The Potential of Self-Assessment and Associated Factors for Delayed Symptomatic Hyponatremia Following Transsphenoidal Surgery: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Eligibility
2.2. Standard Preoperative Evaluation and Inpatient Care
2.3. Outpatient Care
2.4. Statistical Analysis
3. Results
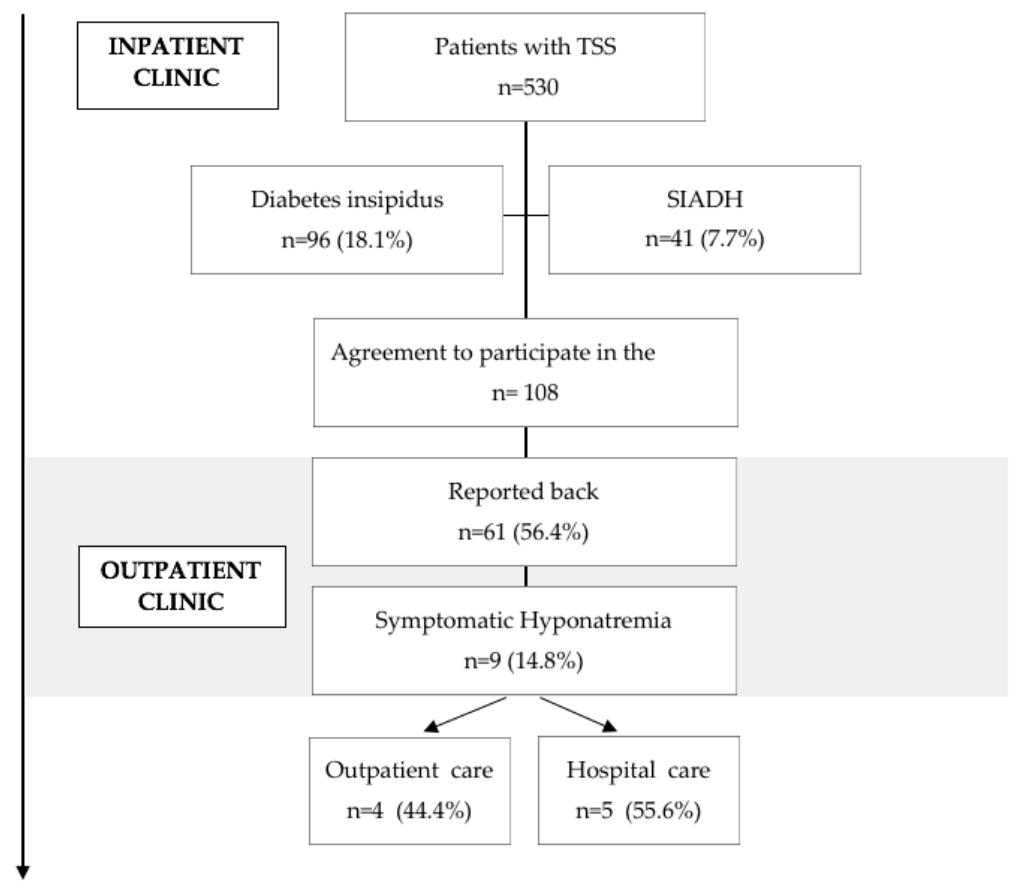
3.1. Study Flow Chart
3.2. Patient and Tumour Characteristics
3.3. Postoperative Hyponatremia after Hospital Discharge
3.4. Factors Associated with DSH
3.5. Clinical Symptoms of DSH
3.6. Correlation Analysis
4. Discussion
4.1. Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Severity, Occurrence and Therapy of the 9 Patients with DSH
| Patient No. | Sodium Nadir | POD | IP Intensive Care Unit (ICU) Outpatient (OP) | Duration of Hospital Stay | Therapy |
| 1 | 122 | 7 | ICU | 7 | Sodium infusion |
| 2 | 118 | 7 | ICU | 5 | Sodium infusions and Prednisolone |
| 3 | 132 | 14 | OP | - | Fluid restriction |
| 4 | 120 | 8 | IP | 6 | multiple doses 3 mg Tolvaptan |
| 5 | 120 | 7 | ICU | 6 | multiple doses 3 mg Tolvaptan |
| 6 | 124 | 11 | IP | 2 | single dose 3 mg Tolvaptan |
| 7 | 125 | 7 | OP | - | Sodium pills |
| 8 | 119 | 9 | OP | - | Fluid restriction |
| 9 | 132 | 8 | OP | - | Fluid restriction |
References
- Kristof, R.A.; Rother, M.; Neuloh, G.; Klingmüller, D. Incidence, clinical manifestations, and course of water and electrolyte metabolism disturbances following transsphenoidal pituitary adenoma surgery: A prospective observational study. J. Neurosurg. 2009, 111, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.S.; Piper, M.; Ludlam, W.G.; Ludlam, W.H.; Fuller, C.J.; Mayberg, M.R. Delayed postoperative hyponatremia after transsphenoidal surgery: Prevalence and associated factors. J. Neurosurg. 2013, 119, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Sane, T.; Rantakari, K.; Poranen, A.; Tähtelä, R.; Välimäki, M.; Pelkonen, R. Hyponatremia after transsphenoidal surgery for pituitary tumors. J. Clin. Endocrinol. Metab. 1994, 79, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Wt, B.; Dj, C.; Si, I.; Ha, Z.; Er, L. A practical method for prevention of readmission for symptomatic hyponatremia following transsphenoidal surgery. Pituitary 2018, 21, 25–31. [Google Scholar] [CrossRef]
- Tomita, Y.; Kurozumi, K.; Inagaki, K.; Kameda, M.; Ishida, J.; Yasuhara, T.; Otsuka, F. Delayed postoperative hyponatremia after endoscopic transsphenoidal surgery for pituitary adenoma. Acta Neurochir. 2019, 161, 707–715. [Google Scholar] [CrossRef]
