Causal Relationships of General and Abdominal Adiposity on Osteoarthritis: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Materials and Methods
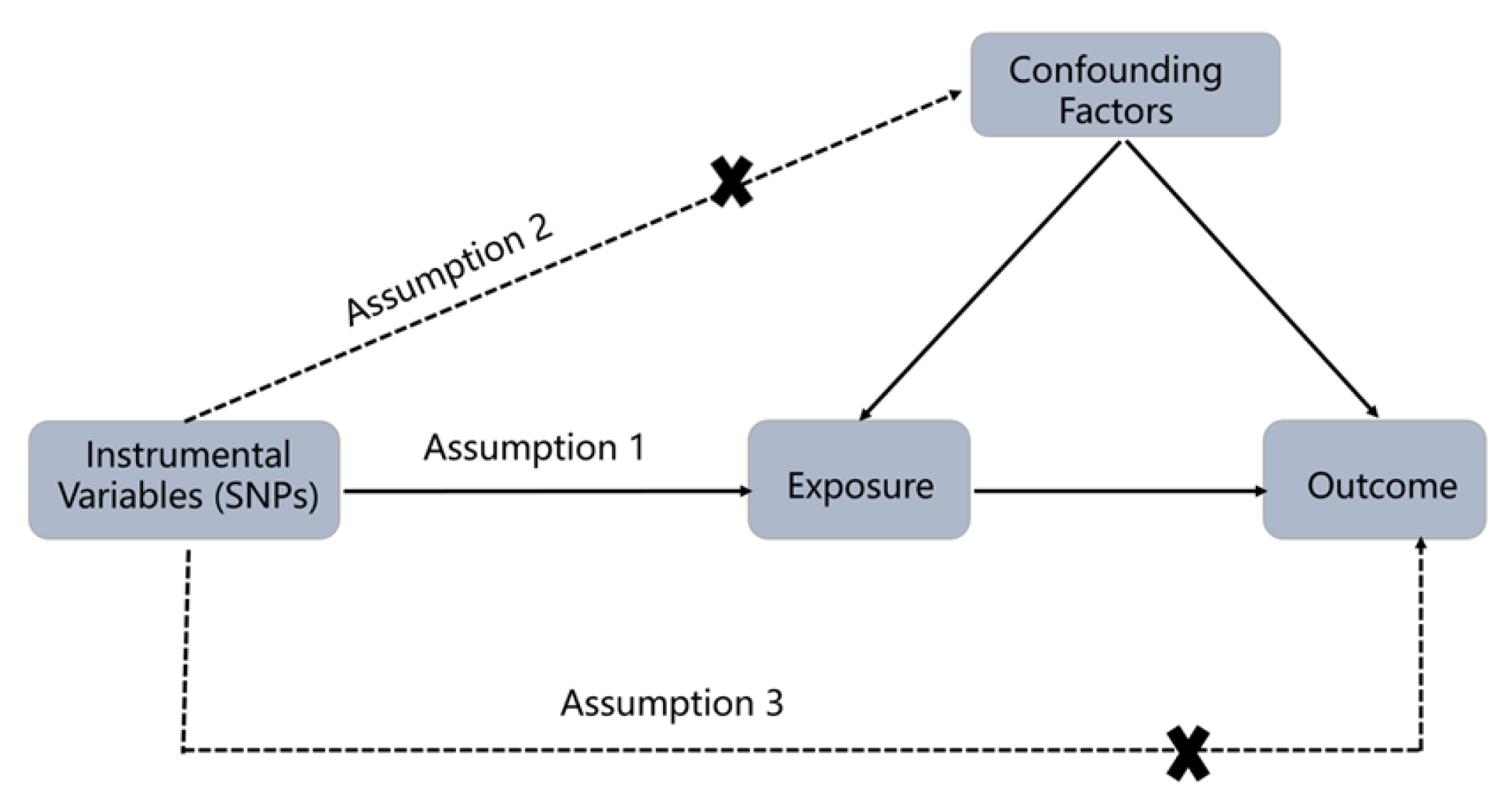
2.1. Study Design
2.2. Data Source
2.3. Selection of Instrumental Variables
2.4. Statistical Analysis
3. Results
3.1. Selected SNPs for This Study
3.2. Causal Relationships between Body Mass Index and Osteoarthritis
3.3. Causal Relationships between Waist Circumference and Osteoarthritis
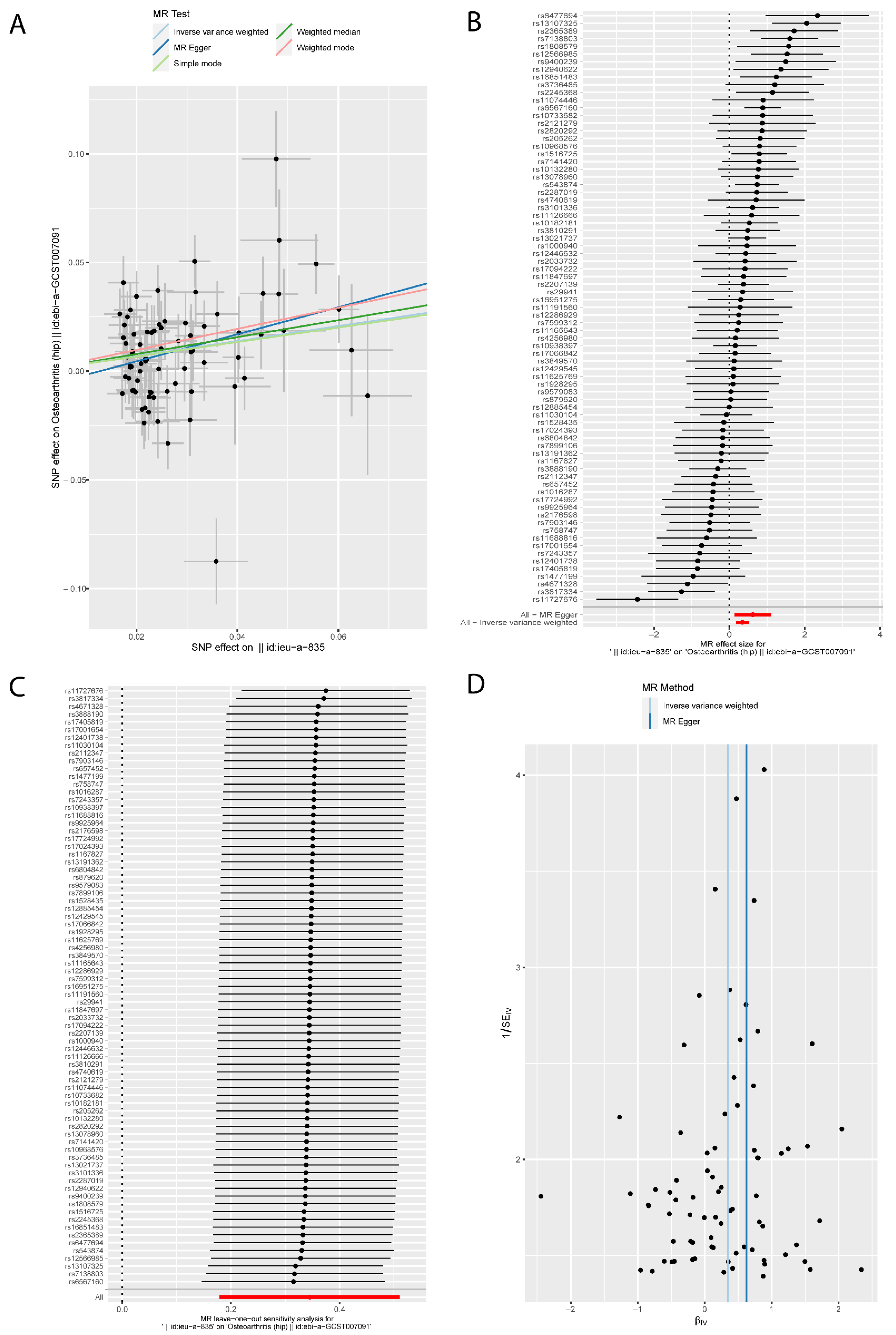
3.4. Causal Relationships between Hip Circumference and Osteoarthritis
3.5. Causal Relationships between Waist-to-Hip Ratio and Osteoarthritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Prim. 2016, 2, 16072. [Google Scholar] [CrossRef]
- Dickson, B.M.; Roelofs, A.J.; Rochford, J.J.; Wilson, H.M.; De Bari, C. The burden of metabolic syndrome on osteoarthritic joints. Arthritis Res. Ther. 2019, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Prim. 2017, 3, 17034. [Google Scholar] [CrossRef]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Reyes, C.; Leyland, K.M.; Peat, G.; Cooper, C.; Arden, N.K.; Prieto-Alhambra, D. Association Between Overweight and Obesity and Risk of Clinically Diagnosed Knee, Hip, and Hand Osteoarthritis: A Population-Based Cohort Study. Arthritis Rheumatol. 2016, 68, 1869–1875. [Google Scholar] [CrossRef]
- Misra, D.; Fielding, R.A.; Felson, D.T.; Niu, J.; Brown, C.; Nevitt, M.; Lewis, C.E.; Torner, J.; Neogi, T.; Study, M. Risk of Knee Osteoarthritis With Obesity, Sarcopenic Obesity, and Sarcopenia. Arthritis Rheumatol. 2019, 71, 232–237. [Google Scholar] [CrossRef]
- Jayedi, A.; Soltani, S.; Zargar, M.S.; Khan, T.A.; Shab-Bidar, S. Central fatness and risk of all cause mortality: Systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ 2020, 370, m3324. [Google Scholar] [CrossRef]
- Yarmolinsky, J.; Wade, K.H.; Richmond, R.C.; Langdon, R.J.; Bull, C.J.; Tilling, K.M.; Relton, C.L.; Lewis, S.J.; Davey Smith, G.; Martin, R.M. Causal Inference in Cancer Epidemiology: What Is the Role of Mendelian Randomization? Cancer Epidemiol. Biomark. Prev. 2018, 27, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.C.