FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review
Abstract
:1. Introduction
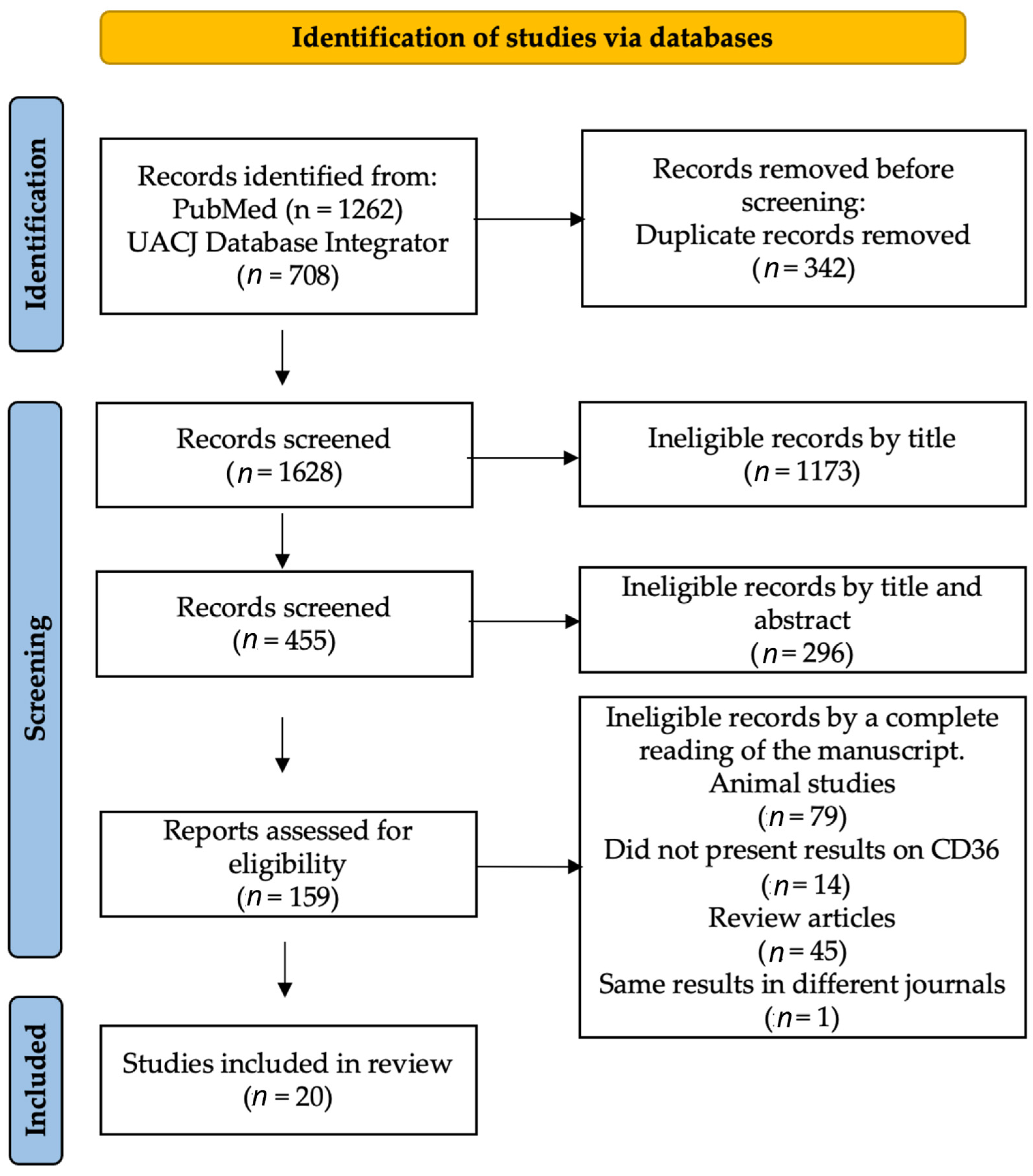
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.2.1. Type of Study
2.2.2. Type of Participants
2.2.3. Type of Interventions
2.3. Outcomes
2.4. Data Synthesis
3. Results
3.1. Descriptive Analysis
3.2. Interventions Analysis
- the lack of information on the eligibility criteria;
- not mentioning the measures taken to avoid or reduce possible bias, and;
- not explaining how to determine the sample size and randomization.
