systemic Inflammatory Response after Early Total Care and Damage Control Procedures for Femoral Shaft Fracture in Severely Injured Patients. J. Trauma Inj. Infect. Crit. Care 2005, 58, 446–454. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Chalidis, B.; Hall, G.M. Surgical stress response. Injury 2006, 37, S3–S9. [Google Scholar] [CrossRef]
- Lasanianos, N.; Kanakaris, N.; Dimitriou, R.; Pape, H.; Giannoudis, P. Second hit phenomenon: Existing evidence of clinical implications. Injury 2011, 42, 617–629. [Google Scholar] [CrossRef]
- George, M.J.; Adams, S.D.; McNutt, M.K.; Love, J.D.; Albarado, R.; Moore, L.J.; Wade, C.E.; Cotton, B.A.; Holcomb, J.B.; Harvin, J.A. The effect of damage control laparotomy on major abdominal complications: A matched analysis. Am. J. Surg. 2017, 216, 56–59. [Google Scholar] [CrossRef]
- Hunt, T.K. Disorders of repair and their management. In Fundamentals of Wound Management; Hunt, T.K., Dunphy, J.E., Eds.; Appleton Century Crofts: New York, USA, 1979; pp. 96–103. [Google Scholar]
- Hunt, T.K. Normal repair. In Fundamentals of Wound Management; Hunt, T.K., Dunphy, J.E., Eds.; Appleton Century Crofts: New York, USA, 1979; pp. 53–63. [Google Scholar]
- Ballantyne, G.H. The experimental basis of intestinal suturing. Effect of surgical technique, inflammation and infection on enteric wound healing. Dis. Colon Rectum 1984, 27, 61–71. [Google Scholar] [CrossRef]
- Ahrendt, G.M.; Gardner, K.; Barbul, A. Loss of colonic structural collagen impairs healing during intra-abdominal sepsis. Arch. Surg. 1994, 129, 1179–1183. [Google Scholar] [CrossRef]
- Kashiwagi, H. The lower limit of tissue blood flow for safe colonic anastomosis: An experimental study using laser doppler velocimetry. Surg. Today 1993, 23, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hiltebrand, L.B.; Krejci, V.; Sigurdsson, G.H. Effects of Dopamine, Dobutamine, and Dopexamine on Microcirculatory Blood Flow in the Gastrointestinal Tract during Sepsis and Anesthesia. Anesthesiology 2004, 100, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Krejci, V.; Hiltebrand, L.B.; Sigurdsson, G.H. Effects of epinephrine, norepinephrine, and phenylephrine on microcirculatory blood flow in the gastrointestinal tract in sepsis*. Crit. Care Med. 2006, 34, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.P.; Galbraith, K.A. Colonic anastomosis in the presence of fecal peritonitis using a disposable skin stapler. J. Investig. Surg. 1998, 11, 267–274. [Google Scholar] [CrossRef]
- Nicholas, J.M.; Rix, E.P.; Easley, K.A.; Feliciano, D.V.; Cava, R.A.; Ingram, W.L.; Parry, N.G.; Rozycki, G.S.; Salomone, J.P.; Tremblay, L.N. Changing Patterns in the Management of Penetrating Abdominal Trauma: The More Things Change, the More They Stay the Same. J. Trauma: Inj. Infect. Crit. Care 2003, 55, 1095–1110. [Google Scholar] [CrossRef]
- Asensio, J.A.; Petrone, P.; Roldán, G.; Kuncir, E.; Ramicone, E.; Chan, L. Has evolution in awareness of guidelines for institution of damage control improved outcome in the management of the posttraumatic open abdomen? Arch. Surg. 2004, 139, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Higa, G.; Friese, R.; O’Keeffe, T.; Wynne, J.; Bowlby, P.; Ziemba, M.; Latifi, R.; Kulvatunyou, N.; Rhee, P. Damage Control Laparotomy: A Vital Tool Once Overused. J. Trauma: Inj. Infect. Crit. Care 2010, 69, 53–59. [Google Scholar] [CrossRef]
