Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS)
Abstract
:1. Introduction
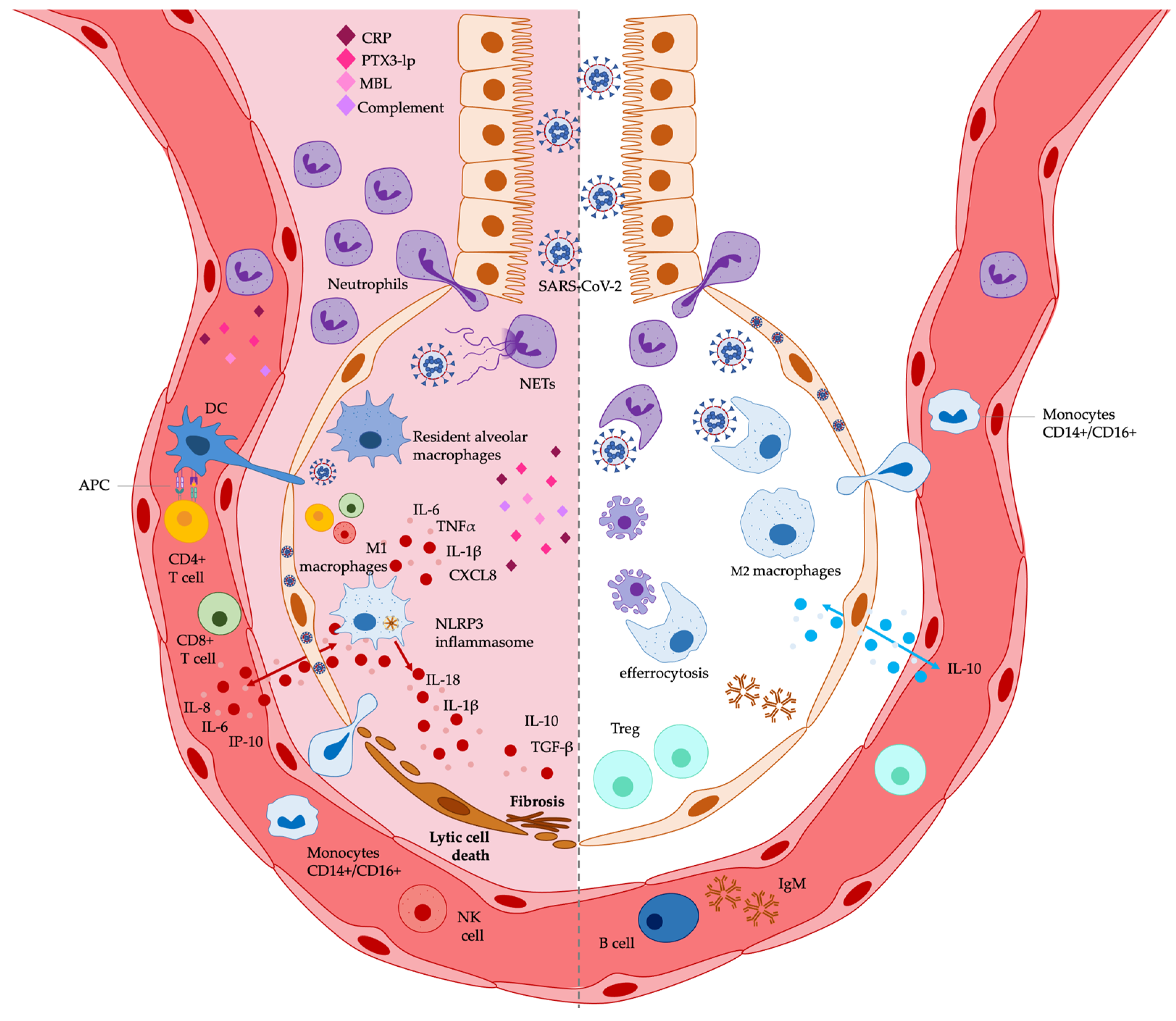
2. The Immune Response in COVID-19 Related ARDS
| Innate Immunity in the Lung | Dysregulation and References |
|---|---|
| Alveolar epithelial cells |
|
| Resident alveolar macrophages and monocytes/macrophages |
|
| Neutrophils | |
| DCs | |
| NK cells | |
| Humoral pattern recognition molecules | |
| Adaptive immunity in the lung | Dysregulation |
| T lymphocytes |
|
| B lymphocytes |
3. n-3 PUFAs and the Immunomodulatory Activity Relevant to COVID-19 Related ARDS
3.1. PUFAs and ARDS: In Vitro Immunomodulatory Effects on Lung Epithelial Cells and Macrophages
3.2. PUFAs and ARDS: Immunomodulation in Animal Lung Experimental Models
3.3. PUFAs and ARDS: Immunomodulation in Human Clinical Trials for Lung Diseases
| Target: Innate Immune Cells and Molecules | Effects and References |
|---|---|
| Alveolar epithelial cells | ↓ NF-κB activation [70] ↓ cytokine production: IL-6, IL-8, IP-10 [69,70,71,94,97] ↑ cytokines production: IL-10 [97] |
| Monocytes/Macrophages | ↓ NF-κB activation [27,72,80] ↓ STAT, IRF, JNK, MAPK signaling [11,77] ↓ cytokine production [11,78,99] ↓ chemokine production [11,94] ↓ NLRP3 inflammasome [74,80] ↓ M1 polarization ↑ M2 polarization [11,73,90,91] ↑ phagocytosis and efferocytosis [11,81,82,86,89] ↓ APC function [11,84] |
| Neutrophils | ↓ migration and tissue infiltration [11,87,88,94] ↑ phagocytosis [11,90,91] ↓ NET formation [11] |
| DCs | ↓ APC function [11,83,84] |
| NK cells | ↓ anti-viral function [11] |
| Humoral pattern recognition molecules | ↓ CRP [20,110] ↓ PTX3 long pentraxin [119] ↓ MBL [120] ↓ Complement [121] |
| Target: adaptive immune cells | Effects and References |
| T lymphocytes | ↓CTL (CD8+) and T helper (CD4+) [99,100] ↓ activation and function [11,97,100] ↑Treg [11,12] |
| B lymphocytes | ↑ ↓ activation * [11,27,98,102] ↑ IgM production [11] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Batah, S.S.; Fabro, A.T. Pulmonary Pathology of ARDS in COVID-19: A Pathological Review for Clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Saguil, A.; Fargo, M.V. Acute Respiratory Distress Syndrome: Diagnosis and Management. Am. Fam Physician 2020, 101, 730–738. [Google Scholar] [PubMed]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune Response in COVID-19: What Is Next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Nunez, P.; Bueno-Cavanillas, A.; San Jose-Saras, D.; Vicente-Guijarro, J.; Fernández Chávez, A.C.; Aranaz-Andrés, J.M.; on behalf of Health Outcomes Research Group of the Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS). How Does Vaccination against SARS-CoV-2 Affect Hospitalized Patients with COVID-19? J. Clin. Med. 2022, 11, 3905. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Piot, P. The Potential Future of the COVID-19 Pandemic: Will SARS-CoV-2 Become a Recurrent Seasonal Infection? JAMA 2021, 325, 1249–1250. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Powell, N.; Chaudhary, S.; Zaidi, A. It Is Time for an Oil Change: Polyunsaturated Fatty Acids and Human Health. Mo. Med. 2021, 118, 426–430. [Google Scholar]
