Uteroplacental–Cerebral Ratio: A Doppler Parameter for Prognostic Prediction of Late-Onset Fetal Growth Restriction: Single Center Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
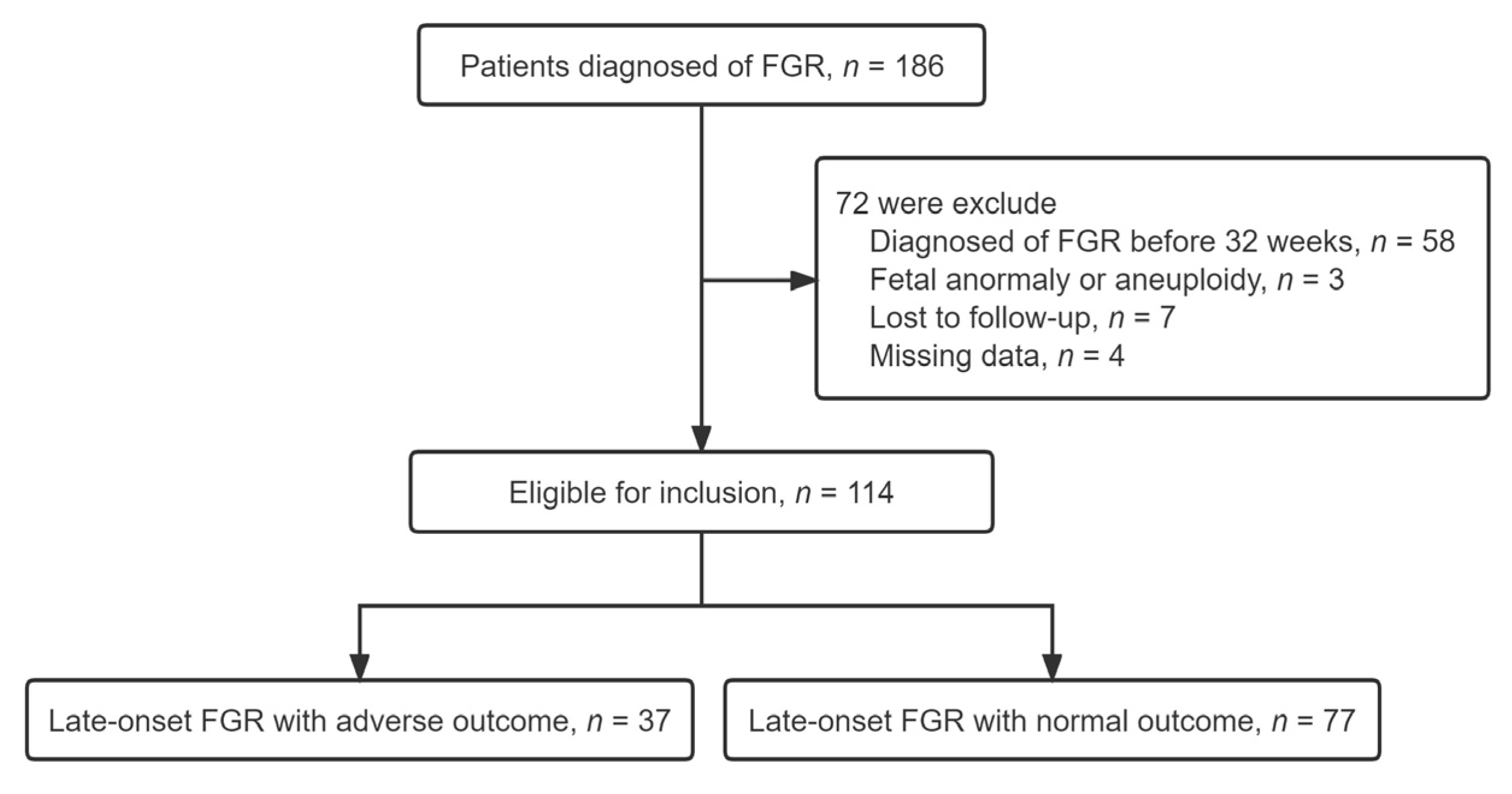
2.1. Study Population
2.2. Doppler Ultrasound Studies
2.3. Clinical Management and Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melamed, N.; Baschat, A.; Yinon, Y.; Athanasiadis, A.; Mecacci, F.; Figueras, F.; Berghella, V.; Nazareth, A.; Tahlak, M.; McIntyre, H.D.; et al. FIGO (International Federation of Gynecology and Obstetrics) initiative on fetal growth: Best practice advice for screening, diagnosis, and management of fetal growth restriction. Int. J. Gynecol. Obstet. 2021, 152, 3–57. [Google Scholar] [CrossRef] [PubMed]
- Figueras, F.; Caradeux, J.; Crispi, F.; Eixarch, E.; Peguero, A.; Gratacos, E. Diagnosis and surveillance of late-onset fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S790–S802.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- MacDonald, T.; McCarthy, E.; Walker, S.P. Shining light in dark corners: Diagnosis and management of late-onset fetal growth restriction. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Romero, R.; Stampalija, T.; Dall’Asta, A.; DeVore, G.; Prefumo, F.; Frusca, T.; Visser, G.H.; Hobbins, J.; Baschat, A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: An evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef]
- Spinillo, A.; Gardella, B.; Adamo, L.; Muscettola, G.; Fiandrino, G.; Cesari, S. Pathologic placental lesions in early and late fetal growth restriction. Acta Obstet. Gynecol. Scand. 2019, 98, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- Geerts, L.; Van Der Merwe, E.; Theron, A.; Rademan, K. Placental insufficiency among high-risk pregnancies with a normal umbilical artery resistance index after 32 weeks. Int. J. Gynecol. Obstet. 2016, 135, 38–42. [Google Scholar] [CrossRef]
- Audette, M.C.; Kingdom, J.C. Screening for fetal growth restriction and placental insufficiency. Semin. Fetal Neonatal Med. 2018, 23, 119–125. [Google Scholar] [CrossRef]
- Meler, E.; Martínez, J.; Boada, D.; Mazarico, E.; Figueras, F. Doppler studies of placental function. Placenta 2021, 108, 91–96. [Google Scholar] [CrossRef]
