Documentation of Drug-Related Problems with ICD-11: Application of the New WHO Code-Set to Clinical Routine Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions of Adverse Drug Reaction and Medication Error
2.2. Study Design and Origin of Drug-Related Problems
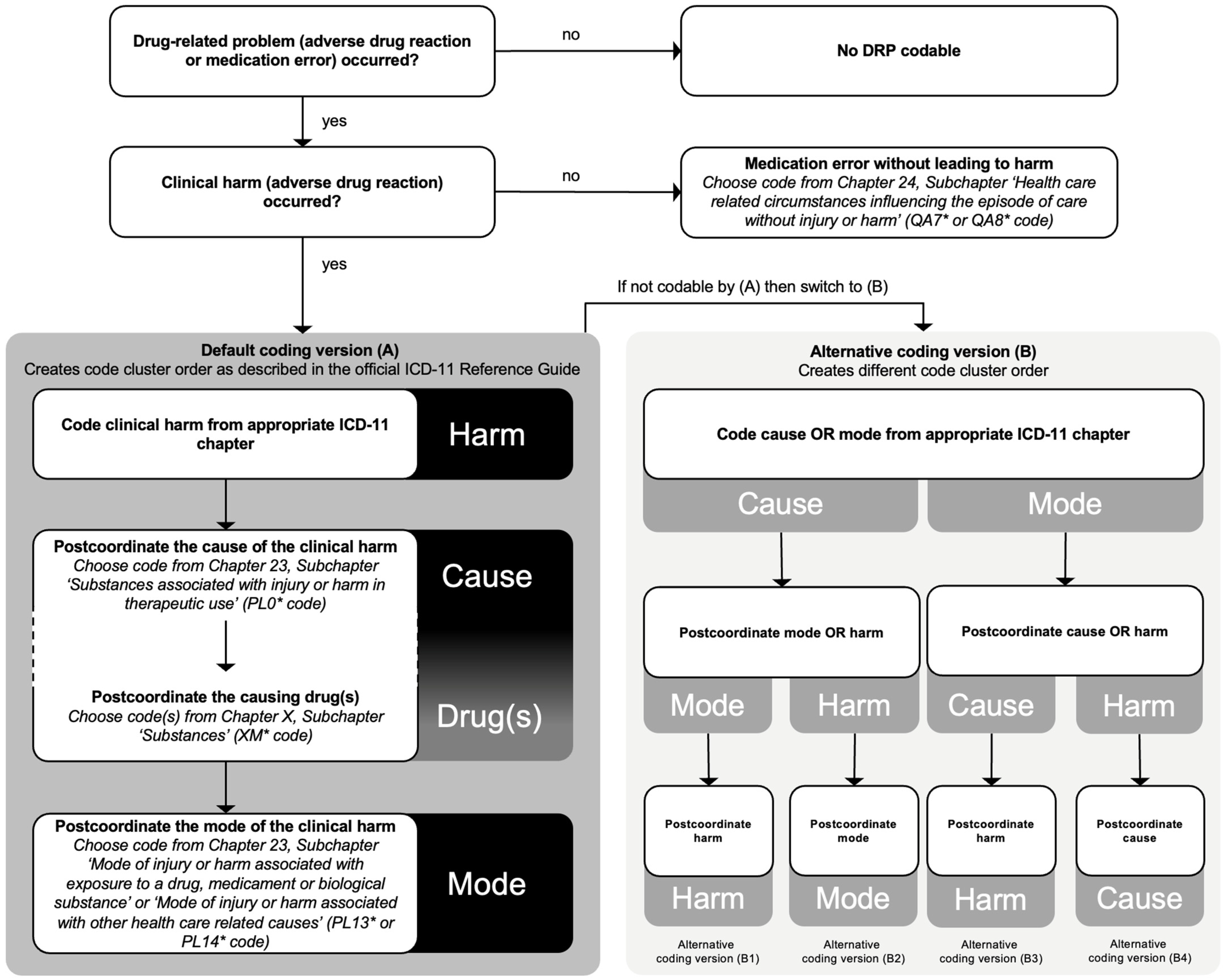
2.3. Coding of the Drug-Related Problems
2.4. Cluster Coding with the Three-Part Quality and Safety Model
2.5. Sanctioning Rules
2.6. Terminology Mapping with ISO/TR 12300:2014
3. Results
3.1. Coding of ADRs Using the Three-Part Quality and Safety Model
3.2. Terminology Matching of the ADRs with the ICD-11 Codes
3.3. Coding and Terminology Matching of the MEs with the ICD-11 Codes
4. Discussion
4.1. Related Work
4.2. Inter-Rater Reliability Using ICD-11
4.3. Errors of Omission and Missing Indication
4.4. Suggestions for Potential Improvements of ICD-11 Coding
4.5. Possible Applications of the New ICD-11 System
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medication without Harm—Global Patient Safety Challenge on Medication Safety; World Health Organization: Geneva, Switzerland, 2017; Licence: CC BY-NC-SA 3.0 IGO; Available online: https://apps.who.int/iris/rest/bitstreams/1083775/retrieve (accessed on 4 November 2022).
