In Search of an Imaging Classification of Adenomyosis: A Role for Elastography?
Abstract
:1. The Tower of Babel
2. Early Attempts to Classify Adenomyosis
3. The Current State of Diagnostic Tools
4. Imaging-Based Classification of Adenomyosis
5. The Physical Limits of TVUS and MRI
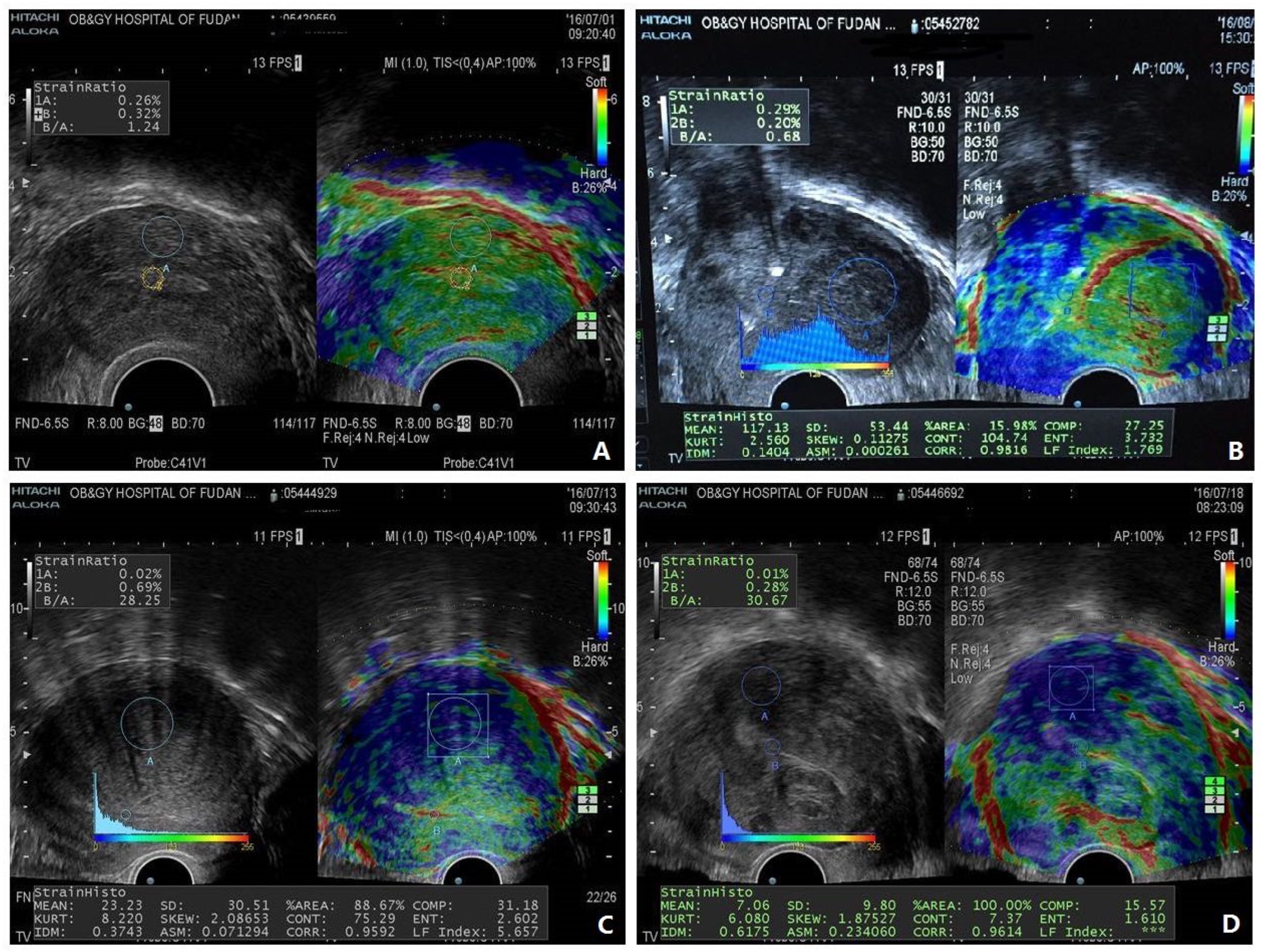
6. The Case for Incorporation of Elastography to Move Forward
7. Emerging Problems with the Utilization of Elastography
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, C.; Brosens, I. Medical and surgical management of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The Impact of Adenomyosis on Women’s Fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [Green Version]
- Gordts, S.; Grimbizis, G.; Campo, R. Symptoms and classification of uterine adenomyosis, including the place of hysteroscopy in diagnosis. Fertil. Steril. 2018, 109, 380–388.e381. [Google Scholar] [CrossRef] [Green Version]
- Alcalde, A.M.; Martinez-Zamora, M.A.; Gracia, M.; Ros, C.; Rius, M.; Castelo-Branco, C.; Carmona, F. Assessment of Quality of Life, Sexual Quality of Life, and Pain Symptoms in Deep Infiltrating Endometriosis Patients With or Without Associated Adenomyosis and the Influence of a Flexible Extended Combined Oral Contraceptive Regimen: Results of a Prospective, Observational Study. J. Sex Med. 2022, 19, 311–318. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Amano, H.; Kurozawa, Y.; Ideno, Y.; Hayashi, K.; Harada, T.; Japan, E.; Children’s Study, G. Adverse obstetrical outcomes for women with endometriosis and adenomyosis: A large cohort of the Japan Environment and Children’s Study. PLoS ONE 2019, 14, e0220256. [Google Scholar] [CrossRef] [Green Version]
- Cope, A.G.; Ainsworth, A.J.; Stewart, E.A. Current and Future Medical Therapies for Adenomyosis. Semin. Reprod. Med. 2020, 38, 151–156. [Google Scholar] [CrossRef]
- Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Scholes, D.; Reed, S.D. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006–2015. Am. J. Obstet. Gynecol. 2020, 223, 94.e1–94.e10. [Google Scholar] [CrossRef]
- Peric, H.; Fraser, I.S. The symptomatology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 547–555. [Google Scholar] [CrossRef]
- Seidman, J.D.; Kjerulff, K.H. Pathologic findings from the Maryland Women’s Health Study: Practice patterns in the diagnosis of adenomyosis. Int. J. Gynecol. Pathol. 1996, 15, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.S.; Hricak, H.; Heinrichs, L.W.; Hendrickson, M.R.; Winkler, M.L.; Bachica, J.A.; Stickler, J.E. Adenomyosis and leiomyoma: Differential diagnosis with MR imaging. Radiology 1987, 163, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Bianchi, S.; Dorta, M.; Arcaini, L.; Zanotti, F.; Carinelli, S. Transvaginal ultrasonography in the diagnosis of diffuse adenomyosis. Fertil. Steril. 1992, 58, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Darai, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordts, S.; Brosens, J.J.; Fusi, L.; Benagiano, G.; Brosens, I. Uterine adenomyosis: A need for uniform terminology and consensus classification. Reprod. Biomed. Online 2008, 17, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Gordts, S.; Bazot, M.; Brosens, I.; Benagiano, G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online 2020, 40, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Canis, M.; Gremeau, A.S.; Bourdel, N. Elusive adenomyosis: A plea for an international classification system to allow artificial intelligence approaches to reset our clinical management. Fertil. Steril. 2018, 110, 1039–1040. [Google Scholar] [CrossRef] [Green Version]