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Shungin, D.; Winkler, T.W.; Croteau-Chonka, D.C.; Ferreira, T.; Locke, A.E.; Mägi, R.; Strawbridge, R.J.; Pers, T.H.; Fischer, K.; Justice, A.E.; et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015, 518, 187–196. [Google Scholar] [CrossRef]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 2016, 45, 1717–1726. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Chu, I.; Lim, A.; Ng, C. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Mudie, K.; Lawlor, D.A.; Pearce, N.; Crampin, A.; Tomlinson, L.; Tafatatha, T.; Musicha, C.; Nitsch, D.; Smeeth, L.; Nyirenda, M.J. How does the association of general and central adiposity with glycaemia and blood pressure differ by gender and area of residence in a Malawian population: A cross-sectional study. Int. J. Epidemiol. 2018, 47, 887–898. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur. J. Clin. Nutr. 2010, 64, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Badley, E.; Zahid, S.; Wilfong, J.; Perruccio, A. The relationship between body mass index and osteoarthritis for single and multi-site osteoarthritis of the hand, hip, or knee: Findings from the CLSA. Arthritis Care Res. 2021, 74, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Holliday, K.; McWilliams, D.; Maciewicz, R.; Muir, K.; Zhang, W.; Doherty, M. Lifetime body mass index, other anthropometric measures of obesity and risk of knee or hip osteoarthritis in the GOAL case-control study. Osteoarthr. Cartil. 2011, 19, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, M.B.; Thoma, L.M.; Master, H.; Voinier, D.; White, D.K. The Association of an Increasing Waist Circumference and Risk of Incident Low Physical Function in Adults with Knee Osteoarthritis. J. Rheumatol. 2020, 47, 1550–1556. [Google Scholar] [CrossRef]
- Batsis, J.; Zbehlik, A.; Barre, L.; Mackenzie, T.; Bartels, S. The impact of waist circumference on function and physical activity in older adults: Longitudinal observational data from the osteoarthritis initiative. Nutr. J. 2014, 13, 81. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Felson, D.T.; Wirth, W.; Dannhauer, T.; Eckstein, F. Is local or central adiposity more strongly associated with incident knee osteoarthritis than the body mass index in men or women? Osteoarthr. Cartil. 2018, 26, 1033–1037. [Google Scholar] [CrossRef]