- Patel, K.S.; Shu Chen, J.; Yuan, F.; Attiah, M.; Wilson, B.; Wang, M.B.; Kim, W. Prediction of post-operative delayed hyponatremia after endoscopic transsphenoidal surgery. Clin. Neurol. Neurosurg. 2019, 182, 87–91. [Google Scholar] [CrossRef]
- Kiran, Z.; Sheikh, A.; Momin, S.N.A.; Majeed, I.; Awan, S.; Rashid, O.; Islam, N. Sodium and water imbalance after sellar, suprasellar, and parasellar surgery. Endocr. Pract. 2017, 23, 309–317. [Google Scholar] [CrossRef]
- Janneck, M.; Burkhardt, T.; Rotermund, R.; Sauer, N.; Flitsch, J.; Aberle, J. Hyponatremia after trans-sphenoidal surgery. Minerva Endocrinol. 2014, 39, 27–31. [Google Scholar]
- Bohl, M.A.; Ahmad, S.; Jahnke, H.; Shepherd, D.; Knecht, L.; White, W.L.; Little, A.S. Delayed Hyponatremia Is the Most Common Cause of 30-Day Unplanned Readmission After Transsphenoidal Surgery for Pituitary Tumors. Neurosurgery 2016, 78, 84–90. [Google Scholar] [CrossRef]
- Yuen, K.C.J.; Ajmal, A.; Correa, R.; Little, A.S. Sodium Perturbations After Pituitary Surgery. Neurosurg. Clin. N. Am. 2019, 30, 515–524. [Google Scholar] [CrossRef]
- Hao, J.; Li, Y.; Zhang, X.; Pang, C.; Wang, Y.; Nigwekar, S.U.; Chen, L. The prevalence and mortality of hyponatremia is seriously underestimated in Chinese general medical patients: An observational retrospective study. BMC Nephrol. 2017, 18, 328. [Google Scholar] [CrossRef]
- Hensen, J.; Henig, A.; Fahlbusch, R.; Meyer, M.; Boehnert, M.; Buchfelder, M. Prevalence, predictors and patterns of postoperative polyuria and hyponatraemia in the immediate course after transsphenoidal surgery for pituitary adenomas. Clin. Endocrinol. 1999, 50, 431–439. [Google Scholar] [CrossRef]
- Cho, D.Y.; Liau, W.R. Comparison of endonasal endoscopic surgery and sublabial microsurgery for prolactinomas. Surg. Neurol. 2002, 58, 371–375. [Google Scholar] [CrossRef]
- White, D.R.; Sonnenburg, R.E.; Ewend, M.G.; Senior, B.A. Safety of minimally invasive pituitary surgery (MIPS) compared with a traditional approach. The Laryngoscope 2004, 114, 1945–1948. [Google Scholar] [CrossRef]
- O’Malley, B.W.; Grady, M.S.; Gabel, B.C.; Cohen, M.A.; Heuer, G.G.; Pisapia, J.; Leibowitz, J.M. Comparison of endoscopic and microscopic removal of pituitary adenomas: Single-surgeon experience and the learning curve. Neurosurg. Focus. 2008, 25, E10. [Google Scholar] [CrossRef]
- Juraschka, K.; Khan, O.H.; Godoy, B.L.; Monsalves, E.; Kilian, A.; Krischek, B.; Zadeh, G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection. J. Neurosurg. 2014, 121, 75–83. [Google Scholar] [CrossRef]
- Zada, G.; Liu, C.Y.; Fishback, D.; Singer, P.A.; Weiss, M.H. Recognition and management of delayed hyponatremia following transsphenoidal pituitary surgery. J. Neurosurg. 2007, 106, 66–71. [Google Scholar] [CrossRef]