- Tellum, T.; Naftalin, J.; Chapron, C.; Dueholm, M.; Guo, S.W.; Hirsch, M.; Larby, E.R.; Munro, M.G.; Saridogan, E.; van der Spuy, Z.M.; et al. Development of a core outcome set and outcome definitions for studies on uterus-sparing treatments of adenomyosis (COSAR): An international multistakeholder-modified Delphi consensus study. Hum. Reprod. 2022, 37, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obs. Gynecol. 2012, 207, 114.e111–114.e117. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsubara, S. A Classification Proposal for Adenomyosis Based on Magnetic Resonance Imaging. Gynecol. Obs. Investig. 2020, 85, 118–126. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Van den Bosch, T.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.J.K.; Groenman, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on revised definitions of Morphological Uterus Sonographic Assessment (MUSA) features of adenomyosis: Results of modified Delphi procedure. Ultrasound Obs. Gynecol. 2022, 60, 118–131. [Google Scholar] [CrossRef]
- Exacoustos, C.; Lazzeri, L.; Martire, F.G.; Russo, C.; Martone, S.; Centini, G.; Piccione, E.; Zupi, E. Ultrasound Findings of Adenomyosis in Adolescents: Type and Grade of the Disease. J. Minim. Invasive Gynecol. 2022, 29, 291–299.e1. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M.; Benagiano, G. Classifying Adenomyosis: Progress and Challenges. Int. J. Environ. Res Public Health 2021, 18, 12386. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G. Classification and Reporting Systems for Adenomyosis. J. Minim. Invasive Gynecol. 2020, 27, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Exacoustos, C.; Morosetti, G.; Conway, F.; Camilli, S.; Martire, F.G.; Lazzeri, L.; Piccione, E.; Zupi, E. New Sonographic Classification of Adenomyosis: Do Type and Degree of Adenomyosis Correlate to Severity of Symptoms? J. Minim. Invasive Gynecol. 2020, 27, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Sammour, A.; Pirwany, I.; Usubutun, A.; Arseneau, J.; Tulandi, T. Correlations between extent and spread of adenomyosis and clinical symptoms. Gynecol. Obs. Investig. 2002, 54, 213–216. [Google Scholar] [CrossRef]
- Zhou, F.; Shi, L.B.; Zhang, S.Y. Ovarian Fibrosis: A Phenomenon of Concern. Chin. Med. J. 2017, 130, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Zaloudek, C.; Hendrickson, M.R. Mesenchymal tumors of the uterus. In Blaustein’s Pathology of the Female Genital Tract, 5th ed.; Kurman, R.J., Ed.; Springer: New York, NY, USA, 2002; pp. 561–615. [Google Scholar]
- Wang, X.; Benagiano, G.; Liu, X.; Guo, S.W. Unveiling the Pathogenesis of Adenomyosis through Animal Models. J. Clin. Med. 2022, 11, 1744. [Google Scholar] [CrossRef]
