Bone Turnover Markers Changes Induced by Plateletpheresis May Be Minimized with Oral Supplementation of Calcium, Minerals, and Vitamin D before the Procedures: A Non-Randomized, Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Blood Samples
2.3. Intervention
2.4. Biomarkers Assessment
- Total calcium and magnesium (tCa, tMg) by colorimetric assay (Vitros, Ortho Clinical Diagnostics, Raritan, NJ, USA);
- Ionized calcium and magnesium (iCa, iMg) by an ion-selective electrode (Stat Profile Prime Plus, Nova Biomedical, Waltham, MA, USA); copper and zinc (Cu, Zn) by atomic absorption spectrophotometry (AAnalyst 400 Perkin Elmer, Wellesley, MA, USA);
- Collagen type-1 C-terminal telopeptide (CTX-1) (DRG International Inc., Springfield, NJ, USA), osteocalcin (OC) and parathyroid hormone (PTH) (Immulite 1000, Siemens, Munich, Germany) and 25-OH Vitamin D, Architect i1000 SR, Abbott Diagnostics (Chicago, IL, USA) by enzyme immunoassay.
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Nutrients Intake
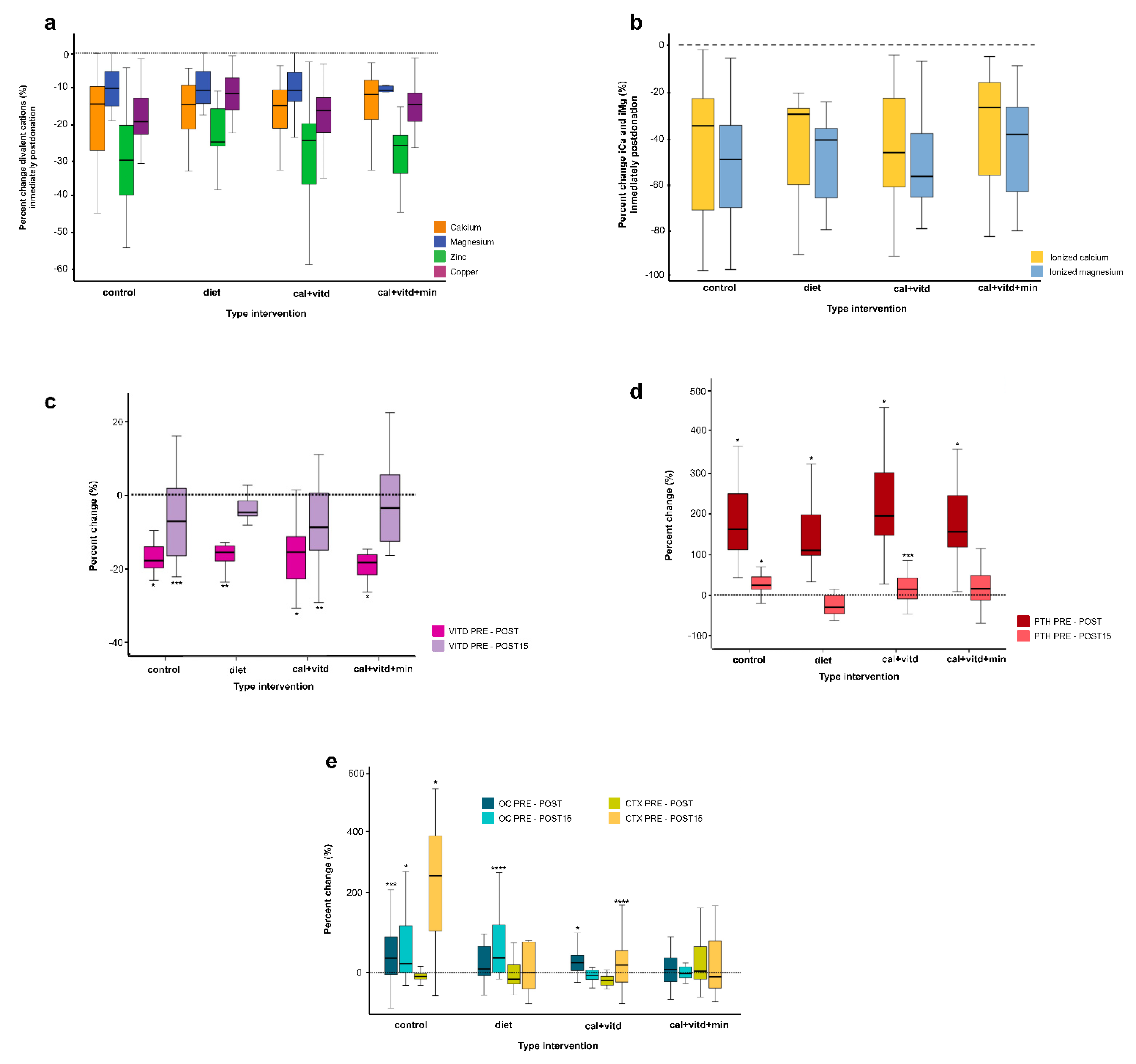
3.3. Metabolic Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winters, J.L. Plasma exchange: Concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematol. Am. Soc. Hematol. Educ. Progr. 2012, 2012, 7–12. [Google Scholar] [CrossRef]
- Amrein, K.; Katschnig, C.; Sipurzynski, S.; Stojakovic, T.; Lanzer, G.; Stach, E.; Pieber, T.R.; Dobnig, H. Apheresis affects bone and mineral metabolism. Bone 2010, 46, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Heuft, H.-G.; Moog, R.; Fischer, E.G.; Zingsem, J.; German and Austrian Plateletpheresis Study Group. Donor safety in triple plateletpheresis: Results from the German and Austrian Plateletpheresis Study Group multicenter trial. Transfusion 2013, 53, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Arepally, G.M. Anticoagulation techniques in apheresis: From heparin to citrate and beyond. J. Clin. Apher. 2012, 27, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Ziman, A.; Smeltzer, B.; Lu, Q.; Goldfinger, D. Moderate and severe adverse events associated with apheresis donations: Incidences and risk factors. Transfusion 2010, 50, 478–486. [Google Scholar] [CrossRef]
- Makar, Y.F.; Butler, M.O.; Cockersole, G.M.; Gabra, G.; Serevitch, J.M. National audit of citrate toxicity in plateletpheresis donors. Transfus. Med. 2002, 12, 187–191. [Google Scholar] [CrossRef]
- Wiltbank, T.B.; Giordano, G.F. The safety profile of automated collections: An analysis of more than 1 million collections. Transfusion 2007, 47, 1002–1005. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [Green Version]
- Huestis, D.; Fletcher, J.; White, R.; Price, M. Citrate anticoagulants for plateletpheresis. Transfusion 1977, 17, 151–155. [Google Scholar] [CrossRef]