- The Fatty Acids and Outcomes Research Consortium (FORCE); Harris, W.S.; Tintle, N.L.; Imamura, F.; Qian, F.; Korat, A.V.A.; Marklund, M.; Djoussé, L.; Bassett, J.K.; Carmichael, P.-H.; et al. Blood N-3 Fatty Acid Levels and Total and Cause-Specific Mortality from 17 Prospective Studies. Nat. Commun 2021, 12, 2329. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yoshida, M.; Arita, M. Omega-3 Fatty Acid-Derived Mediators That Control Inflammation and Tissue Homeostasis. Int. Immunol. 2019, 31, 559–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Parolini, C. Effects of Fish N-3 PUFAs on Intestinal Microbiota and Immune System. Mar. Drugs 2019, 17, 374. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Calder, P.C. N−3 Polyunsaturated Fatty Acids, Inflammation, and Inflammatory Diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Fatty Acids and Inflammation: The Cutting Edge between Food and Pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef]
- Schmidt, E.B.; Møller, J.M.; Svaneborg, N.; Dyerberg, J. Safety Aspects of Fish Oils: Experiences with an n-3 Concentrate of Re-Esterified Triglycerides (Pikasol®). Drug Investig. 1994, 7, 215–220. [Google Scholar] [CrossRef]
- Darwesh, A.M.; Bassiouni, W.; Sosnowski, D.K.; Seubert, J.M. Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications? Pharm. Ther. 2021, 219, 107703. [Google Scholar] [CrossRef]
- Merendino, N.; Costantini, L.; Manzi, L.; Molinari, R.; D’Eliseo, D.; Velotti, F. Dietary ω -3 Polyunsaturated Fatty Acid DHA: A Potential Adjuvant in the Treatment of Cancer. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 Fatty Acids and Cancer Cell Cytotoxicity: Implications for Multi-Targeted Cancer Therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, R.; D’Eliseo, D.; Manzi, L.; Zolla, L.; Velotti, F.; Merendino, N. The N3-Polyunsaturated Fatty Acid Docosahexaenoic Acid Induces Immunogenic Cell Death in Human Cancer Cell Lines via Pre-Apoptotic Calreticulin Exposure. Cancer Immunol Immunother 2011, 60, 1503–1507. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Manzi, L.; Merendino, N.; Velotti, F. Docosahexaenoic Acid Inhibits Invasion of Human RT112 Urinary Bladder and PT45 Pancreatic Carcinoma Cells via Down-Modulation of Granzyme B Expression. J. Nutr. Biochem. 2012, 23, 452–457. [Google Scholar] [CrossRef]
- D’Eliseo, D.; Di Rocco, G.; Loria, R.; Soddu, S.; Santoni, A.; Velotti, F. Epitelial-to-Mesenchimal Transition and Invasion Are Upmodulated by Tumor-Expressed Granzyme B and Inhibited by Docosahexaenoic Acid in Human Colorectal Cancer Cells. J. Exp. Clin. Cancer Res. 2016, 35, 24. [Google Scholar] [CrossRef] [Green Version]
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di Nunno, N.; Di Mizio, G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D.; et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19: A Hypothesis of Work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478. [Google Scholar] [CrossRef]
- Weill, P.; Plissonneau, C.; Legrand, P.; Rioux, V.; Thibault, R. May Omega-3 Fatty Acid Dietary Supplementation Help Reduce Severe Complications in Covid-19 Patients? Biochimie 2020, 179, 275–280. [Google Scholar] [CrossRef]
- Szabó, Z.; Marosvölgyi, T.; Szabó, É.; Bai, P.; Figler, M.; Verzár, Z. The Potential Beneficial Effect of EPA and DHA Supplementation Managing Cytokine Storm in Coronavirus Disease. Front. Physiol. 2020, 11, 752. [Google Scholar] [CrossRef]
- Hammock, B.D.; Wang, W.; Gilligan, M.M.; Panigrahy, D. Eicosanoids. Am. J. Pathol. 2020, 190, 1782–1788. [Google Scholar] [CrossRef]
- Andreakos, E.; Papadaki, M.; Serhan, C.N. Dexamethasone, Pro-resolving Lipid Mediators and Resolution of Inflammation in COVID-19. Allergy 2021, 76, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.M.; Leão, M.d.C.; Santana, T.M.; Pimentel, M.V.d.M.B.; Carlini, G.C.G.; da Silveira, T.F.F.; Gonçalves, R.C.; Castro, I.A. 2Potential Benefits and Risks of Omega-3 Fatty Acids Supplementation to Patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Ratha, S.K.; Renuka, N.; Rawat, I.; Bux, F. Prospective Options of Algae-Derived Nutraceuticals as Supplements to Combat COVID-19 and Human Coronavirus Diseases. Nutrition 2021, 83, 111089. [Google Scholar] [CrossRef] [PubMed]
