Identification of Three Circulating MicroRNAs in Plasma as Clinical Biomarkers for Breast Cancer Detection
Abstract
:1. Introduction
2. Results
2.1. Study Workflow
2.2. Identification of Potential MiRNAs from Public Datasets
2.3. Baseline Characteristics of BC Patients and Healthy Controls in the Validation Cohort
2.4. Evaluation of the Diagnostic Efficacy of MiRNA Biomarkers in the Validation Cohort
2.5. Correlation with Clinicopathology Features
2.6. Functional Enrichment for miRNA Target Genes
3. Discussion
4. Materials and Methods
4.1. Data Acquisition
4.2. Subject Samples for Validation of miRNAs
4.3. Sample Processing and miRNA Extraction
4.4. MiRNA Validation by Quantitative Reverse Transcription (qRT–PCR)
4.5. Target Prediction Analysis and Functional Analysis
4.6. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| ID | BC | Early stage of BC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Youden Index | p | AUC | Sensitivity | Specificity | Youden Index | p | |
| (95%CI) | (%) | (%) | (95%CI) | (%) | (%) | |||||
| CEA | 0.5433 | 30.56 | 82.52 | 0.1307 | 0.277 | 0.5317 | 61.54 | 48.54 | 0.1008 | 0.465 |
| (0.4653–0.6213) | (0.4462–0.6173) | |||||||||
| CA153 | 0.5635 | 21.30 | 97.09 | 0.1838 | 0.111 | 0.5695 | 23.08 | 96.12 | 0.1919 | 0.110 |
| (0.4855–0.6415) | (0.4826–0.6565) | |||||||||
| CEA + CA153 | 0.5679 | 50.00 | 66.02 | 0.1602 | 0.088 | 0.5871 | 55.14 | 66.02 | 0.2115 | 0.045 |
| (0.4907–0.6453) | (0.5004–0.6738) | |||||||||
| Description | Gene ID |
|---|---|
| Calcium signaling pathway | ADRB1, PDGFRA, PDE1A, FGFR3, MYLK2 |
| MAPK signaling pathway | PLA2G4A, PDGFRA, JUN, DUSP6, FGFR3 |
| MicroRNAs in cancer | CDC25A, ZEB1, PDGFRA, ZEB2, FGFR3 |
| Renin secretion | ADRB1, PDE3B, PTGER4, PDE1A |
| Oxytocin signaling pathway | PLA2G4A, JUN, MYLK2, RGS2 |
| cGMP-PKG signaling pathway | ADRB1, PDE3B, MYLK2, RGS2 |
| Focal adhesion | PDGFRA, SHC3, JUN, MYLK2 |
| Chemical carcinogenesis—receptor activation | ADRB1, ESR1, CDC25A, JUN |
| Ras signaling pathway | PLA2G4A, PDGFRA, SHC3, FGFR3 |
| EGFR tyrosine kinase inhibitor resistance | PDGFRA, SHC3, FGFR3 |
| GnRH signaling pathway | PLA2G4A, EGR1, JUN |
| Endocrine resistance | ESR1, SHC3, JUN |
| Choline metabolism in cancer | PLA2G4A, PDGFRA, JUN |
| Purine metabolism | PDE3B, AK5, PDE1A |
| Estrogen signaling pathway | ESR1, SHC3, JUN |
| Apelin signaling pathway | PDE3B, EGR1, MYLK2 |
| Breast cancer | ESR1, SHC3, JUN |
| Phospholipase D signaling pathway | PLA2G4A, PDGFRA, SHC3 |
| Cocaine addiction | FOSB, JUN |
| Regulation of lipolysis in adipocytes | ADRB1, PDE3B |
| Arachidonic acid metabolism | PLA2G4A, PTGIS |
| Inflammatory bowel disease | MAF, JUN |
| Amphetamine addiction | FOSB, JUN |
| Prolactin signaling pathway | ESR1, SHC3 |
| Central carbon metabolism in cancer | PDGFRA, FGFR3 |
References