- Lauerman, M.H.; Dubose, J.; Cunningham, K.; Bruns, B.; Bradley, M.; Diaz, J.; Scalea, T.; Stein, D. Delayed interventions and mortality in trauma damage control laparotomy. Surgery 2016, 160, 1568–1575. [Google Scholar] [CrossRef]
- Harvin, J.A.; Wray, C.J.; Steward, J.; Lawless, R.A.; McNutt, M.K.; Love, J.D.; Moore, L.J.; Wade, C.E.; Cotton, B.A.; Holcomb, J.B. Control the damage: Morbidity and mortality after emergent trauma laparotomy. Am. J. Surg. 2015, 212, 34–39. [Google Scholar] [CrossRef]
- Harvin, J.A.; Maxim, T.; Inaba, K.; Martinez-Aguilar, M.A.; King, D.R.; Choudry, A.J.; Zielinski, M.D.; Akinyeye, S.; Tod, S.R.; Griffin, R.L.; et al. Mortality after emergent trauma laparotomy: A multicenter, retrospective study. J. Trauma Acute Care Surg. 2017, 83, 464–468. [Google Scholar] [CrossRef]
- Weale, R.; Kong, V.; Buitendag, J.; Ras, A.; Blodgett, J.M.; Laing, G.; Bruce, J.; Bekker, W.; Manchev, V.; Clarke, D. Damage control or definitive repair? A retrospective review of abdominal trauma at a major trauma center in South Africa. Trauma Surg. Acute Care Open 2019, 4, e000235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowie, J.M.; Badiee, J.; Calvo, R.Y.; Sise, M.J.; Wessels, L.E.; Butler, W.J.; Dunne, C.E.; Sise, C.B.; Bansal, V. Outcomes after single-look trauma laparotomy: A large population-based study. J. Trauma Acute Care Surg. 2019, 86, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Harvin, J.A.; Adams, S.D.; Dodwad, S.-J.M.; Isbell, K.D.; Pedroza, C.; Green, C.; Tyson, J.E.; Taub, E.A.; Meyer, D.E.; Moore, L.J.; et al. Damage control laparotomy in trauma: A pilot randomized controlled trial. The DCL trial. Trauma Surg. Acute Care Open 2021, 6, e000777. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, A.; Oliveira, R.P.; Friedman, G. Occult hypoperfusion is associated with increased mortality in hemodynamically stable, high-risk, surgical patients. Crit. Care 2004, 8, R60–R65. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H. Chocolate consumption, cognitive function, and Nobel laureates. N. Engl. J. Med. 2012, 367, 1562–1564. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [Green Version]
- Beecher, H.K. Resuscitation and anesthesia. Anesthesiology 1946, 7, 644–650. [Google Scholar] [CrossRef]
- Mullins, R.J.; Trunkey, D.D.; Samuel, D. Gross: Pioneer academic trauma surgeon of 19th century America. J. Trauma 1990, 30, 528–538. [Google Scholar] [CrossRef]
- Gann, D.S.; Drucker, W.R. Hemorrhagic shock. J. Trauma Acute Care Surg. 2013, 75, 888–895. [Google Scholar] [CrossRef]
- Helling, T.S. “A cold and drowsy humor”: Theories of traumatic shock from Bernard to Laborit. J. Trauma Acute Care Surg. 2020, 89, e41–e47. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonanno, F.G. Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies. J. Clin. Med. 2023, 12, 260. https://doi.org/10.3390/jcm12010260
Bonanno FG. Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies. Journal of Clinical Medicine. 2023; 12(1):260. https://doi.org/10.3390/jcm12010260
Chicago/Turabian StyleBonanno, Fabrizio G. 2023. "Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies" Journal of Clinical Medicine 12, no. 1: 260. https://doi.org/10.3390/jcm12010260
APA StyleBonanno, F. G. (2023). Management of Hemorrhagic Shock: Physiology Approach, Timing and Strategies. Journal of Clinical Medicine, 12(1), 260. https://doi.org/10.3390/jcm12010260