- Chen, L.; Yao, F.; Wang, T.; Li, G.; Chen, P.; Bulsara, M.; Zheng, J.; Landao-Bassonga, E.; Firth, M.; Vasantharao, P.; et al. Horizontal fissuring at the osteochondral interface: A novel and unique pathological feature in patients with obesity-related osteoarthritis. Ann. Rheum. Dis. 2020, 79, 811–818. [Google Scholar] [CrossRef]
- Sun, A.R.; Udduttula, A.; Li, J.; Liu, Y.; Ren, P.G.; Zhang, P. Cartilage tissue engineering for obesity-induced osteoarthritis: Physiology, challenges, and future prospects. J. Orthop. Translat. 2021, 26, 3–15. [Google Scholar] [CrossRef]
- Goldring, M.; Otero, M.; Plumb, D.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.; Olivotto, E.; Borzì, R.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Bijsterbosch, J.; Slagboom, P.E.; Rosendaal, F.R.; Huizinga, T.W.J.; Kloppenburg, M. Body mass index and alignment and their interaction as risk factors for progression of knees with radiographic signs of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 1117–1122. [Google Scholar] [CrossRef]
- Runhaar, J.; van Middelkoop, M.; Reijman, M.; Vroegindeweij, D.; Oei, E.H.G.; Bierma-Zeinstra, S.M.A. Malalignment: A possible target for prevention of incident knee osteoarthritis in overweight and obese women. Rheumatology 2014, 53, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Z.; Wang, Y.; Wluka, A.E.; Davies-Tuck, M.L.; Hanna, F.; Urquhart, D.M.; Cicuttini, F.M. Association of obesity and systemic factors with bone marrow lesions at the knee: A systematic review. Semin. Arthritis Rheum. 2014, 43, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.C.; Vullings, J.; van de Loo, F.A.J. Osteoporosis and osteoarthritis are two sides of the same coin paid for obesity. Nutrition 2020, 70, 110486. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Judd, R. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Ait Eldjoudi, D.; Cordero Barreal, A.; Gonzalez-Rodríguez, M.; Ruiz-Fernández, C.; Farrag, Y.; Farrag, M.; Lago, F.; Capuozzo, M.; Gonzalez-Gay, M.A.; Mera Varela, A.; et al. Leptin in Osteoarthritis and Rheumatoid Arthritis: Player or Bystander? Int. J. Mol. Sci. 2022, 23, 2859. [Google Scholar] [CrossRef]