4. Discussion
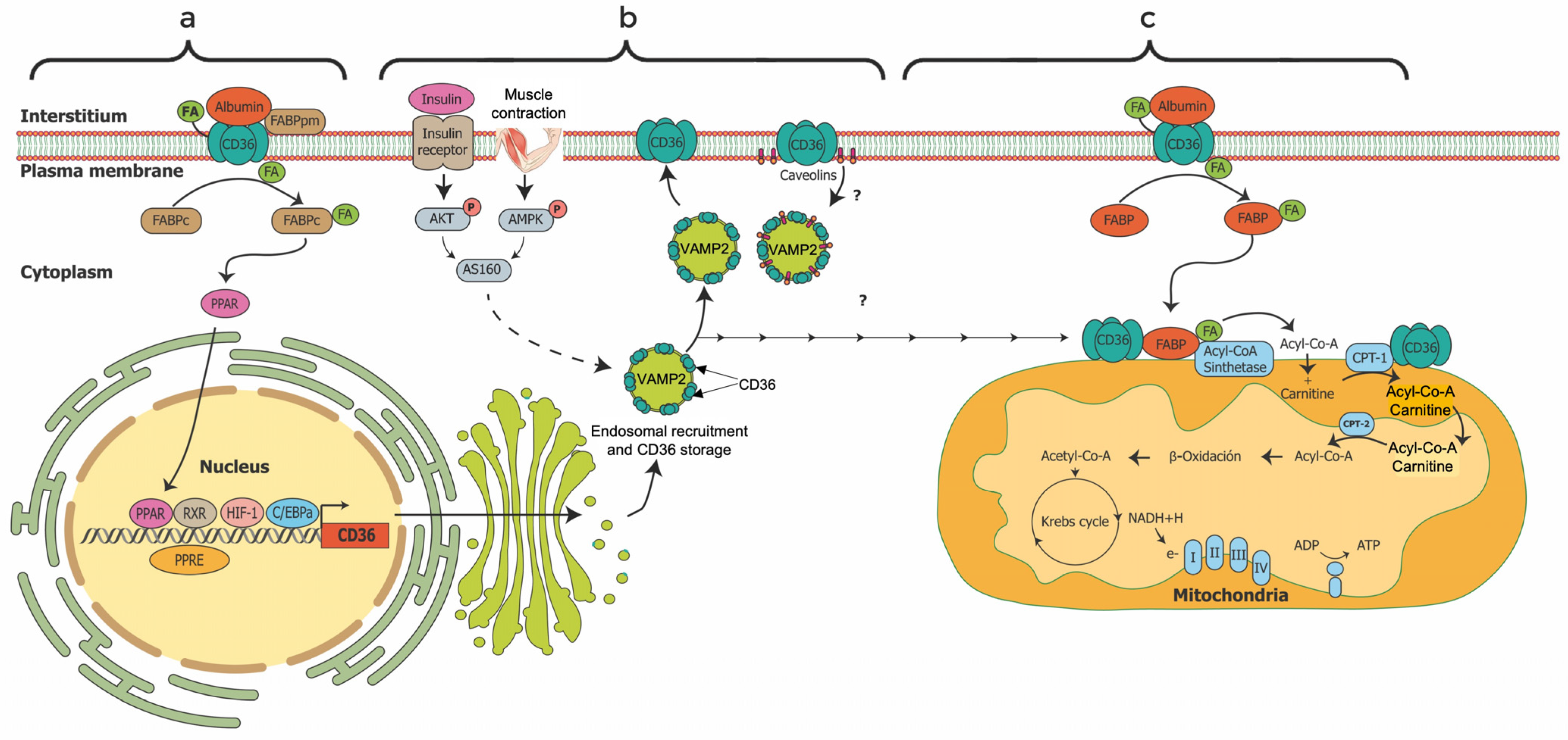
4.1. Characterization and Localization of FAT/CD36
4.2. Regulation and Functions of FAT/CD36
4.3. Gene Expression and Molecular Heterogeneity of FAT/CD36
4.4. Effect of Physical Exercise and Nutrients on FAT/CD36 Regulation and Energy Expenditure
4.4.1. Physical Exercise
4.4.2. Diet
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| β-HAD | β-hydroxyacyl-CoA dehydrogenase |
| AMPK | 5-AMP-activated protein kinase |
| CPT 1 | Carnitine Palmitoyl Transferase 1 |
| CS | Citrate Synthase |
| Cox IV | Cytochrome C oxidase IV |
| ERK | Extracellular Signaling Receptor Kinase. |
| FABP | Fatty Acid-Binding Protein |
| FABP | Plasma Membrane Fatty Acid-Binding Protein. |
| FAs | Fatty acids |
| FAT/CD36/SR-B2 | Fatty Acid Translocase/Cluster of Differentiation 36/Scavenger Receptor Proteins class B2 |
| FATP | Long-chain fatty acid transport protein |
| GLUT | Glucose transport |
| HFD | High fat diet |
| HIIT | High intensity interval training |
| HSL | Hormone-Sensitive Lipase |
| IGT | Impaired glucose tolerance |
| LCFAs | Long-chain Fatty acids |
| LFD | Low fat diet |
| LPL | Lipoprotein Lipase |
| MCT | Monocarboxylate Transporter |
| MFO | Maximal Fat Oxidation |
| PGC | Peroxisome Proliferator-Activated Receptor Gamma Coactivator |
| PDK | Pyruvate Dehydrogenase Kinase |
| PKC | Protein Kinase C |
| PPAR | Peroxisome Proliferator-Activated Receptors |
| PPRE | PPAR response element |
| RER | Respiratory exchange ratio |
| RQ | Quotient respiratory |
| RXR | Retinoid X receptor |
| SNPs | Single Nucleotide polymorphisms |
| UCP | Uncoupled Protein |
| VO2max | Maximal consumption of O2 |
| VT | Ventilatory threshold |
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and Cardiovascular Disease: Revisiting an Old Relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.R., 2nd; Boyle, K.E.; Koves, T.R.; Ilkayeva, O.R.; Muoio, D.M.; Houmard, J.A.; Friedman, J.E. Metabolomic Analysis Reveals Altered Skeletal Muscle Amino Acid and Fatty Acid Handling in Obese Humans. Obes. Silver Spring MD 2015, 23, 981–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines Formedical Care of Patients with Obesity. Endocr. Pract. 2016, 22, 1–203. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Poirier, P.; Després, J.-P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Fock, K.M.; Khoo, J. Diet and Exercise in Management of Obesity and Overweight. J. Gastroenterol. Hepatol. 2013, 28, 59–63. [Google Scholar] [CrossRef]
- Shaw, K.A.; Gennat, H.C.; O’Rourke, P.; Del Mar, C. Exercise for Overweight or Obesity. Cochrane Database Syst. Rev. 2006, 4, CD003817. [Google Scholar] [CrossRef] [Green Version]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Body Composition in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Frandsen, J.; Sahl, R.E.; Rømer, T.; Hansen, M.T.; Nielsen, A.B.; Lie-Olesen, M.M.; Rasmusen, H.K.; Søgaard, D.; Ingersen, A.; Rosenkilde, M.; et al. Extreme Duration Exercise Affects Old and Younger Men Differently. Acta Physiol. Oxf. Engl. 2022, 235, e13816. [Google Scholar] [CrossRef]
- Glatz, J.F.; Nabben, M.; Luiken, J.J. CD36 (SR-B2) as Master Regulator of Cellular Fatty Acid Homeostasis. Curr. Opin. Lipidol. 2022, 33, 103. [Google Scholar] [CrossRef]
- Holloway, G.P.; Bezaire, V.; Heigenhauser, G.J.F.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A.; Spriet, L.L. Mitochondrial Long Chain Fatty Acid Oxidation, Fatty Acid Translocase/CD36 Content and Carnitine Palmitoyltransferase I Activity in Human Skeletal Muscle during Aerobic Exercise. J. Physiol. 2006, 571, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Jauch-Chara, K.; Oltmanns, K.M. Obesity—A Neuropsychological Disease? Systematic Review and Neuropsychological Model. Prog. Neurobiol. 2014, 114, 84–101. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Bavisotto, C.C.; Gammazza, A.M.; Paladino, L.; Carlisi, D.; Cappello, F.; de Macario, E.C.; Macario, A.J.L.; Lauricella, M. Lipid Chaperones and Associated Diseases: A Group of Chaperonopathies Defining a New Nosological Entity with Implications for Medical Research and Practice. Cell Stress Chaperones 2020, 25, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.B.; Peterson, C.M.; Bourgeois, B.; Thomas, D.M.; Gallagher, D.; Strauss, B.; Müller, M.J.; Bosy-Westphal, A. Human Energy Expenditure: Advances in Organ-tissue Prediction Models. Obes. Rev. 2018, 19, 1177–1188. [Google Scholar] [CrossRef]
- Boyle, K.E.; Canham, J.P.; Consitt, L.A.; Zheng, D.; Koves, T.R.; Gavin, T.P.; Holbert, D.; Neufer, P.D.; Ilkayeva, O.; Muoio, D.M.; et al. A High-Fat Diet Elicits Differential Responses in Genes Coordinating Oxidative Metabolism in Skeletal Muscle of Lean and Obese Individuals. J. Clin. Endocrinol. Metab. 2011, 96, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-Function of CD36 and Importance of Fatty Acid Signal Transduction in Fat Metabolism. Annu. Rev. Nutr. 2014, 34, 281. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.C.; Luiken, J.J.F.P. From Fat to FAT (CD36/SR-B2): Understanding the Regulation of Cellular Fatty Acid Uptake. Biochimie 2017, 136, 21–26. [Google Scholar] [CrossRef]