- Bellanger, M.; Zeinomar, N.; Tehranifar, P.; Terry, M.B. Are Global Breast Cancer Incidence and Mortality Patterns Related to Country-Specific Economic Development and Prevention Strategies? J. Glob. Oncol. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun. (Lond.) 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tong, Z.; Chen, K.; Wang, Y.; Liu, P.; Gu, L.; Liu, J.; Yu, J.; Song, F.; Zhao, W.; et al. Interpretation of breast cancer screening guideline for Chinese women. Cancer Biol. Med. 2019, 16, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Y.; Cheng, J. Diagnostic value of molybdenum target combined with DCE-MRI in different types of breast cancer. Oncol. Lett. 2019, 18, 4056–4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahari, Z.; Medjdoub, A.; Sahraoui, T.; Belhabri, L.; El, K.F. Study of Serum Carcinoembryonic Antigen’s Profile for Breast Cancer in Western Algeria: 100 cases. Gulf J. Oncol. 2017, 1, 33–36. [Google Scholar]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [Green Version]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, B.; Mohammadi, A.; Ghasabi, M.; Shirjang, S.; Dehghan, R.; Montazeri, V.; Holmskov, U.; Kazemi, T.; Duijf, P.; Gjerstorff, M.; et al. miR-142-3p as tumor suppressor miRNA in the regulation of tumorigenicity, invasion and migration of human breast cancer by targeting Bach-1 expression. J. Cell. Physiol. 2019, 234, 9816–9825. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Xue, A.; Chi, Y.; Xue, J.; Wang, W.; Zhao, Z.; Fan, M.; Yang, C.H.; Shao, Z.M.; Pfeffer, L.M.; et al. Induction of miRNA-181a by genotoxic treatments promotes chemotherapeutic resistance and metastasis in breast cancer. Oncogene 2016, 35, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Croset, M.; Pantano, F.; Kan, C.; Bonnelye, E.; Descotes, F.; Alix-Panabieres, C.; Lecellier, C.H.; Bachelier, R.; Allioli, N.; Hong, S.S.; et al. miRNA-30 Family Members Inhibit Breast Cancer Invasion, Osteomimicry, and Bone Destruction by Directly Targeting Multiple Bone Metastasis-Associated Genes. Cancer Res. 2018, 78, 5259–5273. [Google Scholar] [CrossRef] [Green Version]
- Shimomura, A.; Shiino, S.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Matsuzaki, J.; Ono, M.; Takeshita, F.; Niida, S.; Shimizu, C.; et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016, 107, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Shiino, S.; Matsuzaki, J.; Shimomura, A.; Kawauchi, J.; Takizawa, S.; Sakamoto, H.; Aoki, Y.; Yoshida, M.; Tamura, K.; Kato, K.; et al. Serum miRNA-based Prediction of Axillary Lymph Node Metastasis in Breast Cancer. Clin. Cancer Res. 2019, 25, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Ashtari, K.; Naderi-Manesh, H. An electrochemical nanobiosensor for plasma miRNA-155, based on graphene oxide and gold nanorod, for early detection of breast cancer. Biosens. Bioelectron. 2016, 77, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, X.; Huang, Z.; Xu, L.; Zhu, W.; Liu, P. An ER-associated miRNA signature predicts prognosis in ER-positive breast cancer. J. Exp. Clin. Cancer Res. 2014, 33, 94. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Atique, N.; Haq, I.U.; Ahmed, Z.; Jabbar, Z.; Nawaz, A.; Aqeel, A.; Akram, R. MicroRNA, a Promising Biomarker for Breast and Ovarian Cancer: A Review. Curr. Protein Pept. Sci. 2021, 22, 599–619. [Google Scholar] [CrossRef]
