The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review
Abstract
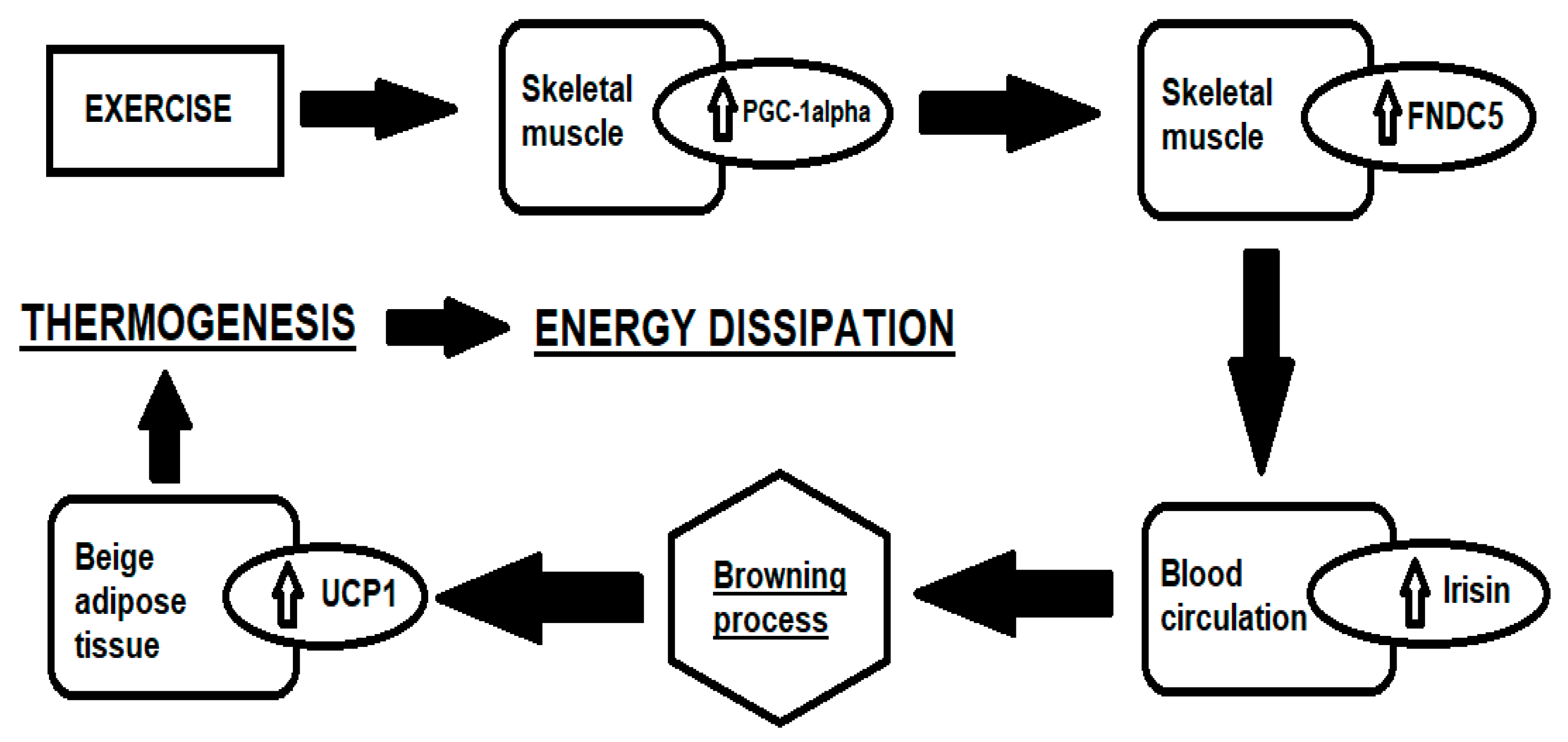
:1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Databases
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
3. Results
3.1. Irisin
3.2. Quality Assessment
4. Discussion
- Exercise intervention increases irisin secretion in individuals with T2D.