- Battaglia, G.M.; Zheng, D.; Hickner, R.C.; Houmard, J.A. Effect of Exercise Training on Metabolic Flexibility in Response to a High-Fat Diet in Obese Individuals. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1440–E1445. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Universidad Autónoma de Ciudad Juárez Biblioteca Virtual (BIVIR): Institutional Database Integrator. Available online: https://www.uacj.mx/bibliotecas/BIVIR/index.html (accessed on 15 December 2022).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int. J. Surg. Lond. Engl. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Bergouignan, A.; Gozansky, W.S.; Barry, D.W.; Leitner, W.; MacLean, P.S.; Hill, J.O.; Draznin, B.; Melanson, E.L. Increasing Dietary Fat Elicits Similar Changes in Fat Oxidation and Markers of Muscle Oxidative Capacity in Lean and Obese Humans. PLoS ONE 2012, 7, e30164. [Google Scholar] [CrossRef] [PubMed]
- Cameron-Smith, D.; Burke, L.M.; Angus, D.J.; Tunstall, R.J.; Cox, G.R.; Bonen, A.; Hawley, J.A.; Hargreaves, M. A Short-Term, High-Fat Diet up-Regulates Lipid Metabolism and Gene Expression in Human Skeletal Muscle. Am. J. Clin. Nutr. 2003, 77, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, I.-S.; Liao, S.-F.; Liu, K.-L.; Liu, H.-Y.; Wu, C.-L.; Huang, C.-Y.; Mallikarjuna, K.; Smith, R.W.; Kuo, C.-H. Effect of Dietary Glycemic Index on Substrate Transporter Gene Expression in Human Skeletal Muscle after Exercise. Eur. J. Clin. Nutr. 2009, 63, 1404–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerling, C.J.; Whitfield, J.; Mukai, K.; Spriet, L.L. Variable Effects of 12 Weeks of Omega-3 Supplementation on Resting Skeletal Muscle Metabolism. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2014, 39, 1083–1091. [Google Scholar] [CrossRef]
- Greene, N.P.; Fluckey, J.D.; Lambert, B.S.; Greene, E.S.; Riechman, S.E.; Crouse, S.F. Regulators of Blood Lipids and Lipoproteins? PPARδ and AMPK, Induced by Exercise, Are Correlated with Lipids and Lipoproteins in Overweight/Obese Men and Women. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1212–E1221. [Google Scholar] [CrossRef] [Green Version]
- Heilbronn, L.K.; Gregersen, S.; Shirkhedkar, D.; Hu, D.; Campbell, L.V. Impaired Fat Oxidation after a Single High-Fat Meal in Insulin-Sensitive Nondiabetic Individuals with a Family History of Type 2 Diabetes. Diabetes 2007, 56, 2046–2053. [Google Scholar] [CrossRef] [Green Version]
- Holloway, G.P.; Thrush, A.B.; Heigenhauser, G.J.F.; Tandon, N.N.; Dyck, D.J.; Bonen, A.; Spriet, L.L. Skeletal Muscle Mitochondrial FAT/CD36 Content and Palmitate Oxidation Are Not Decreased in Obese Women. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1782–E1789. [Google Scholar] [CrossRef] [Green Version]
- Jordy, A.B.; Serup, A.K.; Karstoft, K.; Pilegaard, H.; Kiens, B.; Jeppesen, J. Insulin Sensitivity Is Independent of Lipid Binding Protein Trafficking at the Plasma Membrane in Human Skeletal Muscle: Effect of a 3-Day, High-Fat Diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1136–R1145. [Google Scholar] [CrossRef] [Green Version]
- Kiens, B.; Roepstorff, C.; Glatz, J.F.C.; Bonen, A.; Schjerling, P.; Knudsen, J.; Nielsen, J.N. Lipid-Binding Proteins and Lipoprotein Lipase Activity in Human Skeletal Muscle: Influence of Physical Activity and Gender. J. Appl. Physiol. Bethesda MD 1985 2004, 97, 1209–1218. [Google Scholar] [CrossRef] [Green Version]
- Maunder, E.; Plews, D.J.; Wallis, G.A.; Brick, M.J.; Leigh, W.B.; Chang, W.-L.; Stewart, T.; Watkins, C.M.; Kilding, A.E. Peak Fat Oxidation Is Positively Associated with Vastus Lateralis CD36 Content, Fed-State Exercise Fat Oxidation, and Endurance Performance in Trained Males. Eur. J. Appl. Physiol. 2022, 122, 93–102. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. High-Intensity Aerobic Interval Training Increases Fat and Carbohydrate Metabolic Capacities in Human Skeletal Muscle. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2008, 33, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Horowitz, J.F. Coimmunoprecipitation of FAT/CD36 and CPT I in Skeletal Muscle Increases Proportionally with Fat Oxidation after Endurance Exercise Training. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E254–E260. [Google Scholar] [CrossRef] [PubMed]
- Talanian, J.L.; Galloway, S.D.R.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Two Weeks of High-Intensity Aerobic Interval Training Increases the Capacity for Fat Oxidation during Exercise in Women. J. Appl. Physiol. Bethesda MD 1985 2007, 102, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Talanian, J.L.; Holloway, G.P.; Snook, L.A.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Exercise Training Increases Sarcolemmal and Mitochondrial Fatty Acid Transport Proteins in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E180–E188. [Google Scholar] [CrossRef] [Green Version]