- Massengale, M.; Reichmann, W.M.; Losina, E.; Solomon, D.H.; Katz, J.N. The relationship between hand osteoarthritis and serum leptin concentration in participants of the Third National Health and Nutrition Examination Survey. Arthritis Res. Ther. 2012, 14, R132. [Google Scholar] [CrossRef]
- Kroon, F.P.B.; Veenbrink, A.I.; de Mutsert, R.; Visser, A.W.; van Dijk, K.W.; le Cessie, S.; Rosendaal, F.R.; Kloppenburg, M. The role of leptin and adiponectin as mediators in the relationship between adiposity and hand and knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1761–1767. [Google Scholar] [CrossRef]
- Jiang, M.; He, J.; Sun, Y.; Dong, X.; Yao, J.; Gu, H.; Liu, L. Leptin Induced TLR4 Expression via the JAK2-STAT3 Pathway in Obesity-Related Osteoarthritis. Oxid. Med. Cell. Longev. 2021, 2021, 7385160. [Google Scholar] [CrossRef] [PubMed]


| Exposure | Methods | SNP (n) | OR | OR 95% CI | p-Value |
|---|---|---|---|---|---|
| BMI | MR Egger | 77 | 1.280 | 0.884, 1.854 | 0.195 |
| Weighted median | 77 | 1.615 | 1.403, 1.860 | 2.57 × 10−11 | |
| Inverse variance weighted | 77 | 1.694 | 1.492, 1.923 | 3.96 × 10−16 | |
| Simple mode | 77 | 1.684 | 1.251, 2.267 | 9.51 × 10−4 | |
| Weighted mode | 77 | 1.600 | 1.323, 1.937 | 6.81 × 10−6 | |
| WC | MR Egger | 42 | 1.413 | 0.825, 2.420 | 0.215 |
| Weighted median | 42 | 1.576 | 1.315, 1.889 | 8.65 × 10−7 | |
| Inverse variance weighted | 42 | 1.827 | 1.564, 2.134 | 2.68 × 10−14 | |
| Simple mode | 42 | 1.555 | 1.132, 2.136 | 0.009 | |
| Weighted mode | 42 | 1.610 | 1.247, 2.079 | 7.22 × 10−4 | |
| HC | MR Egger | 51 | 1.699 | 0.910, 3.175 | 0.103 |
| Weighted median | 51 | 1.616 | 1.371, 1.903 | 9.78 × 10−9 | |
| Inverse variance weighted | 51 | 1.610 | 1.357, 1.912 | 5.03 × 10−8 | |
| Simple mode | 51 | 1.673 | 1.231, 2.273 | 0.002 | |
| Weighted mode | 51 | 1.673 | 1.283, 2.181 | 3.96 × 10−4 | |
| WHR | MR Egger | 20 | 1.073 | 0.287, 4.009 | 0.918 |
| Weighted median | 20 | 1.088 | 0.878, 1.350 | 0.440 | |
| Inverse variance weighted | 20 | 1.121 | 0.917, 1.371 | 0.264 | |
| Simple mode | 20 | 1.085 | 0.663, 1.776 | 0.749 | |
| Weighted mode | 20 | 1.061 | 0.680, 1.654 | 0.797 |
| Exposure | Methods | SNP (n) | OR | OR 95% CI | p-Value |
|---|---|---|---|---|---|
| BMI | MR Egger | 77 | 1.864 | 1.144, 3.035 | 0.014 |
| Weighted median | 77 | 1.477 | 1.225, 1.782 | 4.54 × 10−5 | |
