Corin—The Early Marker of Preeclampsia in Pregestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Monitoring of Laboratory and Clinical Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duley, L. The Global Impact of Pre-eclampsia and Eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G. Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granger, J.P.; Alexander, B.T.; Llinas, M.T.; Bennett, W.A.; Khalil, R.A. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 2001, 38, 718–722. [Google Scholar] [CrossRef]
- Ridder, A.; Giorgione, V.; Khalil, A.; Thilaganathan, B. Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function. Int. J. Mol. Sci. 2019, 20, 3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausvater, A.; Giannone, T.; Sandoval, Y.H.G.; Doonan, R.J.; Antonopoulos, C.N.; Matsoukis, I.L.; Ioannis, L.; Petridou, E.T.; Daskalopoulou, S.S. The association between preeclampsia and arterial stiffness. J. Hypertens. 2012, 30, 17–33. [Google Scholar] [CrossRef]
- Hale, S.A.; Badger, G.J.; McBride, C.; Magness, R.; Bernstein, I.M. Prepregnancy Vascular Dysfunction in Women who Subsequently Develop Hypertension During Pregnancy. Pregnancy Hypertens. 2013, 3, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajnerova, O.; Kafka, P.; Kratzerova, T.; Chalupsky, K.; Hampl, V. Pregestational diabetes increases fetoplacental vascular resistance in rats. Placenta 2018, 63, 32–38. [Google Scholar] [CrossRef]
- Hausvater, A.; Giannone, T.; Sandoval, Y.-H.G.; Doonan, R.J.; Antonopoulos, C.N.; Matsoukis, I.L.; Petridou, E.T.; Daskalopoulou, S.S. Pregnancy Outcomes in Women with Long-Duration Type 1 Diabetes-25 Years of Experience. J. Clin. Med. 2020, 9, E3223. [Google Scholar]
- Boroń, D.; Kornacki, J.; Wender-Ozegowska, E. The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology. J. Clin. Med. 2022, 11, 1168. [Google Scholar] [CrossRef]
- Cosmi, E.; Visentin, S.; Fanelli, T.; Mautone, A.J.; Zanardo, V. Aortic intima media thickness in fetuses and children with intrauterine growth restriction. Obstet. Gynecol. 2009, 114, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Pell, J.P.; Walsh, D. Pregnancy complications and maternal risk of ischaemic heart disease: A retrospective cohort study of 129,290 births. Lancet 2001, 357, 2002–2006. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [Green Version]
- Irgens, H.U.; Reisæter, L.; Irgens, L.M.; Lie, R.T.; Roberts, J.M. Long term mortality of mothers and fathers after pre-eclampsia: Population based cohort study. BMJ 2001, 323, 1213–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatjis, C.G.; Greelish, J.P.; Kofinas, A.D.; Stroud, A.; Hashimoto, K.; Rose, J.C. Atrial natriuretic factor maternal and fetal concentrations in severe preeclampsia. Am. J. Obstet. Gynecol. 1989, 161, 1015–1019. [Google Scholar] [CrossRef]