- Donaldson, L.J.; Kelley, E.T.; Dhingra-Kumar, N.; Kieny, M.P.; Sheikh, A. Medication without harm: WHO’s third global patient safety challenge. Lancet 2017, 389, 1680–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laatikainen, O.; Miettunen, J.; Sneck, S.; Lehtiniemi, H.; Tenhunen, O.; Turpeinen, M. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürkle, T.; Müller, F.; Patapovas, A.; Sonst, A.; Pfistermeister, B.; Plank-Kiegele, B.; Dormann, H.; Maas, R. A new approach to identify, classify and count drug-related events. Br. J. Clin. Pharmacol. 2013, 76 (Suppl. S1), 56–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stausberg, J.; Hasford, J. Identification of adverse drug events: The use of ICD-10 coded diagnoses in routine hospital data. Dtsch. Ärzteblatt Int. 2010, 107, 23–29. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems (ICD). Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 4 November 2022).
- Mabon, K.; Steinum, O.; Chute, C.G. Postcoordination of codes in ICD-11. BMC Med. Inform. Decis. Mak. 2022, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Southern, D.A.; Harrison, J.E.; Romano, P.S.; Le Pogam, M.A.; Pincus, H.A.; Ghali, W.A. The three-part model for coding causes and mechanisms of healthcare-related adverse events. BMC Med. Inform. Decis. Mak. 2022, 21, 376. [Google Scholar] [CrossRef] [PubMed]
- Drösler, S.E.; Weber, S.; Chute, C.G. ICD-11 extension codes support detailed clinical abstraction and comprehensive classification. BMC Med. Inform. Decis. Mak. 2021, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.J.; Bernard, B.; Drösler, S.E.; Gurevich, Y.; Harrison, J.; Januel, J.M.; Romano, P.S.; Southern, D.A.; Sundararajan, V.; Quan, H.; et al. A World Health Organization field trial assessing a proposed ICD-11 framework for classifying patient safety events. Int. J. Qual. Health Care 2017, 29, 548–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adverse Drug Reaction. Available online: https://www.ema.europa.eu/en/glossary/adverse-drug-reaction (accessed on 4 November 2022).
- Medication Errors. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medication-errors (accessed on 4 November 2022).
- Hennink, M.M.; Kaiser, B.N.; Marconi, V.C. Code saturation versus meaning saturation: How many interviews are enough? Qual. Health Res. 2017, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- ICD-11 Training Package. Available online: https://icdcdn.who.int/icd11training/index.html (accessed on 5 November 2022).
- ICD-11 Reference Guide. Available online: https://icdcdn.who.int/icd11referenceguide/en/html/index.html (accessed on 4 November 2022).
- Eastwood, C.A.; Khair, S.; Southern, D.A. Decision algorithm for when to use the ICD-11 3-part model for healthcare harms. BMC Med. Inform. Decis. Mak. 2022, 21, 380. [Google Scholar] [CrossRef] [PubMed]
- ICD-11 Coding Tool. Available online: https://icd.who.int/ct11/icd11_mms/en/release (accessed on 4 November 2022).