- Gallo, C.G.; Fiorino, S.; Posabella, G.; Antonacci, D.; Tropeano, A.; Pausini, E.; Pausini, C.; Guarniero, T.; Hong, W.; Giampieri, E.; et al. The Function of Specialized Pro-Resolving Endogenous Lipid Mediators, Vitamins, and Other Micronutrients in the Control of the Inflammatory Processes: Possible Role in Patients with SARS-CoV-2 Related Infection. Prostaglandins Other Lipid Mediat. 2022, 159, 106619. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Alemzadeh, E.; Alemzadeh, E.; Salehiniya, H. The effect of polyunsaturated fatty acids on the severity and mortality of COVID patients: A systematic review. Life Sci. 2022, 299, 120489. [Google Scholar] [CrossRef]
- Ambrosy, A.P.; Malik, U.I.; Thomas, R.C.; Parikh, R.V.; Tan, T.C.; Goh, C.H.; Selby, V.N.; Solomon, M.D.; Avula, H.R.; Fitzpatrick, J.K.; et al. Rationale and Design of the Pragmatic Randomized Trial of Icosapent Ethyl for High Cardiovascular Risk Adults (MITIGATE). Am. Heart J. 2021, 235, 54–64. [Google Scholar] [CrossRef]
- Diaz, R.; Estudios Clínicos Latino América. PREPARE-IT: Prevention and Treatment of COVID19 with EPA in Subjects at Risk-Intervention Trial. ClinicalTrials.gov, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT0446051 (accessed on 24 December 2022).
- Al-Khaled, R.A.; Abu-Samak, M.S. The Effect of Omega-3 Supplements on the Serum Levels of Selected Cytokines Involved in Cytokine Storm of Covid-19; A Randomized Clinical Trial in the Covid-19 Uninfected Jordanian People. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04483271 (accessed on 24 December 2022).
- Arnardottir, H.; Pawelzik, S.-C.; Öhlund Wistbacka, U.; Artiach, G.; Hofmann, R.; Reinholdsson, I.; Braunschweig, F.; Tornvall, P.; Religa, D.; Bäck, M. Stimulating the Resolution of Inflammation Through Omega-3 Polyunsaturated Fatty Acids in COVID-19: Rationale for the COVID-Omega-F Trial. Front. Physiol. 2021, 11, 624657. [Google Scholar] [CrossRef]
- Kosmopoulos, A.; Bhatt, D.L.; Meglis, G.; Verma, R.; Pan, Y.; Quan, A.; Teoh, H.; Verma, M.; Jiao, L.; Wang, R.; et al. A Randomized Trial of Icosapent Ethyl in Ambulatory Patients with COVID-19. iScience 2021, 24, 103040. [Google Scholar] [CrossRef]
- S.L.A. Pharma AG. A Randomized, Double-Blind, Placebo-Controlled Study of Eicosapentaenoic Acid (EPA-FFA) Gastro-Resistant Capsules to Treat Hospitalized Subjects with Confirmed SARS-CoV-2. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04335032 (accessed on 24 December 2022).
- Ingvarson, R.F.; National Hospital of Iceland. Use of a Medical Device, Viruxal Oral and Nasal Spray, for Treating the Symptoms of COVID-19 Via Application to the Naso- and Oropharyngeal Mucosa. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04357990 (accessed on 24 December 2022).
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The Effect of Omega-3 Fatty Acid Supplementation on Clinical and Biochemical Parameters of Critically Ill Patients with COVID-19: A Randomized Clinical Trial. J. Transl Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Hamad Medical Corporation; Rizoli, S. Omega-3 Oil Use in COVID-19 Patients in Qatar: A Randomized Controlled Trial. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04836052 (accessed on 24 December 2022).