- Tihtonen, K.M.; Kööbi, T.; Vuolteenaho, O.; Huhtala, H.S.; Uotila, J.T. Natriuretic peptides and hemodynamics in preeclampsia. Am. J. Obstet. Gynecol. 2007, 196, 328.e1–328.e7. [Google Scholar] [CrossRef]
- Borghi, C.; Esposti, D.D.; Immordino, V.; Cassani, A.; Boschi, S.; Bovicelli, L. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am. J. Obstet. Gynecol. 2000, 183, 140–147. [Google Scholar] [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 8525–8529. Available online: https://pubmed.ncbi.nlm.nih.gov/10880574/ (accessed on 15 September 2022). [CrossRef] [Green Version]
- Zhou, Y.; Wu, Q. Corin in natriuretic peptide processing and hypertension. Curr. Hypertens. Rep. 2014, 16, 415. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Xu-cai, Y.O.; Chen, S.; Wang, W. Corin: New insights into the natriuretic peptide system. Kidney Int. 2009, 75, 142–146. Available online: https://pubmed.ncbi.nlm.nih.gov/18716601/ (accessed on 15 September 2022). [CrossRef] [Green Version]
- Dong, N.; Chen, S.; Yang, J.; He, L.; Liu, P.; Zheng, D.; Li, L.; Zhou, Y.; Ruan, C.; Plow, E.; et al. Plasma soluble corin in patients with heart failure. Circ. Heart Fail. 2010, 3, 207–211. Available online: https://pubmed.ncbi.nlm.nih.gov/20061521/ (accessed on 15 September 2022). [CrossRef] [PubMed]
- Ibebuogu, U.N.; Gladysheva, I.P.; Huong, A.K.; Reed, G.L. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ. Heart Fail. 2011, 4, 114–120. Available online: https://pubmed.ncbi.nlm.nih.gov/21216831/ (accessed on 15 September 2022). [CrossRef] [PubMed] [Green Version]
- Yu, R.; Han, X.; Zhang, X.; Wang, Y.; Wang, T. Circulating soluble corin as a potential biomarker for cardiovascular diseases: A translational review. Clin. Chim. Acta 2018, 485, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.; Kaminski, H.J.; Kastner, C.; Wang, W.; Krämer, S.; Gambaryan, S.; Russwurm, M.; Peters, H.; Wu, Q.; Vandewalle, A.; et al. Decreased renal corin expression contributes to sodium retention in proteinuric kidney diseases. Kidney Int. 2010, 78, 650–659. Available online: https://pubmed.ncbi.nlm.nih.gov/20613715/ (accessed on 15 September 2022). [CrossRef] [PubMed] [Green Version]