- Tunstall, R.J.; Mehan, K.A.; Wadley, G.D.; Collier, G.R.; Bonen, A.; Hargreaves, M.; Cameron-Smith, D. Exercise Training Increases Lipid Metabolism Gene Expression in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E66–E72. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.L.; Hunter, G.R.; Gower, B.A.; Bamman, M.M.; Windham, S.T.; Moellering, D.R.; Fisher, G. Exercise Effects on Mitochondrial Function and Lipid Metabolism during Energy Balance. Med. Sci. Sports Exerc. 2020, 52, 827. [Google Scholar] [CrossRef]
- Bezaire, V.; Bruce, C.R.; Heigenhauser, G.J.F.; Tandon, N.N.; Glatz, J.F.C.; Luiken, J.J.J.F.; Bonen, A.; Spriet, L.L. Identification of Fatty Acid Translocase on Human Skeletal Muscle Mitochondrial Membranes: Essential Role in Fatty Acid Oxidation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E509–E515. [Google Scholar] [CrossRef]
- PrabhuDas, M.R.; Baldwin, C.L.; Bollyky, P.L.; Bowdish, D.M.E.; Drickamer, K.; Febbraio, M.; Herz, J.; Kobzik, L.; Krieger, M.; Loike, J.; et al. A Consensus Definitive Classification of Scavenger Receptors and Their Roles in Health and Disease. J. Immunol. Baltim. MD 1950 2017, 198, 3775–3789. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ruiz, E.; Armesilla, A.L.; Sánchez-Madrid, F.; Vega, M.A. Gene Encoding the Collagen Type I and Thrombospondin Receptor CD36 Is Located on Chromosome 7q11. 2. Genomics 1993, 17, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Rać, M.E.; Safranow, K.; Poncyljusz, W. Molecular Basis of Human CD36 Gene Mutations. Mol. Med. 2007, 13, 288–296. [Google Scholar] [CrossRef]
- Su, X.; Abumrad, N.A. Cellular Fatty Acid Uptake: A Pathway under Construction. Trends Endocrinol. Metab. 2009, 20, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, U.; Elumalai, S.; Moon, J.-S.; Won, K.-C. CD36 Signal Transduction in Metabolic Diseases: Novel Insights and Therapeutic Targeting. Cells 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Jain, S.S.; Rimbaud, S.; Dam, A.; Quadrilatero, J.; Ventura-Clapier, R.; Bonen, A.; Holloway, G.P. FAT/CD36 Is Located on the Outer Mitochondrial Membrane, Upstream of Long-Chain Acyl-CoA Synthetase, and Regulates Palmitate Oxidation. Biochem. J. 2011, 437, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Wu, F.; Chen, M.; Li, Y.; You, M.; Zhang, Y.; Yang, P.; Wei, L.; Ruan, X.Z.; Zhao, L.; et al. Inhibition of Fatty Acid Translocase (FAT/CD36) Palmitoylation Enhances Hepatic Fatty Acid β-Oxidation by Increasing Its Localization to Mitochondria and Interaction with Long-Chain Acyl-CoA Synthetase 1. Antioxid. Redox Signal. 2022, 36, 1081–1100. [Google Scholar] [CrossRef] [PubMed]
- Holloway, G.P. The Role of Protein-Mediated Transport in Regulating Mitochondrial Long-Chain Fatty Acid Oxidation. Appl. Physiol. Nutr. Metab. 2008, 33, 141–142. [Google Scholar] [CrossRef] [Green Version]
- Holloway, G.P.; Bonen, A.; Spriet, L.L. Regulation of Skeletal Muscle Mitochondrial Fatty Acid Metabolism in Lean and Obese Individuals. Am. J. Clin. Nutr. 2009, 89, 455S–462S. [Google Scholar] [CrossRef] [Green Version]
- Arkinstall, M.J.; Tunstall, R.J.; Cameron-Smith, D.; Hawley, J.A. Regulation of Metabolic Genes in Human Skeletal Muscle by Short-Term Exercise and Diet Manipulation. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E25–E31. [Google Scholar] [CrossRef] [Green Version]
- Jordy, A.B.; Kiens, B. Regulation of Exercise-Induced Lipid Metabolism in Skeletal Muscle. Exp. Physiol. 2014, 99, 1586–1592. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.K.; Bonen, A.; Holloway, G.P. A Dual Mechanism of Action for Skeletal Muscle FAT/CD36 during Exercise. Exerc. Sport Sci. Rev. 2012, 40, 211–217. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, M.; Huang, W.; Chen, W.; Zhao, Y.; Schulte, M.L.; Volberding, P.; Gerbec, Z.; Zimmermann, M.T.; Zeighami, A. Mitochondrial Metabolic Reprogramming by CD36 Signaling Drives Macrophage Inflammatory Responses. Circ. Res. 2019, 125, 1087–1102. [Google Scholar] [CrossRef]
- Geloen, A.; Helin, L.; Geeraert, B.; Malaud, E.; Holvoet, P.; Marguerie, G. CD36 Inhibitors Reduce Postprandial Hypertriglyceridemia and Protect against Diabetic Dyslipidemia and Atherosclerosis. PLoS ONE 2012, 7, e37633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoosdally, S.J.; Andress, E.J.; Wooding, C.; Martin, C.A.; Linton, K.J. The Human Scavenger Receptor CD36: Glycosylation Status and Its Role in Trafficking and Function. J. Biol. Chem. 2009, 284, 16277–16288. [Google Scholar] [CrossRef] [Green Version]
- Momken, I.; Chabowski, A.; Dirkx, E.; Nabben, M.; Jain, S.S.; McFarlan, J.T.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. A New Leptin-Mediated Mechanism for Stimulating Fatty Acid Oxidation: A Pivotal Role for Sarcolemmal FAT/CD36. Biochem. J. 2017, 474, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-W.; Wang, J.; Guo, H.; Zhao, Y.-Y.; Sun, H.-H.; Li, Y.-F.; Lai, X.-Y.; Zhao, N.; Wang, X.; Xie, C. CD36 Facilitates Fatty Acid Uptake by Dynamic Palmitoylation-Regulated Endocytosis. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A.; Campbell, S.E.; Benton, C.R.; Chabowski, A.; Coort, S.L.M.; Han, X.-X.; Koonen, D.P.Y.; Glatz, J.F.C.; Luiken, J.J.F.P. Regulation of Fatty Acid Transport by Fatty Acid Translocase/CD36. Proc. Nutr. Soc. 2004, 63, 245–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luiken, J.J.F.P.; Dyck, D.J.; Han, X.