- Lou, Y.; Tu, S.W.; Nyulas, C.; Tudorache, T.; Chalmers, R.J.G.; Musen, M.A. Use of ontology structure and Bayesian models to aid the crowdsourcing of ICD-11 sanctioning rules. J. Biomed. Inform. 2017, 68, 20–34. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 12300:2014; Health Informatics—Principles of Mapping between Terminological Systems. ISO: Geneva, Switzerland, 2014. Available online: https://www.iso.org/standard/51344.html (accessed on 4 November 2022).
- Deutsche Kodierrichtlinien Version 2022. Available online: https://www.dkgev.de/fileadmin/default/DKR_2022.pdf (accessed on 4 November 2022).
- Fung, K.W.; Xu, J.; McConnell-Lamptey, S.; Pickett, D.; Bodenreider, O. Feasibility of replacing the ICD-10-CM with the ICD-11 for morbidity coding: A content analysis. J. Am. Med. Inform. Assoc. 2021, 28, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.M.; Kirley, E.M.; Rosen, M.A.; Winters, B.D. A comparison of two structured taxonomic strategies in capturing adverse events in U.S. hospitals. Health Serv. Res. 2019, 54, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, C.A.; Southern, D.A.; Khair, S.; Doktorchik, C.; Cullen, D.; Ghali, W.A.; Quan, H. Field testing a new ICD coding system: Methods and early experiences with ICD-11 Beta Version 2018. BMC Res. Notes 2022, 15, 343. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Small, S.S.; Wickham, M.E.; Cheng, V.; Balka, E.; Hohl, C.M. The utility of different data standards to document adverse drug event symptoms and diagnoses: Mixed methods study. J Med Internet Res 2021, 23, e27188. [Google Scholar] [CrossRef] [PubMed]
- ZOCOR®/ZOCOR® FORRTE. Fachinformation. Organon Healthcare GmbH, 2021. Available online: https://www.fachinfo.de/api/fachinfo/pdf/004748 (accessed on 4 November 2022).
- The Use of the WHO-UMC System for Standardised Case Causality Assessment. Available online: https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf (accessed on 4 November 2022).
- Classification for Drug Related Problems V9.1. Available online: https://www.pcne.org/upload/files/417_PCNE_classification_V9-1_final.pdf (accessed on 4 November 2022).
- Molokhia, M.; Pathak, A.; Lapeyre-Mestre, M.; Caturla, L.; Montastruc, J.L.; McKeigue, P. Case ascertainment and estimated incidence of drug-induced long-QT syndrome: Study in Southwest France. Br. J. Clin. Pharmacol. 2008, 66, 386–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrendt, C.A.; Heidemann, F.; Rieß, H.C.; Stoberock, K.; Debus, S.E. Registry and health insurance claims data in vascular research and quality improvement. Vasa 2017, 46, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sozialgesetzbuch (SGB) Fünftes Buch (V)—Gesetzliche Krankenversicherung—(Artikel 1 des Gesetzes v. 20. Dezember 1988, BGBl. I S. 2477): §129 Rahmenvertrag Über Die Arzneimittelversorgung, Verordnungsermächtigung. Available online: https://www.gesetze-im-internet.de/sgb_5/__129.html (accessed on 4 November 2022).