- Heidweiller-Schreurs, C.A.V.; De Boer, M.A.; Heymans, M.; Schoonmade, L.J.; Bossuyt, P.M.M.; Mol, B.W.J.; De Groot, C.J.M.; Bax, C.J. Prognostic accuracy of cerebroplacental ratio and middle cerebral artery Doppler for adverse perinatal outcome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 313–322. [Google Scholar] [CrossRef]
- Rizzo, G.; Mappa, I.; Bitsadze, V.; Słodki, M.; Khizroeva, J.; Makatsariya, A.; D’Antonio, F. Role of Doppler ultrasound at time of diagnosis of late-onset fetal growth restriction in predicting adverse perinatal outcome: Prospective cohort study. Ultrasound Obstet. Gynecol. 2019, 55, 793–798. [Google Scholar] [CrossRef]
- Steller, J.G.; Driver, C.; Gumina, D.; Peek, E.; Harper, T.; Hobbins, J.C.; Galan, H.L. Doppler velocimetry discordance between paired umbilical artery vessels and clinical implications in fetal growth restriction. Am. J. Obstet. Gynecol. 2022, 227, 285.e1–285.e7. [Google Scholar] [CrossRef]
- Adefisan, A.S.; Akintayo, A.A.; Awoleke, J.O.; Awolowo, A.T.; Aduloju, O.P. Role of second-trimester uterine artery Doppler indices in the prediction of adverse pregnancy outcomes in a low-risk population. Int. J. Gynecol. Obstet. 2020, 151, 209–213. [Google Scholar] [CrossRef]
- Običan, S.G.; Odibo, L.; Tuuli, M.G.; Rodriguez, A.; Odibo, A.O. Third trimester uterine artery Doppler indices as predictors of preeclampsia and neonatal small for gestational age. J. Matern. Neonatal Med. 2019, 33, 3484–3489. [Google Scholar] [CrossRef]
- Günay, T.; Bilir, R.A.; Hocaoğlu, M.; Bör, E.D.; Özdamar, O.; Turgut, A. The role of abnormal cerebroplacental ratio in predicting adverse fetal outcome in pregnancies with scheduled induction of labor. Int. J. Gynecol. Obstet. 2020, 153, 287–293. [Google Scholar] [CrossRef]
- Akolekar, R.; Syngelaki, A.; Gallo, D.M.; Poon, L.C.; Nicolaides, K.H. Umbilical and fetal middle cerebral artery Doppler at 35–37 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2015, 46, 82–92. [Google Scholar] [CrossRef]
- Bakalis, S.; Akolekar, R.; Gallo, D.M.; Poon, L.C.; Nicolaides, K.H. Umbilical and fetal middle cerebral artery Doppler at 30–34 weeks’ gestation in the prediction of adverse perinatal outcome. Ultrasound Obstet. Gynecol. 2015, 45, 409–420. [Google Scholar] [CrossRef]
- Dall’Asta, A.; Stampalija, T.; Mecacci, F.; Minopoli, M.; Schera, G.B.L.; Cagninelli, G.; Ottaviani, C.; Fantasia, I.; Barbieri, M.; Lisi, F.; et al. Ultrasound prediction of adverse perinatal outcome at diagnosis of late-onset fetal growth restriction. Ultrasound Obstet. Gynecol. 2021, 59, 342–349. [Google Scholar] [CrossRef]