- Togashi, K.; Nishimura, K.; Itoh, K.; Fujisawa, I.; Noma, S.; Kanaoka, M.; Nakano, Y.; Itoh, H.; Ozasa, H.; Fujii, S.; et al. Adenomyosis: Diagnosis with MR imaging. Radiology 1988, 166, 111–114. [Google Scholar] [CrossRef]
- Dartmouth, K. A systematic review with meta-analysis: The common sonographic characteristics of adenomyosis. Ultrasound 2014, 22, 148–157. [Google Scholar] [CrossRef]
- Tellum, T.; Matic, G.V.; Dormagen, J.B.; Nygaard, S.; Viktil, E.; Qvigstad, E.; Lieng, M. Diagnosing adenomyosis with MRI: A prospective study revisiting the junctional zone thickness cutoff of 12 mm as a diagnostic marker. Eur. Radiol. 2019, 29, 6971–6981. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.K.; Hansen, E.S.; Ernst, E.; Dueholm, M. Two- and three-dimensional transvaginal ultrasonography for diagnosis of adenomyosis of the inner myometrium. Reprod. Biomed. Online 2019, 38, 750–760. [Google Scholar] [CrossRef]
- Byun, J.Y.; Kim, S.E.; Choi, B.G.; Ko, G.Y.; Jung, S.E.; Choi, K.H. Diffuse and focal adenomyosis: MR imaging findings. Radiographics 1999, 19, S161–S170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, Y.; Shimada, K.; Fujii, T.; Uchiyama, T.; Yoshimoto, C.; Konishi, N.; Ohbayashi, C.; Kobayashi, H. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS ONE 2017, 12, e0189522. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installe, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obs. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; de Bruijn, A.M.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obs. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Darai, E.; Rouger, J.; Detchev, R.; Cortez, A.; Uzan, S. Limitations of transvaginal sonography for the diagnosis of adenomyosis, with histopathological correlation. Ultrasound Obs. Gynecol. 2002, 20, 605–611. [Google Scholar] [CrossRef]
- Andres, M.P.; Borrelli, G.M.; Ribeiro, J.; Baracat, E.C.; Abrao, M.S.; Kho, R.M. Transvaginal Ultrasound for the Diagnosis of Adenomyosis: Systematic Review and Meta-Analysis. J. Minim. Invasive Gynecol. 2018, 25, 257–264. [Google Scholar] [CrossRef]
- Valentini, A.L.; Speca, S.; Gui, B.; Soglia, G.; Micco, M.; Bonomo, L. Adenomyosis: From the sign to the diagnosis. Imaging, diagnostic pitfalls and differential diagnosis: A pictorial review. Radiol. Med. 2011, 116, 1267–1287. [Google Scholar] [CrossRef]
- Exacoustos, C.; Manganaro, L.; Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract. Res. Clin. Obs. Gynaecol. 2014, 28, 655–681. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Trommelen, L.M.; de Leeuw, R.A.; Tellum, T.; Juffermans, L.J.M.; Griffioen, A.W.; Thomassin-Naggara, I.; Van den Bosch, T.; Huirne, J.A.F. Multidisciplinary view on uterine junctional zone in uteri affected by adenomyosis: Explaining discrepancies between MRI and transvaginal ultrasound images on a microscopic level. Ultrasound Obs. Gynecol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Bunesch, L.; Kido, A.; Togashi, K.; et al. European society of urogenital radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Champaneria, R.; Abedin, P.; Daniels, J.; Balogun, M.; Khan, K.S. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obs. Gynecol. Scand. 2010, 89, 1374–1384. [Google Scholar] [CrossRef]