- Van de Meer, P. Apheresis versus whole-blood-derived platelets: Pros and cons. ISBT Sci. Ser. 2012, 7, 112–116. [Google Scholar] [CrossRef]
- Davenport, A.; Tolwani, A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus 2009, 2, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, K.; Harm, S.; Weber, V.; Hartmann, J. The Role of Ionized Calcium and Magnesium in Regional Citrate Anticoagulation and its Impact on Inflammatory Parameters. Int. J. Artif. Organs 2017, 40, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Passos-Coelho, J.L.; Braine, H.G.; Wright, S.K.; Davis, J.M.; Schepers, K.G.; Huelskamp, A.-M.; Clarke, B.; Noga, S.J.; Kennedy, M.J. Large-Volume Leukapheresis Using Regional Citrate Anticoagulation to Collect Peripheral Blood Progenitor Cells. J. Hematother. 1995, 4, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Leitman, S.F.; Wesley, R.A.; Cecco, S.; Yau, Y.Y.; Starling, J.; Rehak, N.N.; Bolan, C.D. Placebo-controlled study of intravenous magnesium supplementation during large-volume leukapheresis in healthy allogeneic donors. Transfusion 2005, 45, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.; Haynes, S.; Zhao, Y.; Hickson, E.; Linden, J.; Pierre, P.S.; Ducharme, P.; Sulmasy, P.; Graves, M.; Bailey, J.A.; et al. A liquid calcium+vitamin D3 supplement is effective prophylaxis against hypocalcemic toxicity during apheresis platelet donation. J. Clin. Apher. 2017, 33, 60–64. [Google Scholar] [CrossRef]
- Bolan, C.D.; Wesley, R.A.; Yau, Y.Y.; Cecco, S.A.; Starling, J.; Oblitas, J.M.; Rehak, N.N.; Leitman, S.F. Randomized placebo-controlled study of oral calcium carbonate administration in plateletpheresis: I. Associations with donor symptoms. Transfusion 2003, 43, 1403–1413. [Google Scholar] [CrossRef]
- Boot, C.L.; Luken, J.S.; can der Burg, P.J.M.; de Kort, W.L.A.M.; Koopman, M.M.W.; Vrielink, H.; van Schoor, N.M.; Heijer, M.D.; Lips, P. Bone density in apheresis donors and whole blood donors. Vox Sang. 2015, 109, 410–413. [Google Scholar] [CrossRef]
- Chen, Y.; Bieglmayer, C.; Höcker, P.; Dettke, M. Effect of acute citrate load on markers of bone metabolism in healthy volunteers. Vox Sang. 2009, 97, 324–329. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-253-SSA1-2012, Para la Disposición de Sangre Humana y Sus Componentes con Fines Terapéuticos; Secretaria de Salud: Mexico City, Mexico, 2012.
- Sedgwick, P.M. Nonrandomized Trials: Designs and Methodology. Emerg. Themes Epidemiol. 2017, 1–7. [Google Scholar] [CrossRef]
- Barger-Lux, M.J.; Heaney, R.P.; Recker, R.R. Time course of calcium absorption in humans: Evidence for a colonic component. Calcif. Tissue Res. 1989, 44, 308–311. [Google Scholar] [CrossRef]