- Anwar, S.L.; Sari, D.; Kartika, A.I.; Fitria, M.S.; Tanjung, D.S.; Rakhmina, D.; Wardana, T.; Astuti, I.; Haryana, S.M.; Aryandono, T. Upregulation of Circulating MiR-21 Expression as a Potential Biomarker for Therapeutic Monitoring and Clinical Outcome in Breast Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 1223–1228. [Google Scholar] [CrossRef] [Green Version]
- Mar-Aguilar, F.; Luna-Aguirre, C.M.; Moreno-Rocha, J.C.; Araiza-Chavez, J.; Trevino, V.; Rodriguez-Padilla, C.; Resendez-Perez, D. Differential expression of miR-21, miR-125b and miR-191 in breast cancer tissue. Asia Pac. J. Clin. Oncol. 2013, 9, 53–59. [Google Scholar] [CrossRef]
- Luo, Z.B.; Lai, G.E.; Jiang, T.; Cao, C.L.; Peng, T.; Liu, F.E. A Competing Endogenous RNA Network Reveals Novel lncRNA, miRNA and mRNA Biomarkers With Diagnostic and Prognostic Value for Early Breast Cancer. Technol. Cancer Res. Treat. 2020, 19, 1079250941. [Google Scholar] [CrossRef]
- Jusoh, A.R.; Mohan, S.V.; Lu, P.T.; Tengku, D.T.; Haron, J.; Romli, R.C.; Jaafar, H.; Nafi, S.N.; Tuan, S.T.; Yahya, M.M. Plasma Circulating Mirnas Profiling for Identification of Potential Breast Cancer Early Detection Biomarkers. Asian Pac. J. Cancer Prev. 2021, 22, 1375–1381. [Google Scholar] [CrossRef]
- Garrido-Cano, I.; Constancio, V.; Adam-Artigues, A.; Lameirinhas, A.; Simon, S.; Ortega, B.; Martinez, M.T.; Hernando, C.; Bermejo, B.; Lluch, A.; et al. Circulating miR-99a-5p Expression in Plasma: A Potential Biomarker for Early Diagnosis of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 7427. [Google Scholar] [CrossRef]
- Adam-Artigues, A.; Garrido-Cano, I.; Carbonell-Asins, J.A.; Lameirinhas, A.; Simon, S.; Ortega-Morillo, B.; Martinez, M.T.; Hernando, C.; Constancio, V.; Burgues, O.; et al. Identification of a Two-MicroRNA Signature in Plasma as a Novel Biomarker for Very Early Diagnosis of Breast Cancer. Cancers 2021, 13, 2848. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liang, J.; Xu, J.; Li, X.; Xing, S.; Li, H.; Liu, W.; Liu, D.; Xu, J.; Huang, L.; et al. Identification and Validation of Circulating MicroRNA Signatures for Breast Cancer Early Detection Based on Large Scale Tissue-Derived Data. J. Breast Cancer 2018, 21, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Bahmanpour, Z.; Sheervalilou, R.; Khaniani, M.S.; Poursheikhani, A.; Montazeri, V.; Tahmasebivand, M.; Derakhshan, S.M. In Silico and Experimental Analysis of miR-125b-5 and miR-485-5p Expression in Serum of Patients with Breast Cancer. Microrna 2022. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Q.; Liu, Y.; Hao, X.; Zhang, S.; Zhang, J. Analysis of miR-205 and miR-155 expression in the blood of breast cancer patients. Chin. J. Cancer Res. 2013, 25, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, B.; Zhao, H.; Chang, J. The expression and clinical significance of serum miR-205 for breast cancer and its role in detection of human cancers. Int. J. Clin. Exp. Med. 2015, 8, 3034–3043. [Google Scholar]