- Wushouer, H.; Shi, L.; Guan, X.; Cui, Y. China starts to pay for pharmaceutical services. J. Clin. Pharm. Ther. 2022, 47, 1079–1080. [Google Scholar] [CrossRef] [PubMed]


| DRP | Harm | Cause | Drug(s) | Mode | ICD-11 Code Cluster Order as Described in Reference Guide | Alternative ICD-11 Code Cluster Orders |
|---|---|---|---|---|---|---|
| Osteoporosis due to prednisolone treatment without clinically justifiable indication | FB83.13 Drug-induced osteoporosis | PL00 Drugs, medicaments, or biological substances associated with injury or harm in therapeutic use | XM6JJ4 Prednisolone | PL13.Y Other specified mode of injury or harm associated with exposure to a drug, medicament, or biological substance | FB83.13/PL00&XM6JJ4/PL13.Y | FB83.13/PL13.Y/PL00&XM6JJ4 PL00&XM6JJ4/FB83.13/PL13.Y PL00&XM6JJ4/PL13.Y/FB83.13 PL13.Y/FB83.13/PL00&XM6JJ4 PL13.Y/PL00&XM6JJ4/FB83.13 |
| Hypokalaemia due to hydrochlorothiazide treatment | 5C77 Hypokalaemia | PL00 Drugs, medicaments, or biological substances associated with injury or harm in therapeutic use | XM6910 Hydrochlorothiazide | PL13.2 Drug-related injury or harm in the context of correct administration or dosage, as mode of injury or harm | 5C77 (no postcoordination allowed) | PL00&XM6910/5C77/PL13.2 PL00&XM6910/PL13.2/5C77 PL13.2/5C77/PL00&XM6910 PL13.2/PL00&XM6910/5C77 |
| Contraindication: Metformin treatment in combination with GFR < 30 mL/min | N.A. | N.A. | N.A. | QA77 Medication or substance that is known to be contraindicated for the patient without injury or harm | QA77 (no postcoordination allowed) | N.A. |
| Equivalence Degree Scale ISO/TR 12300:2014 | Definition | Example Mapping Case of Source and Target Terms (i.e., ICD-11 Term) |
|---|---|---|
| 1 | Equivalence of meaning: lexical and conceptual; | Hyperkalaemia and Hyperkalaemia |
| 2 | Equivalence of meaning, but with synonym; | Hypocalcaemia and Calcium deficiency |
| 3 | Source term is broader and has less specific meaning than target term; | Leukopenia and Neutropenia |
| 4 | Source term is narrower and has more specific meaning than target term; | QTc interval prolongation and cardiac arrhythmia |
| 5 | No mapping possible. No term was found in the target with some degree of equivalence. | - |
| Harm ICD-11 Code | Meaning | Reason, Which Prevents Full Application of the Three-Part Model |
|---|---|---|
| 1F23.2 | Candidosis of gastrointestinal tract | Postcoordination allowed, but not for the corresponding cause and mode |
| 4B00.0Z | Neutropenia, unspecified | Postcoordincation generally not allowed |
| 5A02.4 | Thyrotoxicosis factitia | Postcoordincation generally not allowed |
| 5B5K.1Z | Calcium deficiency, unspecified | Postcoordincation generally not allowed |
| 5C71 | Hyperosmolality or hypernatraemia | Postcoordincation generally not allowed |
| 5C72 | Hypo-osmolality or hyponatraemia | Postcoordincation generally not allowed |
| 5C76 | Hyperkalaemia | Postcoordincation generally not allowed |
| 5C77 | Hypokalaemia | Postcoordincation generally not allowed |
| 6C44.20 | Sedative, hypnotic or anxiolytic dependence, current use | Postcoordincation for the cause allowed, but not for the corresponding mode |
| 6C4F.5 | Delirium induced by multiple specified psychoactive substances including medications | Postcoordincation for the cause allowed, but not for the corresponding mode |
| 8A63.Y | Seizure due to other acute cause | Postcoordincation for the cause allowed, but not for the corresponding mode |
| BA21 | Orthostatic hypotension | Postcoordincation generally not allowed |
| BD1Z | Heart failure, unspecified | Postcoordination allowed, but not for the corresponding cause and mode |
| BD4Z | Chronic arterial occlusive disease, unspecified | Postcoordination allowed, but not for the corresponding cause and mode |
| DB95.Z | Drug-induced or toxic liver disease, unspecified | Postcoordincation for the cause allowed, but not for the corresponding mode |
| GB60.2 | Acute kidney failure, stage 3 | Postcoordination allowed, but not for the corresponding cause and mode |