- Mejía-Rodríguez, F.; Shamah-Levy, T.; Villalpando, S.; García-Guerra, A.; Méndez-Gómez Humarán, I. Iron, zinc, copper and magnesium deficiencies in Mexican adults from the National Health and Nutrition Survey 2006. Salud Publica Mex. 2013, 55, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, S.A.S.; Canto, N.P.Z.; Dolores, M.T.; Ibarra, A.B.; Hernández, M.C.; Méndez, J.R.L.; Sánchez, L.Z.; López, A.D.A.; Pichón, E.R.; Gómez, L.B.; et al. Hiperparatiroidismo secundario inducido por plaquetoféresis como resultado de la quelación de los cationes divalentes por el citrato. Rev. Mex. Med. Transf. 2013, 6, 12–16. [Google Scholar]
- Mollison, P.L. The introduction of citrate as an anticoagulant for transfusion and of glucose as a red cell preservative. Br. J. Haematol. 2000, 108, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bolan, C.D.; Greer, S.E.; Cecco, S.A.; Oblitas, J.M.; Rehak, N.N.; Leitman, S.F. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion 2001, 41, 1165–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchta, C.; Macher, M.; Bieglmayer, C.; Höcker, P.; Dettke, M. Reduction of adverse citrate reactions during autologous large-volume PBPC apheresis by continuous infusion of calcium-gluconate. Transfusion 2003, 43, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Palfi, M.; Martinsson, L.; Sundström, K. Hypocalcemic symptoms during plateletpheresis using the COBE Spectra: A comparison of oral combination of 600mg calcium+300mg magnesium+100IU vitamin D3 vs. a 1000mg calcium in symptomatic donors. Transfus. Apher. Sci. 2007, 36, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Grau, K.; Vasan, S.K.; Rostgaard, K.; Bialkowski, W.; Norda, R.; Hjalgrim, H.; Edgren, G. No association between frequent apheresis donation and risk of fractures: A retrospective cohort analysis from S weden. Transfusion 2017, 57, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Bialkowski, W.; Blank, R.D.; Zheng, C.; Gottschall, J.L.; Papanek, P.E. Impact of frequent apheresis blood donation on bone density: A prospective, longitudinal, randomized, controlled trial. Bone Rep. 2018, 10, 100188. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhao, W.; Zhang, Y.; Li, L.; Lu, J.; Jiang, L.; Wang, C.; Jia, W. Low serum magnesium levels are associated with impaired peripheral nerve function in type 2 diabetic patients. Sci. Rep. 2016, 6, 32623. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and Type 2 Diabetes: An Update. Int. J. Diabetes Clin. Res. 2015, 2, 1152–1157. [Google Scholar] [CrossRef]
- Chaudhary, D.P.; Sharma, R.; Bansal, D.D. Implications of Magnesium Deficiency in Type 2 Diabetes: A Review. Biol. Trace Elem. Res. 2009, 134, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Bourges, H.; Casanueva, E.; Rosado, J.L. Recomendaciones de Ingestión de Nutrimentos Para La Población Mexicana; Médica Panamericana: Madrid, Spain, 2009. [Google Scholar]