- Cecene, G.; Ak, S.; Eskiler, G.G.; Demirdogen, E.; Erturk, E.; Gokgoz, S.; Polatkan, V.; Egeli, U.; Tunca, B.; Tezcan, G.; et al. Circulating miR-195 as a Therapeutic Biomarker in Turkish Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2016, 17, 4241–4246. [Google Scholar] [PubMed]
- Liu, Y.; Tang, D.; Zheng, S.; Su, R.; Tang, Y. Serum microRNA-195 as a potential diagnostic biomarker for breast cancer: A systematic review and meta-analysis. Int. J. Clin. Exp. Pathol. 2019, 12, 3982–3991. [Google Scholar] [PubMed]
- Farre, P.L.; Duca, R.B.; Massillo, C.; Dalton, G.N.; Grana, K.D.; Gardner, K.; Lacunza, E.; De Siervi, A. MiR-106b-5p: A Master Regulator of Potential Biomarkers for Breast Cancer Aggressiveness and Prognosis. Int. J. Mol. Sci. 2021, 22, 11135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Y.Y.; Xu, Y.; Zhang, L.; Zhu, J.; Si, P.C.; Wang, Y.W.; Ma, R. A two-miRNA signature of upregulated miR-185-5p and miR-362-5p as a blood biomarker for breast cancer. Pathol. Res. Pract. 2021, 222, 153458. [Google Scholar] [CrossRef] [PubMed]
- Ashirbekov, Y.; Abaildayev, A.; Omarbayeva, N.; Botbayev, D.; Belkozhayev, A.; Askandirova, A.; Neupokoyeva, A.; Utegenova, G.; Sharipov, K.; Aitkhozhina, N. Combination of circulating miR-145-5p/miR-191-5p as biomarker for breast cancer detection. PeerJ 2020, 8, e10494. [Google Scholar] [CrossRef] [PubMed]
- Fuso, P.; Di Salvatore, M.; Santonocito, C.; Guarino, D.; Autilio, C.; Mule, A.; Arciuolo, D.; Rinninella, A.; Mignone, F.; Ramundo, M.; et al. Let-7a-5p, miR-100-5p, miR-101-3p, and miR-199a-3p Hyperexpression as Potential Predictive Biomarkers in Early Breast Cancer Patients. J. Pers. Med. 2021, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Mitwally, N.; Soliman, A.S.; Yousef, E. Potential Diagnostic and Prognostic Utility of miR-141, miR-181b1, and miR-23b in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8589. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.; Lobo, J.; Fontes-Sousa, M.; Estevao-Pereira, H.; Salta, S.; Lopes, P.; Coimbra, N.; Antunes, L.; Palma, D.S.S.; Henrique, R.; et al. Predictive and Prognostic Value of Selected MicroRNAs in Luminal Breast Cancer. Front. Genet. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Ji, S.; Shao, G.; Zhang, J.; Zhao, K.; Wang, Z.; Wu, A. Effect of exosome biomarkers for diagnosis and prognosis of breast cancer patients. Clin. Transl. Oncol. 2018, 20, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Shin, V.Y.; Siu, J.M.; Cheuk, I.; Ng, E.K.; Kwong, A. Circulating cell-free miRNAs as biomarker for triple-negative breast cancer. Br. J. Cancer 2015, 112, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhong, J.; Wei, W.; Chen, X.; Liu, J.; Hu, Z. Identification of microRNA expression in sentinel lymph nodes from patients with breast cancer via RNA sequencing for diagnostic accuracy. J. Gene Med. 2019, 21, e3075. [Google Scholar] [CrossRef]