- There is not yet sufficient evidence in the literature to determine which exercise training modality results in a greater secretion of irisin in this population.
- Data on the training intensity are partly discordant but they suggest that high intensity exercise facilitates greater secretion of irisin.
- Several factors, such as timing of the samplings, disease history, and the body composition of the patients, represent a large source of variability among the studies reported.
4.1. Influence of Training Modality and Intensity on Irisin Level in T2D
4.2. Exercise Training Effect on Irisin Secretion in T2D Patients: Sources of Variability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoukry, A.; Shalaby, S.M.; El-Arabi Bdeer, S.; Mahmoud, A.A.; Mousa, M.M.; Khalifa, A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life 2016, 68, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, T.; Elbelt, U.; Stengel, A. Irisin as a muscle-derived hormone stimulating thermogenesis—A critical update. Peptides 2014, 54, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Jiang, W.X.; Lv, Z.T. Lower Circulating Irisin Level in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2016, 48, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Kim, M.-K.; Bae, K.H.; Seo, H.-A.; Jeong, J.-Y.; Lee, W.-K.; Kim, J.-G.; Lee, I.-K.; Park, K.-G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.C.; Jeon, W.S.; Park, C.Y.; Youn, B.S. The ratio of skeletal muscle mass to visceral fat area is a main determinant linking circulating irisin to metabolic phenotype. Cardiovasc. Diabetol. 2016, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.S.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.S.; Coombes, J.S. The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: A systematic review and meta-analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Way, K.L.; Sabag, A.; Sultana, R.N.; Baker, M.K.; Keating, S.E.; Lanting, S.; Gerofi, J.; Chuter, V.H.; Caterson, I.D.; Twigg, S.M.; et al. The effect of low-volume high-intensity interval training on cardiovascular health outcomes in type 2 diabetes: A randomised controlled trial. Int. J. Cardiol. 2020, 320, 148–154. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Dünnwald, T.; Melmer, A.; Gatterer, H.; Salzmann, K.; Ebenbichler, C.; Burtscher, M.; Schobersberger, W.; Grander, W. Supervised Short-term High-intensity Training on Plasma Irisin Concentrations in Type 2 Diabetic Patients. Int. J. Sports Med. 2019, 40, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Enteshary, M.; Esfarjani, F.; Reisi, J. Comparison of the Effects of Two Different Intensities of Combined Training on Irisin, Betatrophin, and Insulin Levels in Women with Type 2 Diabetes. Asian J. Sports Med. 2019, 10, 68943. [Google Scholar] [CrossRef]
- Banitalebi, E.; Kazemi, A.R.; Faramarzi, M.; Nasiri, S.; Haghighi, M.M. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: A randomized controlled trial. Life Sci. 2019, 217, 101–109. [Google Scholar] [CrossRef]
- Bonfante, I.L.P.; Monfort-Pires, M.; Duft, R.G.; Mateus, K.C.D.S.; Júnior, J.C.D.L.; Trombeta, J.C.D.S.; Finardi, E.A.R.; Brunelli, D.T.; Morari, J.; de Lima, J.A.B.; et al. Combined training increases thermogenic fat activity in patients with overweight and type 2 diabetes. Int. J. Obes. 2022, 46, 1145–1154. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Fan, X.; Liang, H.; Han, R. The impact of exercise on serum irisin, osteocalcin, and adiponectin levels and on glycolipid metabolism in patients with type 2 diabetes. Int. J. Clin. Exp. Med. 2020, 13, 7816–7824. Available online: www.ijcem.com (accessed on 12 December 2022).