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The Lipid Mediator Protectin D1 Inhibits Influenza Virus Replication and Improves Severe Influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Vivar-Sierra, A.; Araiza-Macías, M.J.; Hernández-Contreras, J.P.; Vergara-Castañeda, A.; Ramírez-Vélez, G.; Pinto-Almazán, R.; Salazar, J.R.; Loza-Mejía, M.A. In Silico Study of Polyunsaturated Fatty Acids as Potential SARS-CoV-2 Spike Protein Closed Conformation Stabilizers: Epidemiological and Computational Approaches. Molecules 2021, 26, 711. [Google Scholar] [CrossRef]
- Goc, A.; Niedzwiecki, A.; Rath, M. Polyunsaturated ω-3 Fatty Acids Inhibit ACE2-Controlled SARS-CoV-2 Binding and Cellular Entry. Sci. Rep. 2021, 11, 5207. [Google Scholar] [CrossRef]
- Theken, K.N.; Tang, S.Y.; Sengupta, S.; FitzGerald, G.A. The Roles of Lipids in SARS-CoV-2 Viral Replication and the Host Immune Response. J. Lipid Res. 2021, 62, 100129. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Taoufik, Y.; de Goër de Herve, M.-G.; Corgnac, S.; Durrbach, A.; Mami-Chouaib, F. When Immunity Kills: The Lessons of SARS-CoV-2 Outbreak. Front. Immunol. 2021, 12, 692598. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the Human Innate Immune System. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Salvi, V.; Nguyen, H.O.; Sozio, F.; Schioppa, T.; Gaudenzi, C.; Laffranchi, M.; Scapini, P.; Passari, M.; Barbazza, I.; Tiberio, L.; et al. SARS-CoV-2–Associated ssRNAs Activate Inflammation and Immunity via TLR7/8. JCI Insight 2021, 6, e150542. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive Immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic Characteristics of Bronchoalveolar Lavage Fluid and Peripheral Blood Mononuclear Cells in COVID-19 Patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-Cell Landscape of Bronchoalveolar Immune Cells in Patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Kosyreva, A.; Dzhalilova, D.; Lokhonina, A.; Vishnyakova, P.; Fatkhudinov, T. The Role of Macrophages in the Pathogenesis of SARS-CoV-2-Associated Acute Respiratory Distress Syndrome. Front. Immunol. 2021, 12, 682871. [Google Scholar] [CrossRef]
- Wendisch, D.; Dietrich, O.; Mari, T.; von Stillfried, S.; Ibarra, I.L.; Mittermaier, M.; Mache, C.; Chua, R.L.; Knoll, R.; Timm, S.; et al. SARS-CoV-2 Infection Triggers Profibrotic Macrophage Responses and Lung Fibrosis. Cell 2021, 184, 6243–6261.e27. [Google Scholar] [CrossRef]
- German COVID-19 Omics Initiative (DeCOI); Aschenbrenner, A.C.; Mouktaroudi, M.; Krämer, B.; Oestreich, M.; Antonakos, N.; Nuesch-Germano, M.; Gkizeli, K.; Bonaguro, L.; Reusch, N.; et al. Disease Severity-Specific Neutrophil Signatures in Blood Transcriptomes Stratify COVID-19 Patients. Genome Med. 2021, 13, 7. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Zhou, R.; To, K.K.-W.; Wong, Y.-C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.-Y.; Lau, T.T.-K.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef]
- Peruzzi, B.; Bencini, S.; Capone, M.; Mazzoni, A.; Maggi, L.; Salvati, L.; Vanni, A.; Orazzini, C.; Nozzoli, C.; Morettini, A.; et al. Quantitative and Qualitative Alterations of Circulating Myeloid Cells and Plasmacytoid DC in SARS-CoV-2 Infection. Immunology 2020, 161, 345–353. [Google Scholar] [CrossRef]
- Brunetta, E.; Folci, M.; Bottazzi, B.; De Santis, M.; Gritti, G.; Protti, A.; Mapelli, S.N.; Bonovas, S.; Piovani, D.; Leone, R.; et al. Macrophage Expression and Prognostic Significance of the Long Pentraxin PTX3 in COVID-19. Nat. Immunol. 2021, 22, 19–24. [Google Scholar] [CrossRef]
- Stravalaci, M.; Pagani, I.; Paraboschi, E.M.; Pedotti, M.; Doni, A.; Scavello, F.; Mapelli, S.N.; Sironi, M.; Perucchini, C.; Varani, L.; et al. Recognition and Inhibition of SARS-CoV-2 by Humoral Innate Immunity Pattern Recognition Molecules. Nat. Immunol. 2022, 23, 275–286. [Google Scholar] [CrossRef]
- Afzali, B.; Noris, M.; Lambrecht, B.N.; Kemper, C. The State of Complement in COVID-19. Nat. Rev. Immunol. 2022, 22, 77–84. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [Green Version]
- Saedisomeolia, A.; Wood, L.G.; Garg, M.L.; Gibson, P.G.; Wark, P.A.B. Anti-Inflammatory Effects of Long-Chain n -3 PUFA in Rhinovirus-Infected Cultured Airway Epithelial Cells. Br. J. Nutr. 2008, 101, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Cotogni, P.; Muzio, G.; Trombetta, A.; Ranieri, V.M.; Canuto, R.A. Impact of the Ω-3 to Ω-6 Polyunsaturated Fatty Acid Ratio on Cytokine Release in Human Alveolar Cells. J. Parenter. Enter. Nutr. 2011, 35, 114–121. [Google Scholar] [CrossRef]