- Petrelli, A.; Carollo, R.; Cargnelutti, M.; Iovino, F.; Callari, M.; Cimino, D.; Todaro, M.; Mangiapane, L.R.; Giammona, A.; Cordova, A.; et al. By promoting cell differentiation, miR-100 sensitizes basal-like breast cancer stem cells to hormonal therapy. Oncotarget 2015, 6, 2315–2330. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Xiao, R.; He, Y.; He, L.; Xie, C.; Chen, J.; Hong, Y. MicroRNA-100 inhibits breast cancer cell proliferation, invasion and migration by targeting FOXA1. Oncol. Lett. 2021, 22, 816. [Google Scholar] [CrossRef]
- Petrelli, A.; Bellomo, S.E.; Sarotto, I.; Kubatzki, F.; Sgandurra, P.; Maggiorotto, F.; Di Virgilio, M.R.; Ponzone, R.; Geuna, E.; Galizia, D.; et al. MiR-100 is a predictor of endocrine responsiveness and prognosis in patients with operable luminal breast cancer. ESMO Open 2020, 5, e937. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, Y.; Niu, L.; Qiao, Y.; Yan, Y. Mesenchymal stem cell-derived exosome mir-342-3p inhibits metastasis and chemo-resistance of breast cancer through regulating ID4. Genes Genom. 2022, 44, 539–550. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Liu, Y.; Peng, H.; Wu, Y. Extracellular vesicles extracted from bone marrow mesenchymal stem cells carrying MicroRNA-342-3p inhibit the INHBA/IL13Ralpha2 axis to suppress the growth and metastasis of breast cancer. Transl. Oncol. 2022, 18, 101333. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, K.; Goerlitz, D.; Stambouli, N.; Islam, M.; Baroudi, O.; Neili, B.; Benayed, F.; Chivi, S.; Loffredo, C.; Jillson, I.A.; et al. miRNAs in Sera of Tunisian patients discriminate between inflammatory breast cancer and non-inflammatory breast cancer. Springerplus 2014, 3, 636. [Google Scholar] [CrossRef] [PubMed]
- Chamlali, M.; Rodat-Despoix, L.; Ouadid-Ahidouch, H. Store-Independent Calcium Entry and Related Signaling Pathways in Breast Cancer. Genes 2021, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Makena, M.R.; Rao, R. Subtype specific targeting of calcium signaling in breast cancer. Cell Calcium 2020, 85, 102109. [Google Scholar] [CrossRef] [PubMed]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor tyrosine kinases (RTKs) in breast cancer: Signaling, therapeutic implications and challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridrichova, I.; Zmetakova, I. MicroRNAs Contribute to Breast Cancer Invasiveness. Cells 2019, 8, 1361. [Google Scholar] [CrossRef] [Green Version]
- Petri, B.J.; Klinge, C.M. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev. 2020, 39, 837–886. [Google Scholar] [CrossRef]
- Guerit, E.; Arts, F.; Dachy, G.; Boulouadnine, B.; Demoulin, J.B. PDGF receptor mutations in human diseases. Cell. Mol. Life Sci. 2021, 78, 3867–3881. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, F. miR3423p suppresses cell migration and invasion in preeclampsia by targeting plateletderived growth factor receptor alpha. Mol. Med. Rep. 2019, 20, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Wu, R.; Ding, B.; Wu, W. ERBB4 promotes the progression of inflammatory breast cancer through regulating PDGFRA. Transl. Cancer Res. 2020, 9, 3266–3273. [Google Scholar] [CrossRef]