| Inverse variance weighted | 77 | 1.412 | 1.196, 1.666 | 4.58 × 10−5 | |
| Simple mode | 77 | 1.396 | 0.858, 2.270 | 0.183 | |
| Weighted mode | 77 | 1.625 | 1.157, 2.281 | 0.006 | |
| WC | MR Egger | 42 | 2.343 | 1.301, 4.221 | 0.007 |
| Weighted median | 42 | 1.514 | 1.205, 1.902 | 3.7 × 10−4 | |
| Inverse variance weighted | 42 | 1.491 | 1.254, 1.772 | 5.85 × 10−6 | |
| Simple mode | 42 | 1.392 | 0.818, 2.369 | 0.230 | |
| Weighted mode | 42 | 1.606 | 0.987, 2.615 | 0.064 | |
| HC | MR Egger | 51 | 1.629 | 0.854, 3.111 | 0.145 |
| Weighted median | 51 | 1.428 | 1.164, 1.752 | 6.29 × 10−4 | |
| Inverse variance weighted | 51 | 1.439 | 1.205, 1.719 | 5.82 × 10−5 | |
| Simple mode | 51 | 1.657 | 0.936, 2.933 | 0.089 | |
| Weighted mode | 51 | 1.675 | 1.007, 2.786 | 0.053 | |
| WHR | MR Egger | 20 | 0.598 | 0.132, 2.704 | 0.513 |
| Weighted median | 20 | 1.128 | 0.869, 1.465 | 0.364 | |
| Inverse variance weighted | 20 | 1.216 | 0.961, 1.539 | 0.103 | |
| Simple mode | 20 | 1.445 | 0.849, 2.460 | 0.191 | |
| Weighted mode | 20 | 1.025 | 0.630, 1.667 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Cai, Y.; Xiao, M.; Liang, J.; Zhang, G.; Jing, Z.; Zhang, R.; Dang, X. Causal Relationships of General and Abdominal Adiposity on Osteoarthritis: A Two-Sample Mendelian Randomization Study. J. Clin. Med. 2023, 12, 320. https://doi.org/10.3390/jcm12010320
Lyu L, Cai Y, Xiao M, Liang J, Zhang G, Jing Z, Zhang R, Dang X. Causal Relationships of General and Abdominal Adiposity on Osteoarthritis: A Two-Sample Mendelian Randomization Study. Journal of Clinical Medicine. 2023; 12(1):320. https://doi.org/10.3390/jcm12010320
Chicago/Turabian StyleLyu, Leifeng, Yuanqing Cai, Mofan Xiao, Jialin Liang, Guangyang Zhang, Zhaopu Jing, Rupeng Zhang, and Xiaoqian Dang. 2023. "Causal Relationships of General and Abdominal Adiposity on Osteoarthritis: A Two-Sample Mendelian Randomization Study" Journal of Clinical Medicine 12, no. 1: 320. https://doi.org/10.3390/jcm12010320
APA StyleLyu, L., Cai, Y., Xiao, M., Liang, J., Zhang, G., Jing, Z., Zhang, R., & Dang, X. (2023). Causal Relationships of General and Abdominal Adiposity on Osteoarthritis: A Two-Sample Mendelian Randomization Study. Journal of Clinical Medicine, 12(1), 320. https://doi.org/10.3390/jcm12010320