- Jahangiri, A.; Wagner, J.; Tran, M.T.; Miller, L.M.; Tom, M.W.; Kunwar, S.; Aghi, M.K. Factors predicting postoperative hyponatremia and efficacy of hyponatremia management strategies after more than 1000 pituitary operations. J. Neurosurg. 2013, 119, 1478–1483. [Google Scholar] [CrossRef]
- Giuliani, C.; Cangioli, M.; Beck-Peccoz, P.; Faustini-Fustini, M.; Fiaccadori, E.; Peri, A. Awareness and management of hyponatraemia: The Italian Hyponatraemia Survey. J. Endocrinol. Investig. 2013, 36, 693–698. [Google Scholar] [CrossRef]
- Spasovski, G.; Vanholder, R.; Allolio, B.; Annane, D.; Ball, S.; Bichet, D.; Decaux, G.; Fenske, W.; Hoorn, E.J.; Ichai, C.; et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Eur. J. Endocrinol. 2014, 170, G1–G47. [Google Scholar] [CrossRef]
- Cote, D.J.; Alzarea, A.; Acosta, M.A.; Hulou, M.M.; Huang, K.T.; Almutairi, H.; Smith, T.R. Predictors and Rates of Delayed Symptomatic Hyponatremia after Transsphenoidal Surgery: A Systematic Review [corrected]. World Neurosurg. 2016, 88, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Laws, E.R.; Fossett, D. Delayed hyponatremia after transsphenoidal surgery for pituitary adenoma. Report of nine cases. J. Neurosurg. 1995, 83, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Cho, W.H.; Choi, B.K.; Cha, S.H.; Song, G.S.; Choi, C.H. Delayed hyponatremia following transsphenoidal surgery for pituitary adenoma. Neurol. Med. Chir. 2008, 48, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Blount, S.L.; Hwang, J.Y.; Williams, K.; Fong, B.; Yuan, J.; Kim, A.H.; Silverstein, J.M. Early Moderate Fluid Restriction and the Risk of Delayed Hyponatremia Following Transsphenoidal Surgery. J Endocr. Soc. 2021, 5 (Suppl 1), A627. [Google Scholar] [CrossRef]
- Schrier, R.W. Body water homeostasis: Clinical disorders of urinary dilution and concentration. J. Am. Soc. Nephrol. 2006, 17, 1820–1832. [Google Scholar] [CrossRef]
- Forsling, M.L.; Montgomery, H.; Halpin, D.; Windle, R.J.; Treacher, D.F. Daily patterns of secretion of neurohypophysial hormones in man: Effect of age. Exp. Physiol. 1998, 83, 409–418. [Google Scholar] [CrossRef]
- Olson, B.R.; Gumowski, J.; Rubino, D.; Oldfield, E.H. Pathophysiology of hyponatremia after transsphenoidal pituitary surgery. J. Neurosurg. 1997, 87, 499–507. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Obermuller, N. Endocrine testing for the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH). In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: http://www.ncbi.nlm.nih.gov/books/NBK279055/ (accessed on 29 June 2022).