- Joglekar-Javadekar, M.; Van Laere, S.; Bourne, M.; Moalwi, M.; Finetti, P.; Vermeulen, P.B.; Birnbaum, D.; Dirix, L.Y.; Ueno, N.; Carter, M.; et al. Characterization and Targeting of Platelet-Derived Growth Factor Receptor alpha (PDGFRA) in Inflammatory Breast Cancer (IBC). Neoplasia 2017, 19, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ding, Z. Fibroblast growth factor receptors in breast cancer. Tumour Biol. 2017, 39, 1393391294. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Jing, Y.; Cao, Y. Overexpression of miR-100 inhibits growth of osteosarcoma through FGFR3. Tumour Biol. 2015, 36, 8405–8411. [Google Scholar] [CrossRef] [PubMed]
- Blanca, A.; Sanchez-Gonzalez, A.; Requena, M.J.; Carrasco-Valiente, J.; Gomez-Gomez, E.; Cheng, L.; Cimadamore, A.; Montironi, R.; Lopez-Beltran, A. Expression of miR-100 and miR-138 as prognostic biomarkers in non-muscle-invasive bladder cancer. APMIS 2019, 127, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhang, S.; Zuo, L.; Zhou, L. Overexpression of miR-100 inhibits cell proliferation, migration, and chemosensitivity in human glioblastoma through FGFR3. Onco Targets Ther. 2015, 8, 3391–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Chen, B.; Ji, X.X.; Zhou, S.W.; Zheng, D. Overexpression of miR-100 inhibits cancer growth, migration, and chemosensitivity in human NSCLC cells through fibroblast growth factor receptor 3. Tumour Biol. 2016, 37, 15517–15524. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.R.; Morais, D.R.; Reis, S.T.; Viana, N.; Moura, C.; Florez, M.G.; Silva, I.A.; Dip, N.; Srougi, M. MicroRNA 100: A context dependent miRNA in prostate cancer. Clinics (Sao Paulo) 2013, 68, 797–802. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Yu, C.; Wang, M.; Peng, F.; Xiao, J.; Tian, R.; Jiang, J.; Sun, C. MicroRNA-100 regulates pancreatic cancer cells growth and sensitivity to chemotherapy through targeting FGFR3. Tumour Biol. 2014, 35, 11751–11759. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Islam, S.; Saha, T.; Nishat, A.; Biswas, P.K.; Gil, M.; Nkenyereye, L.; El-Sappagh, S.; Islam, M.S.; Cho, S.G. Prognostic role of EGR1 in breast cancer: A systematic review. BMB Rep. 2021, 54, 497–504. [Google Scholar] [CrossRef]
- Di Leva, G.; Piovan, C.; Gasparini, P.; Ngankeu, A.; Taccioli, C.; Briskin, D.; Cheung, D.G.; Bolon, B.; Anderlucci, L.; Alder, H.; et al. Estrogen mediated-activation of miR-191/425 cluster modulates tumorigenicity of breast cancer cells depending on estrogen receptor status. PLoS Genet. 2013, 9, e1003311. [Google Scholar] [CrossRef]
- Lyu, J.H.; Park, D.W.; Huang, B.; Kang, S.H.; Lee, S.J.; Lee, C.; Bae, Y.S.; Lee, J.G.; Baek, S.H. RGS2 suppresses breast cancer cell growth via a MCPIP1-dependent pathway. J. Cell. Biochem. 2015, 116, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Q.; Cao, Y.; Tan, J.; Wang, F.; Jiang, J.; Cao, Y. Downregulation of regulator of G protein signaling 2 expression in breast invasive carcinoma of no special type: Clinicopathological associations and prognostic relevance. Oncol. Lett. 2018, 15, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef] [PubMed]

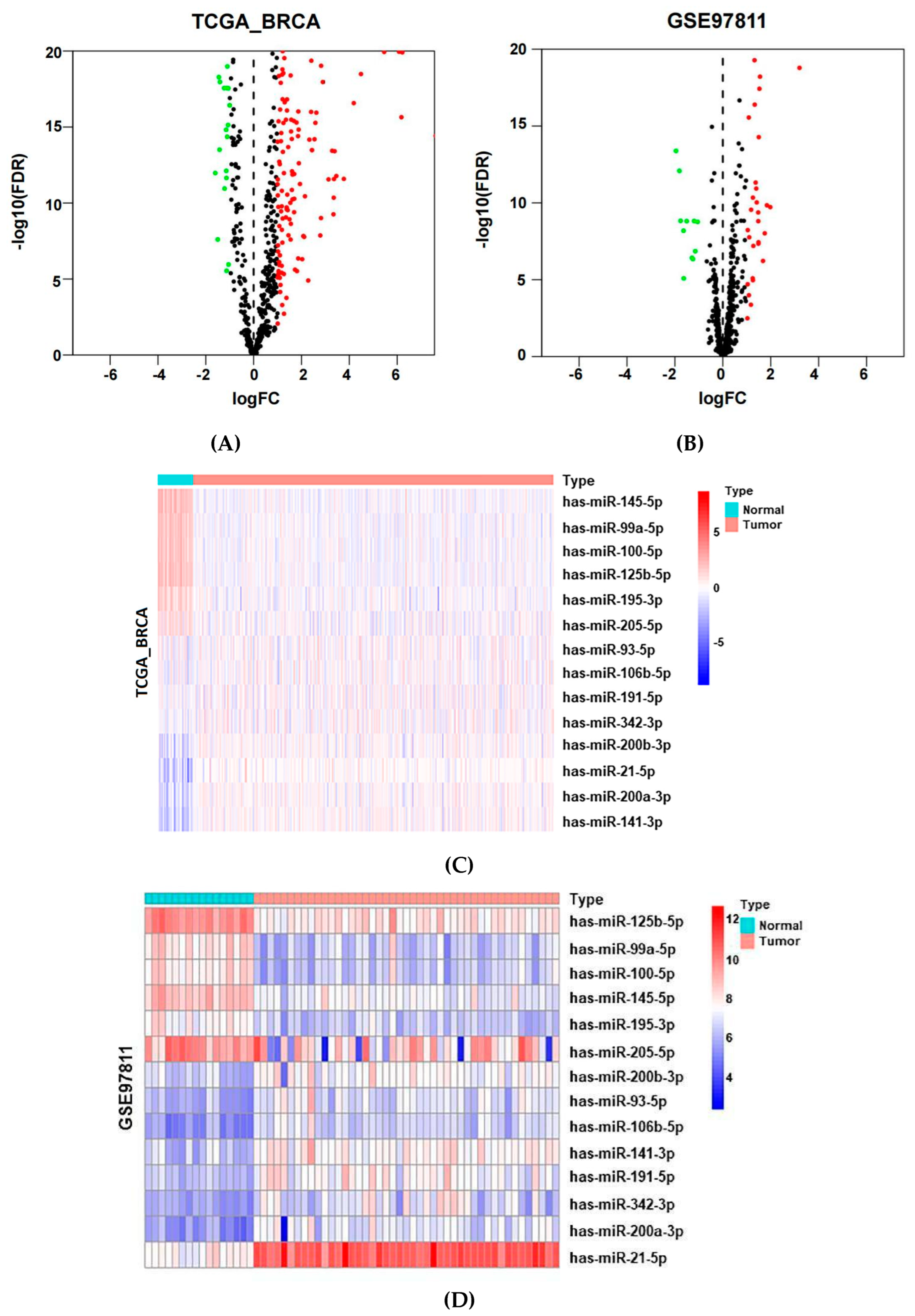


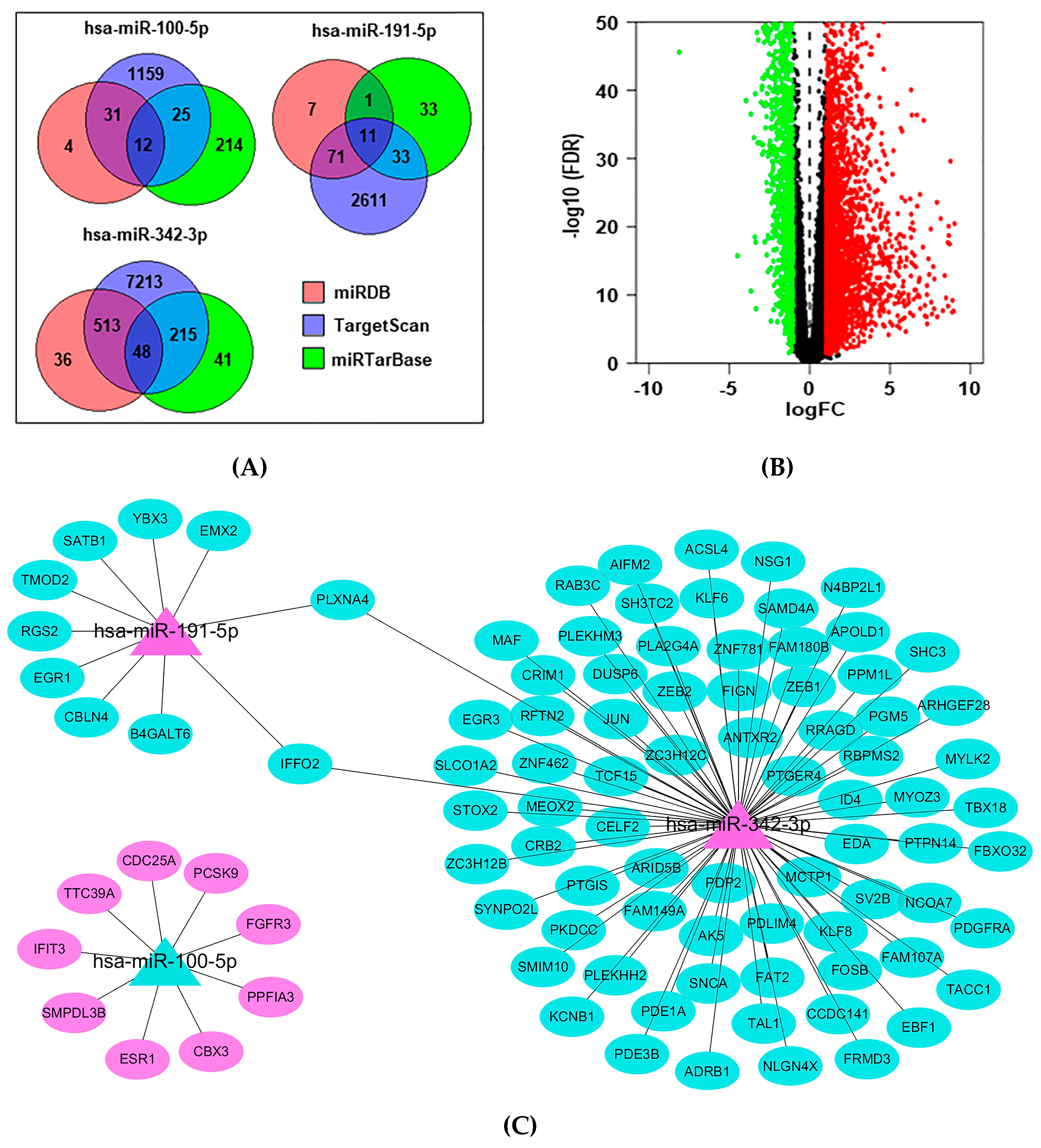

| ID | TCGA_BRCA | GSE97811 | ||||