- Barquera, S.; Hernández-Barrera, L.; Campos-Nonato, I.; Espinosa, J.; Flores, M.; J, A.B.; Rivera, J.A. Energy and nutrient consumption in adults: Analysis of the mexican national health and nutrition survey 2006. Salud Publica Mex. 2009, 51, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Clark, P.; Muñoz-Aguirre, P.; Flores, M.; Talavera, J.O.; Chico-Barba, L.G.; Rivas, R.; Ramírez, P.; Salmerón, J. Dietary patterns are associated with calcium and vitamin D intake in an adult Mexican population. Nutr. Hosp. 2016, 33, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.J.; Yamaguchi, M. Role of endogenous zinc in the enhancement of bone protein synthesis associated with bone growth of newborn rats. J. Bone Miner. Metab. 2001, 19, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.M.; Fraser, W.D.; Jackson, M. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 2002, 61, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Razmandeh, R.; Nasli-Esfahani, E.; Heydarpour, R.; Faridbod, F.; Ganjali, M.R.; Norouzi, P.; Larijani, B.; Khoda-Amorzideh, D. Association of Zinc, Copper and Magnesium with bone mineral density in Iranian postmenopausal women—A case control study. J. Diabetes Metab. Disord. 2014, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Bolan, C.D.; Cecco, S.A.; Wesley, R.A.; Horne, M.; Yau, Y.Y.; Remaley, A.T.; Childs, R.W.; Barrett, A.J.; Rehak, N.N.; Leitman, S.F. Controlled study of citrate effects and response to IV calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion 2002, 42, 935–946. [Google Scholar] [CrossRef]
- Vasu, S.; Leitman, S.F.; Tisdale, J.F.; Hsieh, M.M.; Childs, R.W.; Barrett, A.J.; Fowler, D.H.; Bishop, M.R.; Kang, E.M.; Malech, H.L.; et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood 2008, 112, 2092–2100. [Google Scholar] [CrossRef] [Green Version]
- Mercan, D.; Bastin, G.; Lambermont, M.; Dupont, E. Importance of ionized magnesium measurement for monitoring of citrate- anticoagulated plateletpheresis. Transfusion 1997, 37, 418–422. [Google Scholar] [CrossRef]
- Toffaletti, J.; Nissenson, R.; Endres, D.; McGarry, E.; Mogollon, G. Influence of continuous infusion of citrate on responses of immunoreactive parathyroid hormone, calcium and magnesium components, and other electrolytes in normal adults during plateletapheresis. J. Clin. Endocrinol. Metab. 1985, 60, 874–879. [Google Scholar] [CrossRef]
- Peiris, A.; Youssef, D.; Grant, W. Secondary hyperparathyroidism: Benign bystander or culpable contributor to adverse health outcomes? South Med. J. 2012, 105, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Vetter, T.; Lohse, M.J. Magnesium and the parathyroid. Curr. Opin. Nephrol. Hypertens. 2002, 11, 403–410. [Google Scholar] [CrossRef] [PubMed]