-X.; Tandon, N.N.; Arumugam, Y.; Glatz, J.F.C.; Bonen, A. Insulin Induces the Translocation of the Fatty Acid Transporter FAT/CD36 to the Plasma Membrane. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E491–E495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.-Y.; Lee, T.; Jeong, Y. High-Fat Diet Increases Fat Oxidation and Promotes Skeletal Muscle Fatty Acid Transporter Expression in Exercise-Trained Mice. J. Med. Food 2020, 23, 281–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.E.; Tandon, N.N.; Woldegiorgis, G.; Luiken, J.J.F.P.; Glatz, J.F.C.; Bonen, A. A Novel Function for Fatty Acid Translocase (FAT)/CD36: Involvement in Long Chain Fatty Acid Transfer into the Mitochondria. J. Biol. Chem. 2004, 279, 36235–36241. [Google Scholar] [CrossRef] [Green Version]
- Maréchal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARγ Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef] [Green Version]
- Glatz, J.F.C.; Luiken, J.J.F.P. Dynamic Role of the Transmembrane Glycoprotein CD36 (SR-B2) in Cellular Fatty Acid Uptake and Utilization. J. Lipid Res. 2018, 59, 1084–1093. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK Activation by CD36 Links Fatty Acid Uptake to β-Oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monaco, C.; Whitfield, J.; Jain, S.S.; Spriet, L.L.; Bonen, A.; Holloway, G.P. Activation of AMPKα2 Is Not Required for Mitochondrial FAT/CD36 Accumulation during Exercise. PLoS ONE 2015, 10, e0126122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Jay, A.; Brunaldi, K.; Huang, N.; Hamilton, J.A. CD36 Enhances Fatty Acid Uptake by Increasing the Rate of Intracellular Esterification but Not Transport across the Plasma Membrane. Biochemistry 2013, 52, 7254–7261. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in Chronic Kidney Disease: Novel Insights and Therapeutic Opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Luiken, J.J.; Chanda, D.; Nabben, M.; Neumann, D.; Glatz, J.F. Post-Translational Modifications of CD36 (SR-B2): Implications for Regulation of Myocellular Fatty Acid Uptake. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2016, 1862, 2253–2258. [Google Scholar] [CrossRef]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R. Platelet CD36 Links Hyperlipidemia, Oxidant Stress and a Prothrombotic Phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef] [Green Version]
- Dobri, A.-M.; Dudău, M.; Enciu, A.-M.; Hinescu, M.E. CD36 in Alzheimer’s Disease: An Overview of Molecular Mechanisms and Therapeutic Targeting. Neuroscience 2021, 453, 301–311. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in Human Myocardial Long-Chain Fatty Acid Uptake Is Caused by FAT/CD36 Mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef]
- Jayewardene, A.F.; Mavros, Y.; Gwinn, T.; Hancock, D.P.; Rooney, K.B. Associations between CD36 Gene Polymorphisms and Metabolic Response to a Short-Term Endurance-Training Program in a Young-Adult Population. Appl. Physiol. Nutr. Metab. 2016, 41, 157–167. [Google Scholar] [CrossRef]
- Koonen, D.P.; Jensen, M.K.; Handberg, A. Soluble CD36—A Marker of the (Pathophysiological) Role of CD36 in the Metabolic Syndrome? Arch. Physiol. Biochem. 2011, 117, 57–63. [Google Scholar] [CrossRef]
- Aslankeser, Z.; Balcı, Ş.S. Re-Examination of the Contribution of Substrates to Energy Expenditure during High-Intensity Intermittent Exercise in Endurance Athletes. PeerJ 2017, 5, e3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundsgaard, A.-M.; Kiens, B. Gender Differences in Skeletal Muscle Substrate Metabolism–Molecular Mechanisms and Insulin Sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The Regulation of Fat Metabolism during Aerobic Exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef] [PubMed]
- Pelsers, M.M.A.L.; Stellingwerff, T.; van Loon, L.J.C. The Role of Membrane Fatty-Acid Transporters in Regulating Skeletal Muscle Substrate Use during Exercise. Sports Med. Auckl. NZ 2008, 38, 387–399. [Google Scholar] [CrossRef]
- Yanai, H.; Watanabe, I.; Ishii, K.; Morimoto, M.; Fujiwara, H.; Yoshida, S.; Hui, S.-P.; Matsuno, K.; Chiba, H. Attenuated Aerobic Exercise Capacity in CD36 Deficiency. J. Med. Genet. 2007, 44, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.J.; Kashyap, S.R. Pathogenic Role of Scavenger Receptor CD36 in the Metabolic Syndrome and Diabetes. Metab. Syndr. Relat. Disord. 2011, 9, 239–245. [Google Scholar] [CrossRef]
- Welle, S.; Tawil, R.; Thornton, C.A. Sex-Related Differences in Gene Expression in Human Skeletal Muscle. PLoS ONE 2008, 3, e1385. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.; Choi, J.; Kim, H.J.; Lee, H.; Lim, J.-Y.; Choi, S.-J. Sex-and Fiber-Type-Related Contractile Properties in Human Single Muscle Fiber. J. Exerc. Rehabil. 2019, 15, 537. [Google Scholar] [CrossRef] [Green Version]
- Maher, A.C.; Akhtar, M.; Vockley, J.; Tarnopolsky, M.A. Women Have Higher Protein Content of β-Oxidation Enzymes in Skeletal Muscle than Men. PLoS ONE 2010, 5, e12025. [Google Scholar] [CrossRef] [Green Version]