- Melekoglu, R.; Yilmaz, E.; Yasar, S.; Hatipoglu, I.; Kahveci, B.; Sucu, M. The ability of various cerebroplacental ratio thresholds to predict adverse neonatal outcomes in term fetuses exhibiting late-onset fetal growth restriction. J. Périnat. Med. 2020, 49, 209–215. [Google Scholar] [CrossRef]
- Lees, C.; Stampalija, T.; Baschat, A.A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Louis, G.M.B.; Grewal, J.; Albert, P.S.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Racial/ethnic standards for fetal growth: The NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2015, 213, 449.e1–449.e41. [Google Scholar] [CrossRef] [Green Version]
- Gudmundsson, S.; Flo, K.; Ghosh, G.; Wilsgaard, T.; Acharya, G. Placental pulsatility index: A new, more sensitive parameter for predicting adverse outcome in pregnancies suspected of fetal growth restriction. Acta Obstet. Gynecol. Scand. 2017, 96, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, T.M.; Hui, L.; Robinson, A.J.; Dane, K.M.; Middleton, A.L.; Tong, S.; Walker, S.P. Cerebral–placental–uterine ratio as novel predictor of late fetal growth restriction: Prospective cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Normal Outcome (n = 77) | Adverse Outcome (n = 37) | p |
|---|---|---|---|
| Age (years) | 30.1 ± 4.6 | 29.8 ± 5.7 | 0.804 a |
| BMI (kg/m2) | 28.4 ± 4.5 | 29.3 ± 5.6 | 0.179 a |
| Obstetric history (n, %) | |||
| Nulliparous | 48 (62.3) | 19 (51.3) | 0.265 b |
| Previous FGR | 3 (3.8) | 4 (10.8) | 0.150 c |
| Previous stillbirth | 0(0.0) | 1(2.7) | 0.325 c |
| Pregnancy complication (n, %) | |||
| Gestational diabetes | 6 (7.9) | 3 (8.1) | 0.953 c |
| Liver diseases | 5 (6.9) | 2 (5.4) | 0.821 c |
| Hypertensive diseases | 46 (59.7) | 26 (70.2) | 0.275 b |
| No comorbidity | 12 (15.5) | 4 (10.8) | 0.576 b |
| Gestational age at detection (weeks) | 34.0 ± 1.4 | 33.6 ± 1.5 | 0.108 a |
| Mean gestational age at birth (weeks) | 37.3 ± 1.7 | 35.5 ± 1.5 | <0.001 a |
| Birthweight (g) | 2358.7 ± 296.1 | 2059.7 ± 232.4 | <0.001 a |
| Birthweight percentile (%) | 5.7 ± 2.8 | 4.1 ± 2.2 | 0.004 a |
| Variables | Normal Outcome (n = 77) | Adverse Outcome (n = 37) | p |
|---|---|---|---|
| EFW < 3 centile | 20 (25.97) | 18 (48.46) | 0.016 a |
| MCA PI MoM | 0.84 ± 0.19 | 0.69 ± 0.14 | <0.001 b |
| UA PI MoM | 1.21 ± 0.32 | 1.48 ± 0.48 | <0.001 b |
| Mean UtA PI MoM | 1.59 ± 0.70 | 2.23 ± 0.53 | <0.001 b |