| Study Groups | |||||
|---|---|---|---|---|---|
| CONTROL | DIET | CAL + VITD | CAL + VITD + MIN | p-Value | |
| N | 42 | 13 | 39 | 40 | |
| Males | 26 (62) a | 6 (46) a | 24 (61) a | 19 (48) a | ------ |
| Females | 16 (38) a | 7 (54) a | 15 (39) a | 21 (52) a | ------ |
| Age | 37 (31–45) b | 31 (23–39) b | 30 (24–37) b | 29 (23–32) b | 0.001 c |
| BMI | 29 (4) | 27 (4) | 27 (4) | 27 (4) | 0.019 d |
| Bone fractures (events/life) | 2 (5) | 0 | 0 | 0 | 0.221 d |
| Alcohol intake (cup/day) | 11 (26) | 1 (8) | 2 (5) | 2 (5) | 0.587 d |
| Platelets PRE × 103/µL | 287 (47) | 290 (49) | 260 (43) | 285 (50) | 0.035 d |
| Platelets POST × 103/µL | 179 (28) | 190 (33) | 166 (26) | 178 (34) | 0.088 d |
| Apheresis duration (min) b | 72 (64–81) | 70 (64–80) | 71 (63–79) | 70 (63–81) | 0.595 c |
| Infused citrate (mL) | 293 (39) | 283 (37) | 287 (43) | 281 (44) | 0.624 d |
| Previous donations | 1 (0–2) | 2 (2–4) | 1 (1–1) | 1 (1–2) | 0.065 d |
| Study Group | ||||||
|---|---|---|---|---|---|---|
| Nutrients per Day | CONTROL | DIET | CAL + VITD | CAL + VITD + MIN | RDIs b | p |
| median (P25–P75) | median (P25–P75) | median (P25–P75) | median (P25–P75) | |||
| Energy (Kcal) | 2302 (1778–2730) | 2108 (1735–2471) | 1995 (1752–2356) | 2053 (1698–2632) | 2100F–3100M | 0.641 c |
| Energy adequacy (%) | 80 (66–101) | 83 (64–105) | 67 (57–100) | 80 (62–103) | 100 | 0.641 c |
| Total fat (g) | 84 (63–105) | 71 (65–91) | 79 (56–100) | 80 (60–100) | 210–310 | 0.575 c |
| Total Proteins (%) | 14 (12–15) | 15 (13–16) | 14 (13–16) | 14 (12–16) | 10–15% (Kcal) | 0.986 c |
| Carbohydrates (g) a | 282 (94) | 271 (97) | 266 (110) | 270 (107) | 630–930 | 0.918 d |
| Raw Fiber (g) a | 6 (3) | 6 (2) | 6 (2) | 6 (2) | 18–24 | 0.377 d |
| Calcium (mg) | 606 (463–821) | 679 (436.75–734.36) | 612 (484–877) | 666 (493–824) | 1200 | 0.997 c |
| Magnesium (mg) | 322 (240–385) | 298 (270–383) | 286 (232–365) | 303 (245–383) | 250F–340M | 0.880 c |
| Zinc (mg) | 16 (13–20) | 17 (12–20) | 16 (13–20) | 17 (12–25) | 11F–12N | 0.483 c |
| Copper (mg) | 2 (1–3) | 2 (1–3) | 2 (2–3) | 3 (2–4) | 0.9 | 0.122 c |
| Sodium (mg) | 1824 (1355–2369) | 1728 (1499–2208) | 1482 (1270–2057) | 1515 (1160–2101) | 1600 | 0.302 c |
| Phosphorus (mg) | 1233 (907–1439) | 1128 (1004–1405) | 1140 (909–1489) | 1194 (880–1594) | 580F–700M | 0.988 c |
| Vitamin C (mg) | 126 (68–209) | 179 (110–299) | 157 (106–196) | 118 (91–197) | 57F–84M | 0.256 c |
| Vitamin D (UI) | 111 (69–240) | 138 (88–185) | 165 (105–279) | 142 (91–249) | 525–775 | 0.125 c |
| Metabolic Changes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of Intervention | Biomarker | Time 1 | Time 2 | Time 3 | p-Value | |||