- Roepstorff, C.; Steffensen, C.H.; Madsen, M.; Stallknecht, B.; Kanstrup, I.-L.; Richter, E.A.; Kiens, B. Gender Differences in Substrate Utilization during Submaximal Exercise in Endurance-Trained Subjects. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E435–E447. [Google Scholar] [CrossRef]
- Most, J.; van Can, J.G.P.; van Dijk, J.-W.; Goossens, G.H.; Jocken, J.; Hospers, J.J.; Bendik, I.; Blaak, E.E. A 3-Day EGCG-Supplementation Reduces Interstitial Lactate Concentration in Skeletal Muscle of Overweight Subjects. Sci. Rep. 2015, 5, 17896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmquist, J.K.; Maratos-Flier, E.; Saper, C.B.; Flier, J.S. Unraveling the Central Nervous System Pathways Underlying Responses to Leptin. Nat. Neurosci. 1998, 1, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Sáinz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martínez, J.A. Leptin Resistance and Diet-Induced Obesity: Central and Peripheral Actions of Leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Reseland, J.E.; Anderssen, S.A.; Solvoll, K.; Hjermann, I.; Urdal, P.; Holme, I.; Drevon, C.A. Effect of Long-Term Changes in Diet and Exercise on Plasma Leptin Concentrations. Am. J. Clin. Nutr. 2001, 73, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-Specific Effects of Leptin on Glucose and Lipid Metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Corpeleijn, E.; Pelsers, M.M.A.L.; Soenen, S.; Mensink, M.; Bouwman, F.G.; Kooi, M.E.; Saris, W.H.M.; Glatz, J.F.C.; Blaak, E.E. Insulin Acutely Upregulates Protein Expression of the Fatty Acid Transporter CD36 in Human Skeletal Muscle in Vivo. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 77–83. [Google Scholar]
- Corpeleijn, E.; Petersen, L.; Holst, C.; Saris, W.H.; Astrup, A.; Langin, D.; MacDonald, I.; Martinez, J.A.; Oppert, J.-M.; Polak, J.; et al. Obesity-Related Polymorphisms and Their Associations with the Ability to Regulate Fat Oxidation in Obese Europeans: The NUGENOB Study. Obes. Silver Spring MD 2010, 18, 1369–1377. [Google Scholar] [CrossRef]
- Bokor, S.; Legry, V.; Meirhaeghe, A.; Ruiz, J.R.; Mauro, B.; Widhalm, K.; Manios, Y.; Amouyel, P.; Moreno, L.A.; Molnàr, D.; et al. Single-Nucleotide Polymorphism of CD36 Locus and Obesity in European Adolescents. Obes. Silver Spring MD 2010, 18, 1398–1403. [Google Scholar] [CrossRef]



| Author and Year of Publication | Design/Participats | Purpose of the Study | Characteristics of the Intervention | Output |
|---|---|---|---|---|
| Arkinstall, et al., 2004 [1] | Cross-sectional study.Seven males moderately trained in cycling: age = 35 ± 5 y, BM = 80.3 ± 9.5 kg. | To quantify the acute effect of HCD (77.7%,) and LCD (7.7%) on energy metabolism genes transcription. | Glycogen depletion and HIIT: 2 min 95% and 2 min 55% of VO2 peak. LCD (0.7 g/kg of BM of CHO, 4.4 g/kg BM of fat, 4 g/kg BM of protein) or HCD (10 g/kg BM of CHO, 1 g/kg BM of fat, 1.9 g/kg BM of protein). | FAT/CD36 and UCP3 gene transcriptions in skeletal muscle were increased following an acute LCD. |
| Baker et al., 2015 [3] | Cross-sectional study. Twelve sedentary and healthy caucasian males: age = 19–27 y. 6 lean: BMI ≤ 24.9 kg/m2, and 6 with obesity: BMI≤30 kg/m2 | Investigate the effects of obesity and HFD exposure on fatty acid oxidation and three carboxylic acids cycle intermediates and amino acids in skeletal muscle. | 1 and 5 days of HFD: 65% fat, 15% protein, and 25% carbohydrate and comprise 35% of daily energy intake. | HFD increase skeletal muscle FAT/CD36 levels in obese but not in the lean individuals. |
| Bergouignan et al., 2012 [22] | Cross-sectional study. Nineteen healty participants from 20–45 y: 9 lean men: BMI = 19–25 kg/m2, and 10 females with obesity: BMI = 30–40 kg/m2 | To test that obese non-diabetic humans have an impaired ability to adapt to an HFD. | Isocaloric LFD (20% of energy) and isocaloric HFD (50% of energy). | The expression of skeletal muscle PDK 4, FAT/CD36 and AMPK increased during HFD in both lean and obese individuals during HFD. |
| Cameron-Smith et al., 2003 [23] | Crossover design.Fourteen well-trained males: age = 26.9 ± 1.7 y, BM = 73.7 ± 1.7 kg. | To determine the effect of HFD on genes expression for FATP and β-oxidation in skeletal muscle. | Five days of HFD (>65% lipids) or HCD (70–75% carbohydrate). | FAT/CD36 and β-HAD gene expression and FAT/CD36 gene abundance were greater after HFD vs. HCD. |
| Cheng et al., 2009 [24] | Crossover design. Eight healthy males: 22.5 ± 0.3 y, BMI = 22.7 ± 0.5 kg/m2. | To determine the effect of meal GI on GLUT4 and FAT/CD36 gene expressions in human skeletal muscle after a single bout of exercise. | 60-min cycling exercise at 75% of VO2 max, and an isocaloric meal of HGl or LGl with similar proportions of carbohydrate, fat, and protein. | FAT/CD36 mRNA and protein levels were decreased with the HGI vs. LGI meal but not by acute exercise. GLUT4 mRNA was downregulated by both HGI and LGI diets. |