- Barber, S.M.; Liebelt, B.D.; Baskin, D.S. Incidence, Etiology and Outcomes of Hyponatremia after Transsphenoidal Surgery: Experience with 344 Consecutive Patients at a Single Tertiary Center. J. Clin. Med. 2014, 3, 1199–1219. [Google Scholar] [CrossRef]
- Michelsen, C.F.; Svendsen, M.B.S.; Bagger, M.L.; Konradsen, H. A study on accuracy and precision of fluid volume measurements by nurses, patients and healthy persons in a clinical setting. Nurs. Open 2022, 9, 1303–1310. [Google Scholar] [CrossRef]
- Mann, S.J.; Gerber, L.M. Addressing the problem of inaccuracy of measured 24-hour urine collections due to incomplete collection. J. Clin. Hypertens. 2019, 21, 1626–1634. [Google Scholar] [CrossRef]
- Staiger, R.D.; Sarnthein, J.; Wiesli, P.; Schmid, C.; Bernays, R.L. Prognostic factors for impaired plasma sodium homeostasis after transsphenoidal surgery. Br. J. Neurosurg. 2013, 27, 63–68. [Google Scholar] [CrossRef]
- Torres, A.C.; Wickham, E.P.; Biskobing, D.M. Tolvaptan for the management of syndrome of inappropriate antidiuretic hormone secretion: Lessons learned in titration of dose. Endocr. Pract. 2011, 17, e97–e100. [Google Scholar] [CrossRef]
- Perez-Vega, C.; Tripathi, S.; Domingo, R.A.; Ramos-Fresnedo, A.; Lee, S.J.; Chaichana, K.L.; Samson, S.L. Fluid Restriction After Transsphenoidal Surgery for the Prevention of Delayed Hyponatremia: A Systematic Review and Meta-Analysis. Endocr. Pract. 2021, 27, 966–972. [Google Scholar] [CrossRef]



| Characteristics | Hyponatremic | Nonhyponatremic | p-Value |
|---|---|---|---|
| No. of patients | 9 | 52 | |
| Age (years) | 57 ± 10 | 50 ± 15 | 0.11 |
| Female (%) | 9 (100%) | 39 (75%) | 0.02 |
| BMI (kg/m2) | 24.26 (±2.8) | 27.45 (±5.13) | 0.02 |
| History of hypertension (%) | 4 (44.4%) | 17 (32.7%) | 0.71 |
| History of cardiovascular disease (%) | 1 (11.1%) | 2 (3.8%) | 0.39 |
| History of Diabetes mellitus (%) | 2 (22.2%) | 4 (7.7%) | 0.21 |
| History of thyroid disease (%) | 2 (22.2%) | 16 (30,8%) | 0.71 |
| Nonfunctioning adenoma (%) | 5 (55.5%) | 20 (38.4%) | NA |
| Prolactinoma (%) | 1 (11.1%) | 6 (11.5% | NA |
| Cushing disease (%) | 1 (11.1%) | 11 (21.1%) | NA |
| Acromegaly (%) | 2 (22.2%) | 6 (11.5%) | NA |
| TSHoma | 0 | 1 (1.9%) | NA |
| Rathke cleft cyst | 0 | 2 (3.8%) | NA |
| Chordoma | 0 | 1 (1.9%) | NA |
| Pituicytoma | 0 | 1 (1.9%) | NA |
| Arachnoidal cyst | 0 | 1 (1.9%) | NA |
| Meningeoma | 0 | 1 (1.9%) | NA |
| Cavernous Hemangioma | 0 | 1 (1.9%) | NA |
| Not classified Adenoma | 0 | 1 (1.9%) | NA |
| Tumor size (cm) | 1.9 × 1.5 × 1.5 | 1.5 × 1.2 × 1.5 | NA |
| Coronal | 1.98 ± 0.79 | 1.50 ± 0.78 | 0.12 |
| Sagittal | 1.53 ± 0.54 | 1.27 ± 0.62 | 0.21 |
| Cranio-Caudal | 1.58 ± 0.64 | 1.49 ± 0.94 | 0.70 |
| Serum sodium on POD 1 (mmol/L) | 139 ± 3 | 141 ± 2 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roser, P.; Mende, K.C.; Dimitriadis, G.K.; Mader, M.M.-D.; Aberle, J.; Flitsch, J.; Rotermund, R. The Potential of Self-Assessment and Associated Factors for Delayed Symptomatic Hyponatremia Following Transsphenoidal Surgery: A Single Center Experience. J. Clin. Med. 2023, 12, 306. https://doi.org/10.3390/jcm12010306
Roser P, Mende KC, Dimitriadis GK, Mader MM-D, Aberle J, Flitsch J, Rotermund R. The Potential of Self-Assessment and Associated Factors for Delayed Symptomatic Hyponatremia Following Transsphenoidal Surgery: A Single Center Experience. Journal of Clinical Medicine. 2023; 12(1):306. https://doi.org/10.3390/jcm12010306
Chicago/Turabian StyleRoser, Pia, Klaus Christian Mende, Georgios K. Dimitriadis, Marius Marc-Daniel Mader, Jens Aberle, Jörg Flitsch, and Roman Rotermund. 2023. "The Potential of Self-Assessment and Associated Factors for Delayed Symptomatic Hyponatremia Following Transsphenoidal Surgery: A Single Center Experience" Journal of Clinical Medicine 12, no. 1: 306. https://doi.org/10.3390/jcm12010306
APA StyleRoser, P., Mende, K. C., Dimitriadis, G. K., Mader, M. M.-D., Aberle, J., Flitsch, J., & Rotermund, R. (2023). The Potential of Self-Assessment and Associated Factors for Delayed Symptomatic Hyponatremia Following Transsphenoidal Surgery: A Single Center Experience. Journal of Clinical Medicine, 12(1), 306. https://doi.org/10.3390/jcm12010306