| MA10.0 | Elevation of levels of transaminase or lactic acid dehydrogenase | Postcoordincation generally not allowed |
| MA10.1 | Abnormal levels of other specified serum enzymes | Postcoordincation generally not allowed |
| MB48.0Y | Other specified vertigo | Postcoordincation generally not allowed |
| MC81.1 | Bradycardia, unspecified | Postcoordincation generally not allowed |
| MD11.5 | Dyspnoea | Postcoordincation generally not allowed |
| MD90.0 | Nausea | Postcoordincation generally not allowed |
| ME05.0 | Constipation | Postcoordincation generally not allowed |
| ME24.9Z | Gastrointestinal bleeding, unspecified | Postcoordination allowed, but not for the corresponding cause and mode |
| ME24.A1 | Haemorrhage of anus and rectum | Postcoordination allowed, but not for the corresponding cause and mode |
| ME24.A5 | Haematemesis | Postcoordination allowed, but not for the corresponding cause and mode |
| MF50.3 | Retention of urine | Postcoordincation generally not allowed |
| MG29.00 | Ankle oedema | Postcoordincation generally not allowed |
| ADR | ME | ||||
|---|---|---|---|---|---|
| Equivalence Degree Scale ISO/TR 12300:2014 | Harm (Codable n = 50) | Cause (Codable n = 19) | Drug (Codable n = 32) | Mode (Codable n = 15) | Mode (Codable n = 50) |
| 1 Equivalence of meaning: lexical and conceptual | 20 (40.0%) | 0 (0.0%) | 31 (96.9%) | 0 (0.0%) | 0 (0.0%) |
| 2 Equivalence of meaning, but with synonym | 13 (26.0%) | 19 (100.0%) | 1 (3.1%) | 14 (93.3%) | 41 (82.0%) |
| 3 Source term is broader and has less specific meaning than target term | 4 (8.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 4 Source term is narrower and has more specific meaning than target term | 13 (26.0%) | 0 (0.0%) | 0 (0.0%) | 1 (6.7%) | 9 (18.0%) |
| 5 No mapping possible. No term was found in the target with some degree of equivalence | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| ME without Injury or Harm | Suggested Change |
|---|---|
| Halving of tablets although not allowed | Consider additional code for ‘Inappropriate halving of tablets without injury or harm’ |
| Duplicate prescription | Consider additional code for ‘Duplicate prescription without injury or harm’ |
| Prescription with missing indication | Consider additional code for ‘Medication without clinically justifiable indication without injury or harm’ |
| Wrong dosing interval | Consider splitting the code QA74: Unspecified appropriateness of dosing or administration without injury or harm (‘Inappropriateness of dosing without injury or harm’ and ‘Inappropriateness of administration without injury or harm’) |
| Missing therapeutic drug monitoring | Consider additional code for ‘Clinically required therapeutic drug monitoring omitted or missing without injury or harm’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrikyan, W.; Jung-Poppe, L.; Altenbuchner, A.; Nicolaus, H.F.; Pfistermeister, B.; Dormann, H.; Fromm, M.F.; Maas, R. Documentation of Drug-Related Problems with ICD-11: Application of the New WHO Code-Set to Clinical Routine Data. J. Clin. Med. 2023, 12, 315. https://doi.org/10.3390/jcm12010315
Andrikyan W, Jung-Poppe L, Altenbuchner A, Nicolaus HF, Pfistermeister B, Dormann H, Fromm MF, Maas R. Documentation of Drug-Related Problems with ICD-11: Application of the New WHO Code-Set to Clinical Routine Data. Journal of Clinical Medicine. 2023; 12(1):315. https://doi.org/10.3390/jcm12010315
Chicago/Turabian StyleAndrikyan, Wahram, Lea Jung-Poppe, Anna Altenbuchner, Hagen Fabian Nicolaus, Barbara Pfistermeister, Harald Dormann, Martin F. Fromm, and Renke Maas. 2023. "Documentation of Drug-Related Problems with ICD-11: Application of the New WHO Code-Set to Clinical Routine Data" Journal of Clinical Medicine 12, no. 1: 315. https://doi.org/10.3390/jcm12010315
APA StyleAndrikyan, W., Jung-Poppe, L., Altenbuchner, A., Nicolaus, H. F., Pfistermeister, B., Dormann, H., Fromm, M. F., & Maas, R. (2023). Documentation of Drug-Related Problems with ICD-11: Application of the New WHO Code-Set to Clinical Routine Data. Journal of Clinical Medicine, 12(1), 315. https://doi.org/10.3390/jcm12010315