- Cotogni, P.; Trombetta, A.; Muzio, G.; Maggiora, M.; Canuto, R.A. The Omega-3 Fatty Acid Docosahexaenoic Acid Modulates Inflammatory Mediator Release in Human Alveolar Cells Exposed to Bronchoalveolar Lavage Fluid of ARDS Patients. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential Modulation of Toll-like Receptors by Fatty Acids: Preferential Inhibition by n-3 Polyunsaturated Fatty Acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and Its Precursor Docosahexaenoic Acid Promote Resolution of Adipose Tissue Inflammation by Eliciting Macrophage Polarization toward an M2-Like Phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 Fatty Acids Prevent Inflammation and Metabolic Disorder through Inhibition of NLRP3 Inflammasome Activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.Y.; Lee, H.-N.; Kim, W.; Surh, Y.-J. Docosahexaenoic Acid Induces M2 Macrophage Polarization through Peroxisome Proliferator-Activated Receptor γ Activation. Life Sci. 2015, 120, 39–47. [Google Scholar] [CrossRef]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.; Lichtenstein, A.H. Docosahexaenoic Acid Differentially Affects TNFα and IL-6 Expression in LPS-Stimulated RAW 264.7 Murine Macrophages. Prostaglandins Leukot. Essent. Fat. Acids 2015, 97, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Mildenberger, J.; Johansson, I.; Sergin, I.; Kjøbli, E.; Damås, J.K.; Razani, B.; Flo, T.H.; Bjørkøy, G. N-3 PUFAs Induce Inflammatory Tolerance by Formation of KEAP1-Containing SQSTM1/P62-Bodies and Activation of NFE2L2. Autophagy 2017, 13, 1664–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. A Study of the Differential Effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on Gene Expression Profiles of Stimulated Thp-1 Macrophages. Nutrients 2017, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Schoeniger, A.; Adolph, S.; Fuhrmann, H.; Schumann, J. The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus Equi and Pseudomonas Aeruginosa. Int. J. Mol. Sci. 2011, 12, 7510–7528. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.-N.; Hwang, I.-Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 Free Fatty Acids Suppress Macrophage Inflammasome Activation by Inhibiting NF-ΚB Activation and Enhancing Autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef] [Green Version]
- Adolph, S.; Fuhrmann, H.; Schumann, J. Unsaturated Fatty Acids Promote the Phagocytosis of P. Aeruginosa and R. Equi by RAW264.7 Macrophages. Curr Microbiol 2012, 65, 649–655. [Google Scholar] [CrossRef]
- Davidson, J.; Kerr, A.; Guy, K.; Rotondo, D. Prostaglandin and Fatty Acid Modulation of Escherichia Coli O157 Phagocytosis by Human Monocytic Cells. Immunology 1998, 94, 228–234. [Google Scholar] [CrossRef]
- Sanderson, P.; MacPherson, G.G.; Jenkins, C.H.; Calder, P.C. Dietary Fish Oil Diminishes the Antigen Presentation Activity of Rat Dendritic Cells. J. Leukoc. Biol. 1997, 62, 771–777. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Edidin, M. Immunosuppressive Effects of Polyunsaturated Fatty Acids on Antigen Presentation by Human Leukocyte Antigen Class I Molecules. J. Lipid Res. 2007, 48, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Hansson, G.K. Innate Immunity, Macrophage Activation, and Atherosclerosis. Immunol. Rev. 2007, 219, 187–203. [Google Scholar] [CrossRef]
- Rajasinghe, L.D.; Chauhan, P.S.; Wierenga, K.A.; Evered, A.O.; Harris, S.N.; Bates, M.A.; Gavrilin, M.A.; Pestka, J.J. Omega-3 Docosahexaenoic Acid (DHA) Impedes Silica-Induced Macrophage Corpse Accumulation by Attenuating Cell Death and Potentiating Efferocytosis. Front. Immunol. 2020, 11, 2179. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Whelan, J.; DeMichele, S.J.; Snider, C.C.; Guszcza, J.A.; Claycombe, K.J.; Smith, G.T.; Gregory, T.J.; Karlstad, M.D. Effects of Eicosapentaenoic and Gamma-Linolenic Acid on Lung Permeability and Alveolar Macrophage Eicosanoid Synthesis in Endotoxic Rats. Crit. Care Med. 1997, 25, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Whelan, J.; DeMichele, S.J.; Snider, C.C.; Guszcza, J.A.; Karlstad, M.D. Dietary Fish Oil and Fish and Borage Oil Suppress Intrapulmonary Proinflammatory Eicosanoid Biosynthesis and Attenuate Pulmonary Neutrophil Accumulation in Endotoxic Rats. Crit. Care Med. 1997, 25, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chhibber, S.; Mohan, H.; Sharma, S. Dietary Supplementation with Omega-3 Polyunsaturated Fatty Acids Ameliorates Acute Pneumonia Induced by Klebsiella Pneumoniae in BALB/c Mice. Can. J. Microbiol. 2013, 59, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Körner, A.; Schlegel, M.; Theurer, J.; Frohnmeyer, H.; Adolph, M.; Heijink, M.; Giera, M.; Rosenberger, P.; Mirakaj, V. Resolution of Inflammation and Sepsis Survival Are Improved by Dietary Ω-3 Fatty Acids. Cell Death Differ. 2018, 25, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhaus, S.; Swick, A.G. Specialized proresolving Mediators in Infection and Lung Injury. BioFactors 2021, 47, 6–18. [Google Scholar] [CrossRef]
- Hsiao, H.-M.; Sapinoro, R.E.; Thatcher, T.H.; Croasdell, A.; Levy, E.P.; Fulton, R.A.; Olsen, K.C.; Pollock, S.J.; Serhan, C.N.; Phipps, R.P.; et al. A Novel Anti-Inflammatory and Pro-Resolving Role for Resolvin D1 in Acute Cigarette Smoke-Induced Lung Inflammation. PLoS ONE 2013, 8, e58258. [Google Scholar] [CrossRef] [Green Version]