- Bazot, M.; Darai, E.; Clement de Givry, S.; Boudghene, F.; Uzan, S.; Le Blanche, A.F. Fast breath-hold T2-weighted MR imaging reduces interobserver variability in the diagnosis of adenomyosis. AJR Am. J. Roentgenol. 2003, 180, 1291–1296. [Google Scholar] [CrossRef]
- Bourdon, M.; Oliveira, J.; Marcellin, L.; Santulli, P.; Bordonne, C.; Maitrot Mantelet, L.; Millischer, A.E.; Plu Bureau, G.; Chapron, C. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum. Reprod. 2021, 36, 349–357. [Google Scholar] [CrossRef]
- Sampson, J.A. Perforating hemorrhagic (chocolate) cysts of the ovary. Their importance and especially their relation to pelvic adeno mas of endometrial type. Adenomyoma of the uterus, rectovaginal septum, sigmoid, etc. Arch. Surg. 1921, 3, 245–323. [Google Scholar] [CrossRef] [Green Version]
- Cullen, T.S. Adenomyoma of the Uterus; W.B. Saunders: Philadelphia, PA, USA; London, UK, 1908. [Google Scholar]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsubara, S.; Imanaka, S. Relationship between magnetic resonance imaging-based classification of adenomyosis and disease severity. J. Obs. Gynaecol. Res. 2021, 47, 2251–2260. [Google Scholar] [CrossRef]
- Barrow, J.D. Impossibility: The Limits of Science and the Science of Limits; Oxford Univesity Press: New York, NY, USA, 1999; p. 304. [Google Scholar]
- Ding, D.; Chen, Y.; Liu, X.; Jiang, Z.; Cai, X.; Guo, S.W. Diagnosing Deep Endometriosis Using Transvaginal Elastosonography. Reprod. Sci. 2020, 27, 1411–1422. [Google Scholar] [CrossRef]
- Chapron, C.; Vannuccini, S.; Santulli, P.; Abrao, M.S.; Carmona, F.; Fraser, I.S.; Gordts, S.; Guo, S.W.; Just, P.A.; Noel, J.C.; et al. Diagnosing adenomyosis: An integrated clinical and imaging approach. Hum. Reprod. Update 2020, 26, 392–411. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, B.D.; Mui, K.L.; Driscoll, T.P.; Caliari, S.R.; Mehta, K.D.; Assoian, R.K.; Burdick, J.A.; Mauck, R.L. N-cadherin adhesive interactions modulate matrix mechanosensing and fate commitment of mesenchymal stem cells. Nat. Mater. 2016, 15, 1297–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Z.; Chu, Q.; Jiang, K.; Li, J.; Tang, N. The Strength of Mechanical Forces Determines the Differentiation of Alveolar Epithelial Cells. Dev. Cell 2018, 44, 297–312.e295. [Google Scholar] [CrossRef] [Green Version]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2018, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Peng, R.; Ro, J.Y.; Robboy, S.J. A diagnostically useful histopathologic feature of endometrial polyp: The long axis of endometrial glands arranged parallel to surface epithelium. Am. J. Surg. Pathol. 2004, 28, 1057–1062. [Google Scholar] [CrossRef]
- Lee, W.L.; Liu, C.H.; Cheng, M.; Chang, W.H.; Liu, W.M.; Wang, P.H. Focus on the Primary Prevention of Intrauterine Adhesions: Current Concept and Vision. Int. J. Mol. Sci. 2021, 22, 5175. [Google Scholar] [CrossRef]