| CPR MoM | 0.69 ± 0.24 | 0.49 ± 0.20 | <0.001 b |
| UPCR MoM | 1.74 ± 0.73 | 2.74 ± 0.76 | <0.001 b |
| Variables | B | SE | Adjusted OR | 95%CI | p |
|---|---|---|---|---|---|
| MCA PI MoM | −1.243 | 1.621 | 0.288 | 0.01–6.91 | 0.443 |
| UA PI MoM | 0.385 | 0.751 | 1.469 | 0.33–6.40 | 0.608 |
| Mean UtA PI MoM | 0.286 | 0.555 | 1.330 | 0.44–3.94 | 0.607 |
| CPR MoM | −1.580 | 1.497 | 0.206 | 0.01–3.87 | 0.291 |
| UPCR MoM | 1.557 | 0.377 | 4.744 | 2.26–9.93 | <0.001 |
| Gestational age at delivery | −0.285 | 0.186 | 0.752 | 0.52–1.10 | 0.125 |
| Birthweight percentile (%) | −0.095 | 0.100 | 0.909 | 0.74–1.10 | 0.345 |
| Pre-eclampsia | 0.650 | 0.554 | 1.916 | 0.64–5.67 | 0.240 |
| Variables | AUC (95% CI) | Sensitivity (95% CI)% | Specificity (95% CI)% | PPV (95% CI)% | NPV (95% CI)% | p |
|---|---|---|---|---|---|---|
| MCA PI MoM | 0.705 (0.610–0.789) | 62.0 (50.6–73.1) | 81.0 (64.8–92.0) | 87.3 (75.4–94.8) | 50.8 (37.5–64.1) | <0.001 |
| UA PI MoM | 0.710 (0.615–0.794) | 59.7 (47.9–70.8) | 70.2 (53.0–84.1) | 80.7 (68.1–90.0) | 45.6 (32.4–59.3) | <0.001 |
| Mean UtA PI MoM | 0.753 (0.661–0.831) | 62.5 (50.3–73.6) | 86.4 (71.2–95.5) | 90.0 (78.0–96.7) | 54.2 (40.6–67.4) | <0.001 |
| CPR MoM | 0.765 (0.673–0.841) | 74.0 (62.8–83.4) | 62.1 (44.8–77.5) | 80.3 (69.1–88.8) | 53.5 (37.7–68.8) | <0.001 |
| UPCR MoM | 0.824 (0.739–0.891) | 80.5 (64.0–91.8) | 72.9 (61.4–82.6) | 88.5 (77.7–95.3) | 59.2 (44.2–73.0) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Lv, W.; Zhao, B.; Yao, J.; Yang, Y.; Yin, Z. Uteroplacental–Cerebral Ratio: A Doppler Parameter for Prognostic Prediction of Late-Onset Fetal Growth Restriction: Single Center Prospective Cohort Study. J. Clin. Med. 2023, 12, 275. https://doi.org/10.3390/jcm12010275
Yang Z, Lv W, Zhao B, Yao J, Yang Y, Yin Z. Uteroplacental–Cerebral Ratio: A Doppler Parameter for Prognostic Prediction of Late-Onset Fetal Growth Restriction: Single Center Prospective Cohort Study. Journal of Clinical Medicine. 2023; 12(1):275. https://doi.org/10.3390/jcm12010275
Chicago/Turabian StyleYang, Ziling, Wenjie Lv, Baojing Zhao, Jie Yao, Yuanyuan Yang, and Zongzhi Yin. 2023. "Uteroplacental–Cerebral Ratio: A Doppler Parameter for Prognostic Prediction of Late-Onset Fetal Growth Restriction: Single Center Prospective Cohort Study" Journal of Clinical Medicine 12, no. 1: 275. https://doi.org/10.3390/jcm12010275