- Seki, H.; Fukunaga, K.; Arita, M.; Arai, H.; Nakanishi, H.; Taguchi, R.; Miyasho, T.; Takamiya, R.; Asano, K.; Ishizaka, A.; et al. The Anti-Inflammatory and Proresolving Mediator Resolvin E1 Protects Mice from Bacterial Pneumonia and Acute Lung Injury. J. Immunol. 2010, 184, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-W.; Wang, Q.; Mei, H.-X.; Zheng, S.-X.; Ali, A.M.; Wu, Q.-X.; Ye, Y.; Xu, H.-R.; Xiang, S.-Y.; Jin, S.-W. RvD1 Ameliorates LPS-Induced Acute Lung Injury via the Suppression of Neutrophil Infiltration by Reducing CXCL2 Expression and Release from Resident Alveolar Macrophages. Int. Immunopharmacol. 2019, 76, 105877. [Google Scholar] [CrossRef]
- Tan, W.; Chen, L.; Wang, Y.-X.; Hu, L.-S.; Xiong, W.; Shang, Y.; Yao, S.-L. Protectin DX Exhibits Protective Effects in Mouse Model of Lipopolysaccharide-Induced Acute Lung Injury. Chin. Med. J. 2018, 131, 1167–1173. [Google Scholar] [CrossRef]
- Kocherlakota, C.; Nagaraju, B.; Arjun, N.; Srinath, A.; Kothapalli, K.S.D.; Brenna, J.T. Inhalation of Nebulized Omega-3 Fatty Acids Mitigate LPS-Induced Acute Lung Inflammation in Rats: Implications for Treatment of COPD and COVID-19. Prostaglandins Leukot. Essent. Fat. Acids 2022, 179, 102426. [Google Scholar] [CrossRef] [PubMed]
- McFarland, C.T.; Fan, Y.-Y.; Chapkin, R.S.; Weeks, B.R.; McMurray, D.N. Dietary Polyunsaturated Fatty Acids Modulate Resistance to Mycobacterium Tuberculosis in Guinea Pigs. J. Nutr. 2008, 138, 2123–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byleveld, P.M.; Pang, G.T.; Clancy, R.L.; Roberts, D.C.K. Fish Oil Feeding Delays Influenza Virus Clearance and Impairs Production of Interferon-γ and Virus-Specific Immunoglobulin A in the Lungs of Mice. J. Nutr. 1999, 129, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwerbrock, N.M.J.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish Oil-Fed Mice Have Impaired Resistance to Influenza Infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Kang, K.W.; Kim, S.; Cho, Y.-B.; Ryu, S.R.; Seo, Y.-J.; Lee, S.-M. Endogenous N-3 Polyunsaturated Fatty Acids Are Beneficial to Dampen CD8+ T Cell-Mediated Inflammatory Response upon the Viral Infection in Mice. Int. J. Mol. Sci. 2019, 20, 4510. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.J.B.; Roper, R.L. The Effects of Diets Enriched in Omega-3 Polyunsaturated Fatty Acids on Systemic Vaccinia Virus Infection. Sci. Rep. 2017, 7, 15999. [Google Scholar] [CrossRef] [Green Version]
- Ramon, S.; Baker, S.F.; Sahler, J.M.; Kim, N.; Feldsott, E.A.; Serhan, C.N.; Martínez-Sobrido, L.; Topham, D.J.; Phipps, R.P. The Specialized Proresolving Mediator 17-HDHA Enhances the Antibody-Mediated Immune Response against Influenza Virus: A New Class of Adjuvant? J. Immunol. 2014, 193, 6031–6040. [Google Scholar] [CrossRef] [Green Version]
- Husson, M.-O.; Ley, D.; Portal, C.; Gottrand, M.; Hueso, T.; Desseyn, J.-L.; Gottrand, F. Modulation of Host Defence against Bacterial and Viral Infections by Omega-3 Polyunsaturated Fatty Acids. J. Infect. 2016, 73, 523–535. [Google Scholar] [CrossRef]
- Pontes-Arruda, A.; DeMichele, S.; Seth, A.; Singer, P. The Use of an Inflammation-Modulating Diet in Patients with Acute Lung Injury or Acute Respiratory Distress Syndrome: A Meta-Analysis of Outcome Data. JPEN J. Parenter. Enter. Nutr. 2008, 32, 596–605. [Google Scholar] [CrossRef]
- Rice, T.W. Enteral Omega-3 Fatty Acid, γ-Linolenic Acid, and Antioxidant Supplementation in Acute Lung Injury. JAMA 2011, 306, 1574. [Google Scholar] [CrossRef] [Green Version]
- Sabater, J.; Masclans, J.R.; Sacanell, J.; Chacon, P.; Sabin, P.; Planas, M. Effects of an Omega-3 Fatty Acid-Enriched Lipid Emulsion on Eicosanoid Synthesis in Acute Respiratory Distress Syndrome (ARDS): A Prospective, Randomized, Double-Blind, Parallel Group Study. Nutr. Metab. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bo, L.; Liu, W.; Lu, X.; Jin, F. Enteral Immunomodulatory Diet (Omega-3 Fatty Acid, γ-Linolenic Acid and Antioxidant Supplementation) for Acute Lung Injury and Acute Respiratory Distress Syndrome: An Updated Systematic Review and Meta-Analysis. Nutrients 2015, 7, 5572–5585. [Google Scholar] [CrossRef] [PubMed]
- García de Acilu, M.; Leal, S.; Caralt, B.; Roca, O.; Sabater, J.; Masclans, J.R. The Role of Omega-3 Polyunsaturated Fatty Acids in the Treatment of Patients with Acute Respiratory Distress Syndrome: A Clinical Review. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langlois, P.L.; D’Aragon, F.; Hardy, G.; Manzanares, W. Omega-3 Polyunsaturated Fatty Acids in Critically Ill Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Nutrition 2019, 61, 84–92. [Google Scholar] [CrossRef]
- Hosny, M.; Nahas, R.; Ali, S.; Elshafei, S.A.; Khaled, H. Impact of Oral Omega-3 Fatty Acids Supplementation in Early Sepsis on Clinical Outcome and Immunomodulation. Egypt. J. Crit. Care Med. 2013, 1, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, S.; Zhao, Y.; Luo, Y.; Tong, H.; Su, L. Correlation Analysis of Omega-3 Fatty Acids and Mortality of Sepsis and Sepsis-Induced ARDS in Adults: Data from Previous Randomized Controlled Trials. Nutr. J. 2018, 17, 57. [Google Scholar] [CrossRef] [Green Version]