- Nie, J.; Zhao, C.; Lagana, A.S.; Liu, X.; Guo, S.W. Identification of lesional attributes of dysmenorrhea severity and the serum antimullerian hormone levels in women with ovarian endometriomas. Fertil. Steril. 2022, 118, 191–202. [Google Scholar] [CrossRef]
- Kim, T.H.; Yoo, J.Y.; Choi, K.C.; Shin, J.H.; Leach, R.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Yoon, H.G.; Jeong, J.W. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci. Transl. Med. 2019, 11, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.W.; Li, K.N.; Yi, A.J.; Wang, B.; Wei, Q.; Wu, G.G.; Dietrich, C.F. Ultrasound elastography. Endosc. Ultrasound 2022, 11. [Google Scholar] [CrossRef]
- Mariappan, Y.K.; Glaser, K.J.; Ehman, R.L. Magnetic resonance elastography: A review. Clin. Anat. 2010, 23, 497–511. [Google Scholar] [CrossRef] [Green Version]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.H.; Cosgrove, D.; et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: Basic principles and terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, D.; Wei, H.; Yang, Z.; Zhang, Y.; Ma, S.; Zhou, X. Application of transvaginal sonographic elastography to distinguish endometrial cancer from benign masses. Am. J. Transl. Res. 2019, 11, 1049–1057. [Google Scholar] [PubMed]
- Shao, J.; Shi, G.; Qi, Z.; Zheng, J.; Chen, S. Advancements in the Application of Ultrasound Elastography in the Cervix. Ultrasound Med. Biol. 2021, 47, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.; Salomoni, S.E.; Stafford, R.E.; Hall, L.M.; Hodges, P.W. Validation of shear wave elastography as a noninvasive measure of pelvic floor muscle stiffness. Neurourol. Urodyn 2022, 41, 1620–1628. [Google Scholar] [CrossRef]
- Liu, X.; Ding, D.; Ren, Y.; Guo, S.W. Transvaginal Elastosonography as an Imaging Technique for Diagnosing Adenomyosis. Reprod. Sci. 2018, 25, 498–514. [Google Scholar] [CrossRef]
- Stoelinga, B.; Hehenkamp, W.J.; Brolmann, H.A.; Huirne, J.A. Real-time elastography for assessment of uterine disorders. Ultrasound Obs. Gynecol. 2014, 43, 218–226. [Google Scholar] [CrossRef]
- Stoelinga, B.; Hehenkamp, W.J.K.; Nieuwenhuis, L.L.; Conijn, M.M.A.; van Waesberghe, J.; Brolmann, H.A.M.; Huirne, J.A.F. Accuracy and Reproducibility of Sonoelastography for the Assessment of Fibroids and Adenomyosis, with Magnetic Resonance Imaging as Reference Standard. Ultrasound Med. Biol. 2018, 44, 1654–1663. [Google Scholar] [CrossRef]
- Acar, S.; Millar, E.; Mitkova, M.; Mitkov, V. Value of ultrasound shear wave elastography in the diagnosis of adenomyosis. Ultrasound 2016, 24, 205–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, B.; VanBuren, W.M.; Burnett, T.L.; Knudsen, J.M. Transvaginal Ultrasound Vibro-elastography for Measuring Uterine Viscoelasticity: A Phantom Study. Ultrasound Med. Biol. 2019, 45, 617–622. [Google Scholar] [CrossRef]
- Gorgulu, F.F.; Okcu, N.T. Which imaging method is better for the differentiation of adenomyosis and uterine fibroids? J. Gynecol. Obs. Hum. Reprod. 2021, 50, 102002. [Google Scholar] [CrossRef] [PubMed]
- Vora, Z.; Manchanda, S.; Sharma, R.; Das, C.J.; Hari, S.; Mathur, S.; Kumar, S.; Kachhawa, G.; Khan, M.A. Transvaginal Shear Wave Elastography for Assessment of Endometrial and Subendometrial Pathologies: A Prospective Pilot Study. J. Ultrasound Med. 2022, 41, 61–70. [Google Scholar] [CrossRef]