|---|---|---|---|---|---|---|
| logFC | p | FDR | logFC | p | FDR | |
| hsa-miR-145-5p | −2.29 | 6.23 × 10−215 | 8.75 × 10−213 | −1.51 | 1.00 × 10−10 | 1.55 × 10−09 |
| hsa-miR-99a-5p | −1.96 | 1.19 × 10−97 | 4.41 × 10−96 | −1.76 | 9.04 × 10−11 | 1.47 × 10−09 |
| hsa-miR-125b-5p | −1.83 | 2.24 × 10−118 | 1.31 × 10−116 | −1.81 | 1.98 × 10−14 | 8.25 × 10−13 |
| hsa-miR-100-5p | −1.76 | 1.90 × 10−85 | 5.82 × 10−84 | −1.64 | 4.96 × 10−10 | 6.48 × 10−09 |
| hsa-miR-205-5p | −1.41 | 3.23 × 10−19 | 1.09 × 10−18 | −1.63 | 1.26 × 10−06 | 8.13 × 10−06 |
| hsa-miR-195-5p | −1.24 | 7.99 × 10−44 | 8.65 × 10−43 | −1.29 | 4.28 × 10−08 | 3.75 × 10−07 |
| hsa-miR-93-5p | 1.26 | 6.90 × 10−34 | 5.11 × 10−33 | 1.26 | 1.78 × 10−12 | 4.56 × 10−11 |
| hsa-miR-191-5p | 1.32 | 3.36 × 10−36 | 2.78 × 10−35 | 1.49 | 4.25 × 10−09 | 4.49 × 10−08 |
| hsa-miR-106b-5p | 1.34 | 6.83 × 10−49 | 8.89 × 10−48 | 1.37 | 1.58 × 10−13 | 4.77 × 10−12 |
| hsa-miR-342-3p | 1.68 | 4.42 × 10−34 | 3.34 × 10−33 | 1.68 | 7.28 × 10−08 | 5.99 × 10−07 |
| hsa-miR-200b-3p | 1.83 | 1.20 × 10−48 | 1.54 × 10−47 | 1.27 | 6.15 × 10−09 | 6.30 × 10−08 |
| hsa-miR-21-5p | 2.28 | 1.70 × 10−126 | 1.20 × 10−124 | 3.20 | 7.37 × 10−22 | 1.64 × 10−19 |
| hsa-miR-200a-3p | 2.35 | 1.84 × 10−63 | 3.81 × 10−62 | 1.84 | 6.24 × 10−12 | 1.43 × 10−10 |
| hsa-miR-141-3p | 2.7 | 5.05 × 10−83 | 1.48 × 10−81 | 1.48 | 1.02 × 10−10 | 1.55 × 10−09 |
| ID | Healthy Controls (mean ± SD) | Breast Cancer Patients (mean ± SD) | p |
|---|---|---|---|
| hsa-miR-145-5p | 2.12 ± 1.07 | 4.78 ± 1.19 | <0.001 |
| hsa-miR-99a-5p | 2.01 ± 1.07 | 4.75 ± 1.32 | <0.001 |
| hsa-miR-125b-5p | 2.28 ± 0.83 | 4.49 ± 1.16 | <0.001 |
| hsa-miR-100-5p | 1.97 ± 1.08 | 4.60 ± 1.33 | <0.001 |
| hsa-miR-205-5p | 2.40 ± 0.95 | 4.68 ± 1.14 | <0.001 |
| hsa-miR-195-5p | 1.92 ± 1.02 | 4.35 ± 1.19 | <0.001 |
| hsa-miR-93-5p | 2.29 ± 1.12 | 4.82 ± 1.11 | <0.001 |
| hsa-miR-191-5p | 2.37 ± 1.23 | 6.20 ± 1.38 | <0.001 |
| hsa-miR-106b-5p | 2.06 ± 1.08 | 4.60 ± 1.10 | <0.001 |
| hsa-miR-342-3p | 2.86 ± 0.70 | 4.79 ± 1.03 | <0.001 |
| hsa-miR-200b-3p | 2.02 ± 1.02 | 4.53 ± 1.27 | <0.001 |
| hsa-miR-21-5p | 2.07 ± 1.14 | 4.85 ± 1.20 | <0.001 |
| hsa-miR-200a-3p | 1.89 ± 1.03 | 4.43 ± 1.28 | <0.001 |
| hsa-miR-141-3p | 1.81 ± 1.02 | 4.24 ± 1.25 | <0.001 |
| Variable | Breast Cancer | Healthy Control | p |
|---|---|---|---|
| Number | 108 | 103 | |
| Age (mean ± SD) | 52.79 ± 9.55 | 50.01 ± 13.49 | 0.087 |
| Molecular subtype, N (%) | |||
| Luminal A | 26 (24.07) | NA | |
| Luminal B | 37 (34.26) | NA | |
| HER2 | 27 (25.00) | NA | |
| TNBC | 18 (16.67) | NA | |
| Stage, N (%) | |||
| I | 9 (8.33) | NA | |
| II | 69 (63.89) | NA | |
| III | 30 (27.78) | NA | |
| T, N (%) | |||
| T1 | 51 (47.22) | NA | |
| T2 | 53 (49.07) | NA | |
| T3 | 4 (3.70) | NA | |
| N, N (%) | |||
| N0 | 80 (74.07) | NA | |
| N1 | 21 (19.44) | NA | |
| N2 | 4 (3.70) | NA | |
| N3 | 3 (2.78) | NA | |
| CEA (μg/L) (median, range) | 1.24 (0.92, 2.05) | 1.17 (0.82, 1.75) | 0.296 |
| CA153 (U/mL) (median, range) | 8.40 (5.63, 12.27) | 9.36 (7.52, 12.10) | 0.076 |