| Pre-Donation | Post-Donation | Post-Donation 15 Days | Time 1 vs. Time 2 a | Time 1 vs. Time 3 a | Time 2 vs. Time 3 a | All Times b | ||
| Control | Ca c | 9.7 (9.4–10.0) | 8.2 (6.9–8.7) | nd | <0.001 | na | na | na |
| iCa d | 1.14 (1.06–1.17) | 0.70 (0.34–0.87) | nd | <0.001 | na | na | na | |
| Mg c | 2.1 (2.0–2.2) | 1.9 (1.8–2.0) | nd | <0.001 | na | na | na | |
| iMg d | 0.54 (0.43–0.55) | 0.25 (0.15–0.33) | nd | <0.001 | na | na | na | |
| Zn e | 96.4 (83.5–126.9) | 70.1 (63.9–71.9) | 94.3 (84.3–107.8) | <0.001 | 0.534 | 0.003 | <0.001 | |
| Cu e | 102.2 (88.1–117.3) | 85.9 (74.3–94.7) | 102.5 (92.4–118.1) | <0.001 | 0.794 | 0.003 | <0.001 | |
| PTH f | 41.0 (35.50–44.10) | 97.90 (78.80–138) | 50.0 (42.40–64.60) | <0.001 | <0.001 | <0.001 | <0.001 | |
| VIT D g | 22.55 (17.5–26.9) | 18.55 (14.72–21.6) | 21.50 (18.40–27.25) | <0.001 | 0.010 | <0.001 | <0.001 | |
| OC g | 3.2 (1.5–6.38) | 4.1 (2.1–7.1) | 5.59 (4.24–7.3) | 0.010 | 0.001 | 0.469 | 0.013 | |
| CTX g | 0.24 (0.20–0.28) | 0.21 (0.19–0.27) | 0.86 (0.42–1.25) | <0.001 | <0.001 | <0.001 | <0.001 | |
| Diet | Ca c | 9.8 (9.5–9.9) | 8.3 (7.9–9.0) | nd | 0.001 | na | na | na |
| iCa d | 1.19 (1.15–1.21) | 0.82 (0.48–0.87) | nd | 0.001 | na | na | na | |
| Mg c | 2.0 (1.9–2.2) | 1.9 (1.8–2.0) | nd | 0.002 | na | na | na | |
| iMg d | 0.54 (0.50–0.59) | 0.31 (0.21–0.35) | nd | 0.001 | na | na | na | |
| Zn e | 89.0 (80.4–99.5) | 71.4 (66.3–76.4) | 83.8 (72.6–89.0) | 0.001 | 0.893 | 0.043 | 0.001 | |
| Cu e | 120.5 (112.1–133.6) | 108.9 (101.0–116.0) | 103.9 (96.2–107.3) | 0.001 | 0.008 | 0.173 | 0.001 | |
| PTH f | 41.1 (27.7–48.5) | 88.1 (79–123) | 25.7 (22.72–37.25) | 0.001 | 0.015 | 0.002 | <0.001 | |
| VIT D g | 20.5 (17.2–25.0) | 16.9 (13.9–21.9) | 19.90 (15.20–23.75) | 0.001 | 0.173 | 0.008 | 0.001 | |
| OC g | 5.7 (3.1–13.7) | 6.0 (3.7–13.25) | 5.6 (4.1–10.77) | 0.507 | 0.028 | 0.575 | 0.741 | |
| CTX g | 0.22 (0.16–0.51) | 0.37 (0.16–0.42) | 0.37 (0.10–1.04) | 0.600 | 0.445 | 0.241 | 0.497 | |
| Cal + vitd | Ca c | 9.5 (9.3–10.0) | 8.2 (7.7–8.6) | nd | <0.001 | na | na | na |
| iCa d | 1.17 (1.05–1.19) | 0.60 (0.42–0.85) | nd | <0.001 | na | na | na | |
| Mg c | 2.0 (1.9–2.1) | 1.8 (1.7–1.9) | nd | <0.001 | na | na | na | |
| iMg d | 0.53 (0.49–0.58) | 0.21 (0.16–0.34) | nd | <0.001 | na | na | na | |
| Zn e | 93.3 (84.6–104.2) | 67.2 (58.7–77.9) | 90.1 (81.6–100.2) | <0.001 | 0.447 | <0.001 | <0.001 | |
| Cu e | 93.1 (78.6- 114.5) | 78.2 (66.7–93.6) | 88.0 (75.8–103.9) | <0.001 | 0.586 | 0.006 | <0.001 | |
| PTH f | 34.1 (26.5–43.7) | 101 (77.1–141.0) | 33.35 (25.37–50.15) | <0.001 | 0.015 | <0.001 | <0.001 | |