- Orishchak, I.K.; Makarchuk, O.M.; Henyk, N.I.; Ostrovska, O.M.; Havryliuk, H.M. Sonoelastography evaluation in the diagnosis of endometrial pathology combined with chronic endometritis in infertile women. J. Med. Life 2022, 15, 397–404. [Google Scholar] [CrossRef]
- Sasaran, V.; Turdean, S.; Marginean, C.; Gliga, M.; Ilyes, L.; Grama, O.; Puscasiu, L. Transvaginal Ultrasound Combined with Strain-Ratio Elastography for the Concomitant Diagnosis of Uterine Fibroids and Adenomyosis: A Pilot Study. J. Clin. Med. 2022, 11, 3757. [Google Scholar] [CrossRef]
- Sasaran, V.; Turdean, S.; Gliga, M.; Ilyes, L.; Grama, O.; Muntean, M.; Puscasiu, L. Value of Strain-Ratio Elastography in the Diagnosis and Differentiation of Uterine Fibroids and Adenomyosis. J. Pers. Med. 2021, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Flores, V.A.; Vanhie, A.; Dang, T.; Taylor, H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018, 103, 4561–4568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, Q.; Guo, S.W. Histological and Immunohistochemical Characterization of the Similarity and Difference Between Ovarian Endometriomas and Deep Infiltrating Endometriosis. Reprod. Sci. 2018, 25, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Critchley, H.; Fu, Z.; Guo, S.W. How does the extent of fibrosis in adenomyosis lesions contribute to heavy menstrual bleeding? Reprod. Med. Biol. 2022, 21, e12442. [Google Scholar] [CrossRef]
- Critchley, H.O.; Maybin, J.A. Molecular and cellular causes of abnormal uterine bleeding of endometrial origin. Semin. Reprod. Med. 2011, 29, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Pongpunprut, S.; Panburana, P.; Wibulpolprasert, P.; Waiyaput, W.; Sroyraya, M.; Chansoon, T.; Sophonsritsuk, A. A Comparison of Shear Wave Elastography between Normal Myometrium, Uterine Fibroids, and Adenomyosis: A Cross-Sectional Study. Int. J. Fertil. Steril. 2022, 16, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 2020, 19, e13259. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.D.; Vijayvergia, M.; Miller, F.H.; Carroll, T.; Fasanati, C.; Shea, L.D.; Brinson, L.C.; Woodruff, T.K. Multi-modal magnetic resonance elastography for noninvasive assessment of ovarian tissue rigidity in vivo. Acta Biomater. 2015, 13, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czuczwar, P.; Wozniak, S.; Szkodziak, P.; Kudla, M.J.; Pyra, K.; Paszkowski, T. Elastography Improves the Diagnostic Accuracy of Sonography in Differentiating Endometrial Polyps and Submucosal Fibroids. J. Ultrasound Med. 2016, 35, 2389–2395. [Google Scholar] [CrossRef]
- Troiano, R.N.; Flynn, S.D.; McCarthy, S. Cystic adenomyosis of the uterus: MRI. J. Magn. Reson. Imaging 1998, 8, 1198–1202. [Google Scholar] [CrossRef]
- Kerbage, Y.; Dericquebourg, S.; Collinet, P.; Verpillat, P.; Giraudet, G.; Rubod, C. Cystic adenomyoma surgery. J. Gynecol. Obs. Hum. Reprod. 2022, 51, 102313. [Google Scholar] [CrossRef]
- Peyron, N.; Jacquemier, E.; Charlot, M.; Devouassoux, M.; Raudrant, D.; Golfier, F.; Rousset, P. Accessory cavitated uterine mass: MRI features and surgical correlations of a rare but under-recognised entity. Eur. Radiol. 2019, 29, 1144–1152. [Google Scholar] [CrossRef]