| Frandsen et al., 2022 [9] | Prospective study.Fourteen well- trained cyclists male.Seven younger: age = 30 ± 5 y, BM~77 kg, and 7 older: age 65 ± 6 y, BM~70 kg. | To examines the physiologic and metabolic impact of repeated prolonged moderate intensity exercise (7–10 h/day for 15 consecutive days at ~63% HR maximal in two cohorts of younger and old cyclists. | 3000 km of cycling from Copenhagen, Denmark, to Palermo, Italy over 15 days. | Fifteen days of extreme endurance exercise kept skeletal muscle FAT/CD36 and FABP4 unchanged. |
| Gerling et al., 2014 [25] | Prospective simple blind randomized study. Thirty healthy, recreationally active males, age~21 y, BMI ~24.6 km/m2. 21 intake omega-3 and 9 placebo. | To examine the effects of EPA and DHA supplementation on whole-body RMR and the content of proteins involved in fat metabolism in human skeletal muscle. | 12 weeks of 3.0 g/day of EPA and DHA or olive oil. | Twelve weeks of 3.0 g/day of EPA and DHA did no change whole-body fat oxidation, and skeletal muscle mitochondrial content of FAT/CD36, FABP4, FATP 1, and FATP4. |
| Greene et al., 2012 [26] | Prospective study.Sixteen sedentary overweight and obese partipants: 9 men, age = 41 ± 2 y, BMI = 32.0 ± 2.3 kg/m2; 7 women, age 52 ± 2 y BMI = 31.9 ± 1.7 kg/m2. | To determine the association of skeletal muscle PPAR content with blood lipids and lipoproteins before and after exercise. | A single session or 12-week program (3 session/day) of land or water treadmill training. 250–500 kcal/ session, 60–85% of VO2 max. | A single exercise session, but not exercise training, increases the FAT/CD36, PPAR, PGC-1, and LPL content in skeletal muscle. |
| Heilbronn et al., 2007 [27] | Cross-sectional study.Five men and twelve women sedentary and nonsmoking. 9 with (FHD-2): age = 46 ± 6 y, BMI= 26.6 ± 5.3 kg/m2, and 8 without FHD-2: age= 41 ± 7 y, BMI = 26.7 ± 5.3 kg/m2 | To examine whole-body glucose and fat oxidation after a prolonged fast and in response to refeeding a single high-fat meal or high-carbohydrate meal meal in both groups. | 1000-kcal meal, 76% of high-fat, or 76% of high-carbohydrate. | After a single high-fat meal, the individuals with FHD-2 increase the respiratory quotient and decrease FAT/CD36, CPT1, and PGC1α gene expression in skeletal muscle. |
| Holloway et al., 2006 [11] | Cross-sectional study.Fiveteen healthy, recreationally active individuals. 10 males and 5 females: age = 22 ± 1 y, BMI = 24 ± 1 kg/m2. | To investigate the effects of exercise on CPTI, palmitoyl-CoA and Malonil-CoA kinetics, on the presence and functional significance of FAT/CD36 on skeletal muscle mitochondria. | 120 min of cycling at ~60% VO2 peak | Whole body fat and palmitate oxidation rates in isolated mitochondria progressively increased during exercise and were correlated (r = 0.78). Skeletal muscle FAT/CD36 protein increased by 63% during exercise and was correlated with mitochondrial palmitate oxidation rates (r = 0.52). |
| Holloway et al., 2007 [28] | Cross-sectional study.Eighteen nondiabetic women.9 lean: age = 47 ± 3 y, BMI < 27 kg/m2; and 9 with obesity: age = 45 ± 3 y, BMI ≥ 30 kg/m2. | To examine whether the obesity-related decreases in skeletal muscle lipid oxidation are attributable to (1) a reduction in mitochondrial content and/or (2) an intrinsic defect in mitochondria, and (3) whether there are reductions in the content of mitochondrial FATP | NA | Skeletal muscle FAT/CD36 did not differ in lean and obese individuals but was correlated with mitochondrial fatty acid oxidation (r = 0.67). Obesity did not alter the ability of isolated mitochondria to oxidize palmitate; however, fatty acid oxidation was reduced at the whole muscle level by 28% in the obese. |
| Jordy et al., 2014 [29] | Cross-sectional study.Eighteen healthy, moderately trained males: age = 24.4 ± 0.7 y, BMI = 23.3 ± 0.5 kg/m2 | To investigate lipid-induced regulation of FABP4 in human skeletal muscle and the impact on insulin sensitivity. | A hypercaloric (175% of estimated daily energy intake) of HFD (77% from fat) or HCD (80% from carbohydrate) for 3 days. | Three days of a HFD (77% fat) decreased insulin sensitivity but was not associated with a relocation of FAT/CD36 or FABP4 protein to the skeletal muscle sarcolemma. FAT/CD36 and FABP4 mRNA, but not the proteins, were upregulated by increased fatty acid availability. |
| Kiens et al., 2004 [30] | Cross-sectional study.Forty six healthy and nonsmoking individuals.24 eumenorrheic women: age~26.3 y, body fat~21.4%; and 22 men: age~25.3 y, body fat~13.9%. | To evaluate whether a physical exercise test and sex, influence the FABP4 and skeletal muscle LPL. | An exercise test on a bicycle ergometer at 60% VO2 peak for 90 min. | A single 90-min exercise bout increased FAT/CD36 mRNA (25%) and FABP4 mRNA (15%) muscle levels in male and female. FAT/CD36 protein level was 49% higher in women than in men, irrespective of training status. FAT/CD36 mRNA was only higher in untrained women. |
| Maunder et al., 2022 [31] | Cross-sectional.Seventeen endurance-trained male cyclists and triathletes: age 34 ± 7 y, BMI = 24.5 kg/m2. | To assess relationships between PFO measured during fasted incremental cycling, skeletal muscle FAT/CD36 abundance, endurance performance, and fat oxidation rates during prolonged moderate-intensity fed-state exercise. | Incremental cycling exercise test to evaluate the PFO. | FAT/CD36 abundance in skeletal muscle correlated with PFO (R = 0.68) and Citrate Synthase activity (R = 0.84). |