| VIT D g | 22.4 (17.6–25.40) | 18.0 (14.4–21.8) | 20.60 (16.17–24.77) | <0.001 | 0.002 | 0.012 | <0.001 | |
| OC g | 6.8 (4.6–10.0) | 8.9 (5.6–12.1) | 5.91 (4.9–8.9) | 0.001 | 0.466 | 0.008 | <0.001 | |
| CTX g | 0.38 (0.30–0.55) | 0.32 (0.24–0.42) | 0.42 (0.22–0.92) | <0.001 | 0.026 | 0.006 | 0.007 | |
| Cal + vitd + min | Ca c | 9.9 (9.5–10.3) | 8.6 (8.1–9.0) | nd | <0.001 | na | na | na |
| iCa d | 1.15 (1.1–1.18) | 0.79 (0.50–0.95) | nd | <0.001 | na | na | na | |
| Mg c | 2.0 (2.0–2.1 | 1.9 (1.8–2.0) | nd | <0.001 | na | na | na | |
| iMg d | 0.54 (0.48–0.56) | 0.29 (0.19–0.36) | nd | <0.001 | na | na | na | |
| Zn e | 98.9 (91.1–106.9) | 74.9 (68.7–78.3) | 98.3 (86.7–107.7) | <0.001 | 0.219 | <0.001 | <0.001 | |
| Cu e | 110.9 (884.3–126.1) | 94.6 (69.4–108.0) | 89.3 (78.1–97.1) | <0.001 | 0.859 | 0.086 | 0.012 | |
| PTH f | 32.8 (25.1–48.8) | 91.3 (68.72–133.5) | 36.90 (27.52–46.90) | <0.001 | 0.083 | <0.001 | <0.001 | |
| VIT D g | 19.0 (14.52–23.6) | 15.4 (11.6–19.1) | 18.90 (17.67–24.80) | <0.001 | 0.221 | 0.001 | <0.001 | |
| OC g | 5.4 (3.4–8.0) | 6.4 (3.6–8.6) | 5.9 (5.49–7.72) | 0.267 | 0.984 | 0.687 | 0.692 | |
| CTX g | 0.40 (0.23–0.70) | 0.46 (0.35–0.64) | 0.40 (0.18–0.71) | 0.648 | 0.451 | 0.696 | 0.756 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos-Galeana, E.; Tolentino-Dolores, M.C.; Morales-Hernández, R.M.; Sámano, R.; Chico-Barba, G.; Fernández-Sánchez, E.; Zamora-Sánchez, L.J.; Alonso-López, A.D.; López-Martínez, H.; Alba-Rosales, T.; et al. Bone Turnover Markers Changes Induced by Plateletpheresis May Be Minimized with Oral Supplementation of Calcium, Minerals, and Vitamin D before the Procedures: A Non-Randomized, Controlled Study. J. Clin. Med. 2023, 12, 281. https://doi.org/10.3390/jcm12010281
Barrientos-Galeana E, Tolentino-Dolores MC, Morales-Hernández RM, Sámano R, Chico-Barba G, Fernández-Sánchez E, Zamora-Sánchez LJ, Alonso-López AD, López-Martínez H, Alba-Rosales T, et al. Bone Turnover Markers Changes Induced by Plateletpheresis May Be Minimized with Oral Supplementation of Calcium, Minerals, and Vitamin D before the Procedures: A Non-Randomized, Controlled Study. Journal of Clinical Medicine. 2023; 12(1):281. https://doi.org/10.3390/jcm12010281
Chicago/Turabian StyleBarrientos-Galeana, Edgar, Mari Cruz Tolentino-Dolores, Rosa María Morales-Hernández, Reyna Sámano, Gabriela Chico-Barba, Emmanuel Fernández-Sánchez, Lizbeth Jazmín Zamora-Sánchez, Alma Delia Alonso-López, Heriberto López-Martínez, Tania Alba-Rosales, and et al. 2023. "Bone Turnover Markers Changes Induced by Plateletpheresis May Be Minimized with Oral Supplementation of Calcium, Minerals, and Vitamin D before the Procedures: A Non-Randomized, Controlled Study" Journal of Clinical Medicine 12, no. 1: 281. https://doi.org/10.3390/jcm12010281