- D’Orsi, C.J.; Kopans, D.B. Mammography interpretation: The BI-RADS method. Am. Fam. Physician 1997, 55, 1548–1550. [Google Scholar]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef]
- Tatsumi, C.; Kudo, M.; Ueshima, K.; Kitai, S.; Ishikawa, E.; Yada, N.; Hagiwara, S.; Inoue, T.; Minami, Y.; Chung, H.; et al. Non-invasive evaluation of hepatic fibrosis for type C chronic hepatitis. Intervirology 2010, 53, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, C.; Kudo, M.; Ueshima, K.; Kitai, S.; Takahashi, S.; Inoue, T.; Minami, Y.; Chung, H.; Maekawa, K.; Fujimoto, K.; et al. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology 2008, 51 (Suppl. S1), 27–33. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kato, M.; Kudo, M.; Yada, N.; Shiina, T.; Ueshima, K.; Yamada, Y.; Ishida, T.; Azuma, M.; Yamasaki, M.; et al. Novel image analysis method using ultrasound elastography for noninvasive evaluation of hepatic fibrosis in patients with chronic hepatitis C. Oncology 2013, 84 (Suppl. S1), 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ehman, R.L. Magnetic resonance elastography: From invention to standard of care. Abdom. Radiol. 2022, 47, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.L.; Chew, L.E.; Han, N.R.; Ooi, C.C.; Yeo, Y.C.; Chew, S.H.; Wong, W.L.; Tang, P.H.; Teo, S.Y. The Characteristics of Real-time Transvaginal Sono-elastography in Endometrial Cancer. J. Med. Ultrasound 2022, 30, 101–108. [Google Scholar] [CrossRef]
- Evans, A.; Rauchhaus, P.; Whelehan, P.; Thomson, K.; Purdie, C.A.; Jordan, L.B.; Michie, C.O.; Thompson, A.; Vinnicombe, S. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res. Treat. 2014, 143, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Athanasiou, A.; Latorre-Ossa, H.; Criton, A.; Tardivon, A.; Gennisson, J.L.; Tanter, M. Feasibility of Imaging and Treatment Monitoring of Breast Lesions with Three-Dimensional Shear Wave Elastography. Ultraschall Med. 2017, 38, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Schwarb, H.; Johnson, C.L.; McGarry, M.D.J.; Cohen, N.J. Medial temporal lobe viscoelasticity and relational memory performance. Neuroimage 2016, 132, 534–541. [Google Scholar] [CrossRef]


| Author(s) and Year of Publication | Imaging Platform | Proposed Classification | Rationale | Remarks |
|---|---|---|---|---|
| Kishi et al. (2012) [19] | MRI | Four subtypes: I: intrinsic; II: extrinsic; III: intramural; IV: indeterminate | Based on Sampson’s observation as well as clinical observations | Subtypes I and II appear to have different pathogenesis, symptomology, and severity |
| Van den Bosch et al. (2015) [36] Revised in Harmsen et al. (2022) [21] (The MUSA standard) | TVUS | Direct features: Cysts, hyperechogenic islands, echogenic subendometrial lines and buds. Indirect features: Asymmetrical thickening, globular uterus, irregular JZ, fan-shaped shadowing, translesional vascularity, interrupted JZ. | Based on expert consensus through several rounds of modified Delphi procedure | A welcome step towards the establishment of standardized terminology, with the goal to build a uniformly accepted or validated system to diagnose or classify the severity of adenomyosis based on imaging findings. |