| 2−ΔΔCq of hsa-miR-100-5p | 1.34 (0.79, 2.33) | 0.89 (0.55, 1.73) | 0.0033 |
| (median, range) | |||
| 2−ΔΔCq of hsa-miR-191-5p | 11.49 (7.35, 21.61) | 1.09 (0.63, 1.81) | <0.001 |
| (median, range) | |||
| 2−ΔΔCq of hsa-miR-342-3p | 2.81 (2.07, 4.04) | 1.07 (0.65, 1.48) | <0.001 |
| (median, range) |
| ID | BC | Early Stage of BC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Sensitivity | Specificity | Youden Index | p | AUC | Sensitivity | Specificity | Youden Index | p | |
| (95%CI) | (%) | (%) | (95%CI) | (%) | (%) | |||||
| hsa-miR-100-5p | 0.6171 | 72.22 | 54.37 | 0.2659 | 0.003 | 0.6254 | 74.36 | 54.37 | 0.2873 | 0.004 |
| (A) | (0.5411–0.6932) | (0.5444–0.7064) | ||||||||
| hsa-miR-191-5p | 0.9549 | 86.11 | 97.09 | 0.8320 | <0.001 | 0.9551 | 84.62 | 97.09 | 0.8170 | <0.001 |
| (B) | (0.9246–0.9852) | (0.9235–0.9868) | ||||||||
| hsa-miR-342-3p | 0.8969 | 75.93 | 92.23 | 0.6816 | <0.001 | 0.8950 | 89.74 | 76.70 | 0.6644 | <0.001 |
| (C) | (0.8510–0.9428) | (0.8440–0.9460) | ||||||||
| (A + B) * | 0.9615 | 93.52 | 93.20 | 0.8672 | <0.001 | 0.9556 | 92.31 | 93.20 | 0.8551 | <0.001 |
| (0.9326–0.9905) | (0.9193–0.9919) | |||||||||
| (B + C) * | 0.949 | 85.19 | 97.09 | 0.8227 | <0.001 | 0.9482 | 84.62 | 95.15 | 0.7976 | <0.001 |
| (0.9157–0.9823) | (0.9124–0.9840) | |||||||||
| (A + C) * | 0.9187 | 78.70 | 94.17 | 0.7288 | <0.001 | 0.9142 | 92.31 | 81.55 | 0.7386 | <0.001 |
| (0.8781–0.9594) | (0.8660–0.9624) | |||||||||
| (A + B + C) * | 0.9504 | 92.59 | 95.15 | 0.8774 | <0.001 | 0.9431 | 88.46 | 97.09 | 0.8555 | <0.001 |
| (0.9162–0.9845) | (0.9012–0.9850) | |||||||||
| Name | Primer Sequence |
|---|---|
| hsa-miR-100-5p stem-loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAG |
| hsa-miR-100-5p forward primer | GCGAACCCGTAGATCCGAA |
| hsa-miR-191-5p stem-loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCAGCTG |
| hsa-miR-191-5p forward primer | CGCAACGGAATCCCAAAAG |
| hsa-miR-342-3p stem-loop primer | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACGGGT |
| hsa-miR-342-3p forward primer | GCGTCTCACACAGAAATCGC |
| U6 forward primer | CTCGCTTCGGCAGCACA |
| U6 reverse primer | AACGCTTCACGAATTTGCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Li, L.; Yang, M.; Wang, X.; Zhang, H.; Wu, N.; Jia, K.; Wang, J.; Li, M.; Wei, L.; et al. Identification of Three Circulating MicroRNAs in Plasma as Clinical Biomarkers for Breast Cancer Detection. J. Clin. Med. 2023, 12, 322. https://doi.org/10.3390/jcm12010322
Wang S, Li L, Yang M, Wang X, Zhang H, Wu N, Jia K, Wang J, Li M, Wei L, et al. Identification of Three Circulating MicroRNAs in Plasma as Clinical Biomarkers for Breast Cancer Detection. Journal of Clinical Medicine. 2023; 12(1):322. https://doi.org/10.3390/jcm12010322
Chicago/Turabian StyleWang, Shuang, Lijuan Li, Mengmeng Yang, Xiaoyan Wang, Huan Zhang, Nan Wu, Kaichao Jia, Junchao Wang, Menghui Li, Lijuan Wei, and et al. 2023. "Identification of Three Circulating MicroRNAs in Plasma as Clinical Biomarkers for Breast Cancer Detection" Journal of Clinical Medicine 12, no. 1: 322. https://doi.org/10.3390/jcm12010322