- Badrov, M.B.; Park, S.Y.; Yoo, J.-K.; Hieda, M.; Okada, Y.; Jarvis, S.S.; Stickford, A.S.; Best, S.A.; Nelson, D.B.; Fu, Q. Role of Corin in Blood Pressure Regulation in Normotensive and Hypertensive Pregnancy. Hypertension 2019, 73, 432–439. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, W.; Dong, N.; Lou, J.; Srinivasan, D.K.; Cheng, W.; Huang, X.; Liu, M.; Fang, C.; Peng, J.; et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012, 484, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta 2013, 34, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, J.; Nishizawa, H.; Kambayashi, A.; Ito, M.; Noda, Y.; Terasawa, S.; Kato, T.; Miyamura, H.; Shiogama, K.; Sekiya, T.; et al. Increased levels of soluble corin in pre-eclampsia and fetal growth restriction. Placenta 2016, 48, 20–25. [Google Scholar] [CrossRef]
- Wender-Ożegowska, E.; Bomba-Opoń, D.; Brązert, J.; Celewicz, Z.; Czajkowski, K.; Gutaj, P.; Malinowska-Polubiec, A.; Zawiejska, A.; Wielgoś, M. Standards of Polish Society of Gynecologists and Obstetricians in management of women with diabetes. Ginekol. Polska 2018, 89, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Hare, J.W.; White, P. Gestational Diabetes and the White Classification. Diabetes Care 1980, 3, 394. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [PubMed]
- Kornacki, J.; Boroń, D.; Gutaj, P.; Mantaj, U.; Wirstlein, P.; Wender-Ozegowska, E. Diagnosis of preeclampsia in women with diabetic kidney disease. Hypertens. Pregnancy 2021, 40, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Maiz, N.; Garcia-Mandujano, R.; Elkhouli, M.; Nicolaides, K.H. Longitudinal changes in maternal corin and mid-regional proatrial natriuretic peptide in women at risk of pre-eclampsia. Ultrasound Obstet. Gynecol. 2015, 45, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Y.; Dong, Y.; Liu, W.; Li, H.; Song, W. Correlation between N-terminal pro-atrial natriuretic peptide, corin, and target organ damage in hypertensive disorders of pregnancy. J. Clin. Hypertens. 2022, 24, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Degrelle, S.A.; Chissey, A.; Stepanian, A.; Fournier, T.; Guibourdenche, J.; Mandelbrot, L.; Tsatsaris, V. Placental Overexpression of Soluble CORIN in Preeclampsia. Am. J. Pathol. 2020, 190, 970–976. [Google Scholar] [CrossRef]
- Knappe, S.; Wu, F.; Masikat, M.R.; Morser, J.; Wu, Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: Design and characterization of a soluble corin. J. Biol. Chem. 2003, 278, 52363–52370. [Google Scholar] [CrossRef] [Green Version]
- Figueras, F.; Gratacós, E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014, 36, 86–98. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Torbe, A.; Borowski, D.; Breborowicz, G.; Czajkowski, K.; Huras, H.; Kajdy, A.; Kalinka, J.; Kosinska-Kaczynska, K.; Leszczynska-Gorzelak, B.; et al. Polish Society of Gynecologists and Obstetricians Recommendations on diagnosis and management of fetal growth restriction. Ginekol. Polska 2020, 91, 634–643. [Google Scholar] [CrossRef]