| Bazot and Darai (2018) [14] | MRI | Three subtypes: -Internal -External -Adenomyoma | Based on Sampson’s observation as well as clinical observations | Different subtypes appear to have different pathogenesis, symptomatology, and severity |
| Gordts et al. (2018) [5] | MRI/TVUS/hysteroscopy | Important parameters to be included in a classification system: Affected area (inner or outer myometrium), localization (anterior, posterior, or fundus), pattern (diffuse or focal), type (muscular or cystic), volume or size (expressed as <1/3, <2/3, >2/3 or in cm) | These parameters are potentially related to symptomatology and/or severity | Included parameters are important for accurate diagnosis and, through grading, may be associated with disease severity. |
| Van den Bosch et al. (2019) [37] | TVUS | Location (anterior, posterior, left or right lateral side, or fundus), differentiation (focal, diffuse, or mixed type), cysticity (cystic or non-cystic), uterine layer involvement ( I: involving inner/sub-endometrial myometrium; II: involvement of middle myometrium; III: involvement of outer/sub-serosal myometrium), extent (<1/4, ≥1/4 but ≤1/2, >1/2 myometrium), and size. | Based on consensus among sonographers, and consistent with the previous MUSA consensus. | A welcome first step towards an internationally accepted classification and reporting system |
| Kobayashi and Matsubara (2020) [20] | MRI | Five main categories: (1) affected area (internal vs. external), (2) pattern (diffuse, focal); (3) size (<1/3, <2/3, or >2/3 of uterine wall); and (4) localization (anterior, posterior, left lateral, right lateral, and fundus); (5) concomitant pathologies (none, PE, OE, DE, UF, others) | Adopted from previous proposals of classification | Combined all important features of adenomyosis that may be useful for proper classification |
| Exacoustos et al. (2020) [25] | TVUS | Type (focal, diffuse, or adenomyomas), Extension of the lesion in the myometrium | Empirical observations | These variables seem to correlate with the severity of symptoms and infertility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-W.; Benagiano, G.; Bazot, M. In Search of an Imaging Classification of Adenomyosis: A Role for Elastography? J. Clin. Med. 2023, 12, 287. https://doi.org/10.3390/jcm12010287
Guo S-W, Benagiano G, Bazot M. In Search of an Imaging Classification of Adenomyosis: A Role for Elastography? Journal of Clinical Medicine. 2023; 12(1):287. https://doi.org/10.3390/jcm12010287
Chicago/Turabian StyleGuo, Sun-Wei, Giuseppe Benagiano, and Marc Bazot. 2023. "In Search of an Imaging Classification of Adenomyosis: A Role for Elastography?" Journal of Clinical Medicine 12, no. 1: 287. https://doi.org/10.3390/jcm12010287
APA StyleGuo, S.-W., Benagiano, G., & Bazot, M. (2023). In Search of an Imaging Classification of Adenomyosis: A Role for Elastography? Journal of Clinical Medicine, 12(1), 287. https://doi.org/10.3390/jcm12010287