- Dirjayanto, V.J. Evidence on the Efficacy of Omega-3 Polyunsaturated Fatty Acids as an Adjunct Therapy for Chronic Obstructive Pulmonary Disease. J. Asian Med. Stud. Assoc. 2021, 9. [Google Scholar] [CrossRef]
- Stapleton, R.D.; Martin, T.R.; Weiss, N.S.; Crowley, J.J.; Gundel, S.J.; Nathens, A.B.; Akhtar, S.R.; Ruzinski, J.T.; Caldwell, E.; Curtis, J.R.; et al. A Phase II Randomized Placebo-Controlled Trial of Omega-3 Fatty Acids for the Treatment of Acute Lung Injury*. Crit. Care Med. 2011, 39, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Dushianthan, A.; Cusack, R.; Burgess, V.A.; Grocott, M.P.; Calder, P. Immunonutrition for Adults With ARDS: Results From a Cochrane Systematic Review and Meta-Analysis. Respir. Care 2020, 65, 99–110. [Google Scholar] [CrossRef]
- Bistrian, B.R. Parenteral Fish-Oil Emulsions in Critically Ill COVID-19 Emulsions. J. Parenter. Enter. Nutr. 2020, 44, 1168. [Google Scholar] [CrossRef]
- Torrinhas, R.S.; Calder, P.C.; Lemos, G.O.; Waitzberg, D.L. Parenteral Fish Oil: An Adjuvant Pharmacotherapy for Coronavirus Disease 2019? Nutrition 2021, 81, 110900. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.; Gokorsch, S.; Fegbeutel, C.; Hattar, K.; Rosseau, S.; Walmrath, D.; Seeger, W.; Grimminger, F. Parenteral Nutrition with Fish Oil Modulates Cytokine Response in Patients with Sepsis. Am. J. Respir. Crit. Care Med. 2003, 167, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Gupta, A.; Bhatnagar, S.; Goyal, J.; Baweja, H. Efficacy and Safety of Parenteral Omega 3 Fatty Acids in Ventilated Patients with Acute Lung Injury. Indian J. Crit. Care Med. 2011, 15, 108–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, S.; Marchioli, R.; Mozaffarian, D.; Bernasconi, R.; Milani, V.; Dragani, L.; Tacconi, M.; Marfisi, R.M.; Borgese, L.; Cirrincione, V.; et al. Plasma n-3 polyunsaturated fatty acids in chronic heart failure in the GISSI-Heart Failure Trial: Relation with fish intake, circulating biomarkers, and mortality. Am. Heart J. 2013, 165, 208–215.e4. [Google Scholar] [CrossRef]
- Arshad, A.; Chung, W.; Isherwood, J.; Steward, W.; Metcalfe, M.; Dennison, A. Restoration of mannose-binding lectin complement activity is associated with improved outcome in patients with advanced pancreatic cancer treated with gemcitabine and intravenous ω-3 fish oil. JPEN J. Parenter Enter. Nutr. 2014, 38, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, J.; Lin, Z.; Hua, Q.; Zhang, W.; Ye, L.; Wu, G.; Du, J.; Xia, J.; Chu, M.; et al. Resolvin D1 Alleviates the Lung Ischemia Reperfusion Injury via Complement, Immunoglobulin, TLR4, and Inflammatory Factors in Rats. Inflammation 2016, 39, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
| Study Identifier and References | Intervention(s) | Title of the Study and Proposal | Recruitment Status |
|---|---|---|---|
| NCT04505098 Kaiser Permanente Northern California (KPNC). Phase 4 [36] | Icosapent ethyl (IPE)/Vascepa 2 g by mouth twice daily for at least 6 months. | MITIGATE: A Pragmatic Randomized Trial of Icosapent Ethyl for High-Cardiovascular Risk Adults. A prospective, open-label, parallel-group, randomized, pragmatic clinical trial designed to evaluate the real-world clinical effectiveness of pre-treatment with IPE/Vascepa®, compared to the usual standard of care to prevent and reduce the sequelae of laboratory-confirmed viral upper respiratory infection (URI)-related (i.e., COVID-19, influenza, and other known viral respiratory pathogens) morbidity and mortality in a high-risk cohort of adults with established atherosclerotic cardiovascular disease. | Active, not recruiting. Estimated enrollment: 39,600 participants, 50 years and older, with no prior history of confirmed COVID-19. |
| NCT04460651 Estudios Clínicos Latino América. Phase 3 [37] | Icosapent ethyl (IPE)/Vascepa 8 g (4 capsules every 12 h) for the first 3 days, followed by 4 g (2 capsules every 12 h) thereafter for 4–60 days. | PREPARE-IT: Prevention of COVID-19 With EPA in Subjects at Risk Intervention Trial. The trial intended to reduce infection rates and subsequent morbidity and mortality among subjects at high risk of COVID-19 infection. | Completed. Enrollment: 18 years and older, any subject that is circulating and exposed to the public. |
| NCT04483271 Mahmoud Suleiman Abu-Samak; Applied Science Private University, Jordan. (Not applicable phase) [38] | Dietary Supplement: 300 mg of omega-3 fatty acid/daily for 2 months. | The Effect of Omega-3 Supplements on the Serum Levels of Selected Cytokines (IL-1, IL-6, TNF) Involved in Cytokine Storm of Covid-19. A randomized clinical trial designed to evaluate the effect of daily 300 mg of omega-3 fatty acid supplements on the immune health status of uninfected people with Covid-19 as a part of preventive health care. | Enrolling. Estimated enrollment: 100 participants in the age range of 30 to 66 years without a medical diagnosis of COVID-19 infection. |