- Wang, W.; Cui, Y.; Shen, J.; Jiang, J.; Chen, S.; Peng, J.; Wu, Q. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension 2012, 60, 1352–1358. [Google Scholar] [CrossRef] [Green Version]
- Melo, L.G.; Veress, A.T.; Chong, C.K.; Pang, S.C.; Flynn, T.G.; Sonnenberg, H. Salt-sensitive hypertension in ANP knockout mice: Potential role of abnormal plasma renin activity. Am. J. Physiol. Integr. Comp. Physiol. 1998, 274, R255–R261. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.Y.; Knudson, O.; Wu, F.; Morser, J.; Dole, W.P.; Wu, Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. USA 2005, 102, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abassi, Z.; Kinaneh, S.; Skarzinski, G.; Cinnamon, E.; Smith, Y.; Bursztyn, M.; Ariel, I. Aberrant corin and PCSK6 in placentas of the maternal hyperinsulinemia IUGR rat model. Pregnancy Hypertens. 2020, 21, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dries, D.L.; Victor, R.G.; Rame, J.E.; Cooper, R.S.; Wu, X.; Zhu, X.; Leonard, D.; Ho, S.-I.; Wu, Q.; Post, W.; et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 2005, 112, 2403–2410. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liao, X.; Fukuda, K.; Knappe, S.; Wu, F.; Dries, D.L.; Qin, J.; Wu, Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ. Res. 2008, 103, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Zaki, M.A.; El-Banawy, S.E.-D.S.; El-Gammal, H.H. Plasma soluble corin and N-terminal pro-atrial natriuretic peptide levels in pregnancy induced hypertension. Pregnancy Hypertens. 2012, 2, 48–52. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Yu, Q.; Zhang, P.; Han, X.; Peng, H. Increased Serum Soluble Corin in Mid Pregnancy Is Associated with Hypertensive Disorders of Pregnancy. J. Women’s Health 2015, 24, 572–577. [Google Scholar] [CrossRef]
- Liu, M.; Wang, R.B.; Xing, J.H.; Tang, Y.X. Nested Case–Control Study of Corin Combined with sFlt-1/PLGF in Predicting the Risk of Preeclampsia. Int. J. Gen. Med. 2021, 14, 2313–2320. [Google Scholar] [CrossRef]
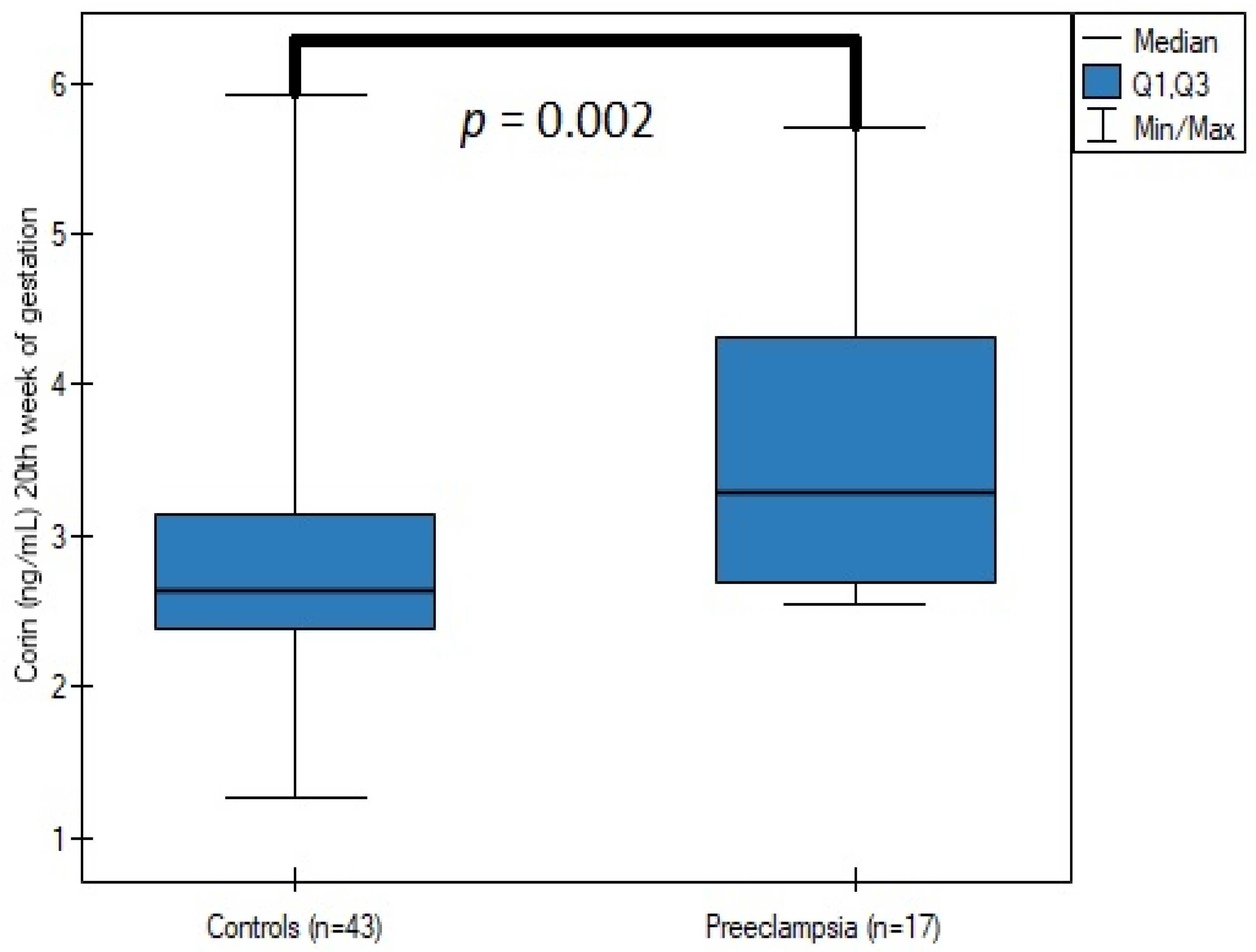

| Parameter | Controls (n = 43) | Preeclampsia (n = 17) | p |
|---|---|---|---|
| Maternal age | 30.47 ± 5.67 | 32 ± 6.24 | 0.36 |
| Maternal height (m) | 1.65 ± 0.077 | 1.63 ± 0.055 | 0.44 |
| Maternal bmi at admission (kg/m2) | 24.28 ± 6.25 | 24.85 ± 3.88 | 0.72 |
| Maternal bmi at delivery (kg/m2) | 27.53 (25.21–31.06) | 28.42 (25.8–30.86) | 0.74 |
| Weight gain (kg) | 10.6 (7.3–15) | 9 (6–14) | 0.72 |
| Nulliparous | 26 (60.47%) | 13 (76.47%) | 0.38 |
| Vascular complications at admission | 14 (32.56%) | 10 (58.82%) | 0.11 |
| Age at diabetes diagnosis (years) | 9 (7–12.5) | 11 (9–14) | 0.18 |
| Diabetes duration (years) | 20 (16–23) | 22 (18–23) | 0.56 |