| Perry et al., 2008 [32] | Prospective study.Three females and 5 males: age = 24 ± 1 y, BMI~22.7 kg/m2. | To investigate the ability of 6 wk of HIIT (18 h at 90% of VO2 peak) to increase the whole-body CHO and Fat oxidation. | 6 weeks of cycle-ergometer HIIT: ~1 h of 10 × 4 min intervals at ~90% of peak oxygen consumption (VO2 peak), separated by 2 min rest, 3 day·week–1 | Eighteen sesions, six weaks of HIIT (3 d/wk) increases fat oxidation (60%) and FAT/CD36, B-HAD, FABP4 (14–30%) protein content. |
| Schenk and Horowitz, 2006 [33] | Prospective study.Fiveteen abdominally obese premenopausal health women: age~30 y, BMI = 30–40 kg/m2, waist circumference >100 cm. | To determine the effect of an endurance exercise training and weight loss program (12%) on fat oxidation and the colocalization of the fatty acid translocase FAT/CD36 with CPTI in human skeletal muscle. | Caloric intake 500–800kcal/day below that required to maintain body weight. 45 min 3 d/wk of stationary bicycle at 70–85% of HRmax. | FAT/CD36 and CPT1 proteins coimmunoprecipitate in skeletal muscle. Weight loss program (diet + exercise) increased the coimmunoprecipitate of these proteins (25%) and total fat oxidation (R2 = 0.857). |
| Talanian et al., 2007 [34] | Prospective study.Eight healthy recreationally active women: age = 22.1 ± 0.2 y, BM = 65 ± 2.2 kg. | To examine the effects of seven HIIT sessions over 2 wk on skeletal muscle fuel content, mitochondrial enzyme activities, fatty acid transport proteins, VO2 peak, and whole body metabolic, hormonal, and cardiovascular responses | Seven HIIT supervised sessions in 13 days: ten 4-min cycling bouts at 90% of VO2 peak separated by 2 min of rest. | There were increased whole body fat oxidation and muscle skeletal FABP4, whereas FAT/CD36 content was unaffected. |
| Talanian et al., 2010 [35] | Prospective study.Ten healthy females: age= 22 ± 1 y, BM = 65 ± 2 kg, VO2 peak = 2.82 ± 0.14 L/min. | To determine whether HIIT increased total skeletal muscle, sarcolemmal, and mitochondrial FABP contents. | Three days/wk, completing 18 supervised training sessions in 6 wk: ten 4-min cycling bouts at 90% VO2 peak separated by 2 min of rest. | HIIT (3 d/wk for 6 wk) increased the mitochondrial (51%) and whole skeletal muscle amount (10%) of FAT/CD36 without alterations in sarcolemmal content. Whole muscle FABP4 increased in 48%. Sarcolemmal FABP4 increased in 23%, whereas mitochondrial FABP4 was unaltered. |
| Tunstall et al., 2002 [36] | Cross-sectional and prospective study.Seven heathy untrained individuals: 3 male and 4 female, age = 20–42 y, BMI = 17–26 kg/m2 | To study the effects of a single bout of exercise and exercise training on the expression of genes necessary for the transport and β-oxidation of FAs, together with the gene expression of transcription factors implicated in the regulation of FA homeostasis. | 9-day of 60 min cycling per day at 63% VO2 peak. | Nine consecutive days of aerobic training (63% of VO2 peak) increased total lipid oxidation and expression of FAT/CD36 and CPT I mRNA during 1-h cycling bout in skeletal muscle. |
| Warren et al., 2020 [37] | Prospective study.Fourteen premenopausal women: age = 31.2 ± 6.7 y, BMI = 26.6 ± 5.1 kg/m2 | To assess the effects of aerobic exercise training on skeletal muscle mitochondrial function and markers of lipid metabolism. | Three days per week for 8–16 weeks: 20–40 min, 67–80% of maximal HR. | 8–16 weeks of aerobic exercise training increase skeletal muscle FAT/CD36 content and mitochondrial respiratory capacity proportionally. |
| Post-Translational Modifications | Impact |
|---|---|
| Glycosylation | Correct folding, stability, transport to the cell surface, and function of the protein. |
| Acetylation | The consequences for FAT/CD36 expression and/or functioning have not yet been investigated. |
| Phosphorylation | This process is linked to FAT/CD36 functioning and regulation of FA utilization in the heart and muscle. |
| Ubiquitination | Considered to trigger the degradation of FAT/CD36 proteins by directing them to proteasomes in skeletal muscle and adipocyte. |
| Palmitoylation | Have an impact on the subcellular localization, membrane interactions, and subcellular trafficking of proteins. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Jiménez, A.; Zavala-Lira, R.A.; Moreno-Brito, V.; González-Rodríguez, E. FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review. J. Clin. Med. 2023, 12, 318. https://doi.org/10.3390/jcm12010318
Ramos-Jiménez A, Zavala-Lira RA, Moreno-Brito V, González-Rodríguez E. FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review. Journal of Clinical Medicine. 2023; 12(1):318. https://doi.org/10.3390/jcm12010318
Chicago/Turabian StyleRamos-Jiménez, Arnulfo, Ruth A. Zavala-Lira, Verónica Moreno-Brito, and Everardo González-Rodríguez. 2023. "FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review" Journal of Clinical Medicine 12, no. 1: 318. https://doi.org/10.3390/jcm12010318