| Study Identifier and References | Intervention(s) | Title of the Study and Proposal | Recruitment Status |
|---|---|---|---|
| NCT04647604 Karolinska University Hospital. Phase 2 [39] | Omegaven® (2 mL/kg/day, equivalent to 6 g DHA+EPA in a 70 kg individual) once daily i.v. for 5 days; 7 patients/each group received concomitant corticoids | Resolving inflammatory storm in COVID-19 patients by Omega-3 Polyunsaturated Fatty Acids. | Completed. 22 older participants with COVID-19 diagnosis and requiring hospitalization. |
| NCT04412018 Canadian Medical and Surgical Knowledge Translation Research Group. Phase 2 [40] | Icosapent ethyl (IPE)/Vascepa: 4 g by mouth twice daily for 3 days; then 2 g twice daily for the subsequent 11 days | VASCEPA-COVID-19: An Investigation on the Effects of Icosapent Ethyl (VascepaTM) on Inflammatory Biomarkers in Individuals With COVID-19. | Completed. 100 participants |
| NCT04460651 Estudios Clínicos Latino América, Argentina. Phase 3 [37] | Icosapent ethyl (IPE)/Vascepa 8 g (4 capsules every 12 h) for the first 3 days; followed by 4 g (2 capsules every 12 h) thereafter days 4–28. | PREPARE-IT: Treatment of COVID-19 With EPA in Subjects at Risk—Intervention Trial. The PREPARE-IT investigator-initiated trial program is a simple, pragmatic, therapeutic strategy evaluating pure icosapent ethyl (IPE) at initially higher doses intended to reduce the hospitalization rate and complications in patients with a positive diagnosis of COVID-19. | Completed. 2000 participants, 40 years or older; enrolled no more than 7 days from onset of symptoms and without clear indication of hospitalization |
| NCT04335032 S.L.A. Pharma AG. Phase 3 [41] | EPA-Free Fatty Acid 500 mg gastro-resistant capsules twice daily (2 g daily) for 28 days | A Randomised, Double-blind, Placebo-Controlled Study of Eicosapentaenoic Acid (EPA-FFA) Gastroresistant Capsules to Treat Hospitalised Subjects with Confirmed COVID-19 (SARS-CoV-2). Treatment failure is defined as the additional or alternative treatment required, or intubation and invasive ventilation, or transfer to the intensive care unit, or death. Reduction of CRP, IL-6, pro-inflammatory cytokines, and chemokines, as well as increased IFN-γ, will be determined. | Recruiting. Estimated enrollment: 284 participants, 18 years and older. |
| NCT04357990 National Hospital of Iceland (Landspítali). Not applicable Phase [42] | Viruxal Oral and Nasal Spray (Class I CE marked medical device, by Kerecis hf, containing Omega-3 Viruxide) for 14 days. | KONS-COVID-19: Viruxal Oral and Nasal Spray, for Treating the Symptoms of COVID-19. Patients will have their symptoms recorded until no further symptoms are reported, up to a maximum of 28 days follow-up. | Recruiting. Estimated enrollment: 128 participants with mild to moderate symptoms of COVID-19 |
| IRCT20151226025699N3 National Nutrition and Food Technology Research Institute, Iran [43] | 1 capsule of omega-3 daily, produced by Omid persina Damavand company, Iran (1000 mg Omega-3-DHA and EPA for each capsule, containing of EPAs+DHAs), for 14 days | The effect of omega-3 supplementation on inflammatory and biochemical markers in critically ill patients with COVID-19 a randomized clinical trial. WBC, Neutrophils, Lymphocytes, LDH, CPK, CBC, CRP: 14 days after the intervention will be determined. | Completed. 50 critically ill COVID-19 patients, from 35 to 85 years old. |
| NCT04836052 Hamad Medical Corporation (HMC). Phase 3 [44] | Omega-3-oil 2 g PO/NGT/ OGT twice daily for 28 days | Omega-3 Oil Use in COVID-19 Patients in Qatar: a Randomized Controlled Trial. Patients admitted to ICU in HMC on any kind of oxygen support will get omega-3-oil 2 g PO/NGT/ OGT twice daily for 28 days or till ICU discharge or till death | Recruiting. Estimated Enrollment: 372 participants, 18 years and older |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velotti, F.; Costantini, L.; Merendino, N. Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS). J. Clin. Med. 2023, 12, 304. https://doi.org/10.3390/jcm12010304
Velotti F, Costantini L, Merendino N. Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS). Journal of Clinical Medicine. 2023; 12(1):304. https://doi.org/10.3390/jcm12010304
Chicago/Turabian StyleVelotti, Francesca, Lara Costantini, and Nicolò Merendino. 2023. "Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS)" Journal of Clinical Medicine 12, no. 1: 304. https://doi.org/10.3390/jcm12010304
APA StyleVelotti, F., Costantini, L., & Merendino, N. (2023). Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs) for Immunomodulation in COVID-19 Related Acute Respiratory Distress Syndrome (ARDS). Journal of Clinical Medicine, 12(1), 304. https://doi.org/10.3390/jcm12010304