| Treatment with the insulin pump | 37 (86.05%) | 13 (76.47%) | 0.37 |
| Chronic hypertension | 4 (9.3%) | 8 (47.06%) | 0.001 |
| Fetal FGR | 1 (2.38%) | 4 (23.53%) | 0.008 |
| Gestational age at delivery (weeks) | 38 (37–38) | 35 (33–37) | 0.00003 |
| Newborns’weight | 3348.3 ± 524.9 | 2591.7 ± 907.3 | 0.0002 |
| Cesarean section | 34 (85%) | 17 (100%) | 0.09 |
| Emergency cesarian section | 8 (23.53%) | 6 (35.29%) | 0.37 |
| Parameter | Controls (n = 43) | Preeclampsia (n = 17) | p |
|---|---|---|---|
| Corin—18th–22nd week of gestation (ng/mL) | 2.64 (2.382–3.142) | 3.286 (2.684–4.31) | 0.002 |
| Corin—28th–32nd week of gestation (ng/mL) | 2.93 (2.652–4.174) | 2.754(2.668–3.537) | 0.55 |
| Hba1c—I trimester (%) | 6.50 (6.09–7.31) | 6.61 (6.29–7.27) | 0.46 |
| Hba1c—II trimester (%) | 5.66 ± 0.65 | 5.99 ± 0.95 | 0.13 |
| Hba1c—III trimester (%) | 5.88 ± 0.61 | 6.09 ± 0.77 | 0.27 |
| Triglycerides— I trimester (mg/dL) | 64.6 (52.75–85.35) | 65.7 (46.6–82.6) | 0.84 |
| Triglycerides— II trimester (mg/dL) | 132.6 (107.2–158.1) | 117.2 (102.5–178.1) | 0.94 |
| Triglycerides—III trimester (mg/dL) | 280.61 ± 86.41 | 241.71 ± 51.43 | 0.09 |
| Proteinuria—I trimester (g/24 h) | 0.18 (0.14–0.21) | 0.3 (0.2–0.73) | 0.003 |
| Proteinuria—II trimester (g/24 h) | 0.16 (0.14–0.22) | 0.34 (0.15-0.91) | 0.02 |
| Proteinuria—III trimester (g/24 h)) | 0.22 (0.16–0.31) | 0.78 (0.38–1.82) | 0.000001 |
| Creatinine clearance—I trimester (mL/min) | 126.79 ± 41.24 | 130.59 ± 53.64 | 0.77 |
| Creatinine clearance—II trimester (mL/min) | 138.74 ± 43.15 | 112.01 ± 53.7 | 0.054 |
| Creatinine clearance—III trimester (mL/min) | 127.87 ± 37.64 | 99.26 ± 41.8 | 0.01 |
| Serum creatinine—I trimester (mg/dL) | 0.57 (0.52–0.62) | 0.62 (0.52–0.79) | 0.34 |
| Serum creatinine—II trimester (mg/dL) | 0.595 (0.49–0.62) | 0.62 (0.49–0.72) | 0.13 |
| Serum creatinine—III trimester (mg/dL) | 0.6 (0.52–0.69) | 0.7 (0.64–0.81) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroń, D.; Kornacki, J.; Gutaj, P.; Mantaj, U.; Wirstlein, P.; Wender-Ozegowska, E. Corin—The Early Marker of Preeclampsia in Pregestational Diabetes Mellitus. J. Clin. Med. 2023, 12, 61. https://doi.org/10.3390/jcm12010061
Boroń D, Kornacki J, Gutaj P, Mantaj U, Wirstlein P, Wender-Ozegowska E. Corin—The Early Marker of Preeclampsia in Pregestational Diabetes Mellitus. Journal of Clinical Medicine. 2023; 12(1):61. https://doi.org/10.3390/jcm12010061
Chicago/Turabian StyleBoroń, Daniel, Jakub Kornacki, Paweł Gutaj, Urszula Mantaj, Przemysław Wirstlein, and Ewa Wender-Ozegowska. 2023. "Corin—The Early Marker of Preeclampsia in Pregestational Diabetes Mellitus" Journal of Clinical Medicine 12, no. 1: 61. https://doi.org/10.3390/jcm12010061