- Motahari Rad, M.; Bijeh, N.; Attarzadeh Hosseini, S.R.; Raouf Saeb, A. The effect of two concurrent exercise modalities on serum concentrations of FGF21, irisin, follistatin, and myostatin in men with type 2 diabetes mellitus. Arch. Physiol. Biochem. 2020, 12, 1829649. [Google Scholar] [CrossRef]
- Fatouros, I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef]
- Cosio, P.L.; Crespo-Posadas, M.; Velarde-Sotres, Á.; Pelaez, M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2476. [Google Scholar] [CrossRef]
- Rahimi, G.R.M.; Hejazi, K.; Hofmeister, M. The effect of exercise interventions on Irisin level: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2022, 21, 524–539. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; So, B.; Son, J.S.; Yoon, D.; Song, W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: A pilot study. Physiol. Res. 2016, 65, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Rioux, B.V.; Goulet, E.D.B.; Johanssen, N.M.; Swift, D.L.; Bouchard, D.R.; Loewen, H.; Sénéchal, M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand. J. Med. Sci. Sports 2018, 28, 16–28. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef]
- Gamas, L.; Matafome, P.; Seicą, R. Irisin and Myonectin Regulation in the Insulin Resistant Muscle: Implications to Adipose Tissue: Muscle Crosstalk. J. Diabetes Res. 2015, 2015, 359159. [Google Scholar] [CrossRef] [Green Version]
- Parada-Sánchez, S.G.; Macias-Cervantes, M.H.; Pérezvázquez, V.; Vargas-Ortiz, K. The Effects of Different Types of Exercise on Circulating Irisin Levels in Healthy Individuals and in People with Overweight, Metabolic Syndrome and Type 2 Diabetes. Physiol. Res. 2022, 71, 457–475. [Google Scholar] [CrossRef]
- Qiu, S.; Bosnyák, E.; Treff, G.; Steinacker, J.M.; Nieß, A.M.; Krüger, K.; Mooren, F.C.; Zügel, M.; Schumann, U. Acute exercise-induced irisin release in healthy adults: Associations with training status and exercise mode. Eur. J. Sport Sci. 2018, 18, 1226–1233. [Google Scholar] [CrossRef]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef]
- Kraemer, R.R.; Shockett, P.; Webb, N.D.; Shah, U.; Castracane, V.D. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm. Metab. Res. 2014, 46, 150–154. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Cai, X.; Sun, Z.; Schumann, U.; Zügel, M.; Steinacker, J.M. Chronic Exercise Training and Circulating Irisin in Adults: A Meta-Analysis. Sports Med. 2015, 45, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A.; Baar, K.; Davidsen, P.K.; Atherton, P.J. Is irisin a human exercise gene? Nature 2012, 488, E9–E10. [Google Scholar] [CrossRef] [PubMed]
- Vecchiato, M.; Quinto, G.; Palermi, S.; Foccardi, G.; Mazzucato, B.; Battista, F.; Duregon, F.; Michieletto, F.; Neunhaeuserer, D.; Ermolao, A. Are Gyms a Feasible Setting for Exercise Training Interventions in Patients with Cardiovascular Risk Factors? An Italian 10-Years Cross-Sectional Survey Comparison. Int. J. Environ. Res. Public Health 2022, 19, 2407. [Google Scholar] [CrossRef] [PubMed]
- Micielska, K.; Kortas, J.A.; Gmiat, A.; Jaworska, J.; Kozlowska, M.; Lysak-Radomska, A.; Rodziewicz-Flis, E.; Zychowska, M.; Ziemann, E. Habitually inactive physically—A proposed procedure of counteracting cognitive decline in women with diminished insulin sensitivity through a high-intensity circuit training program. Physiol. Behav. 2021, 229, 113235. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [Green Version]
- Wen, M.S.; Wang, C.Y.; Lin, S.L.; Hung, K.C. Decrease in Irisin in Patients with Chronic Kidney Disease. PLoS ONE 2013, 8, e64025. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-J.; Zhang, X.-F.; Ma, Z.-M.; Pan, L.-L.; Chen, Z.; Han, H.-W.; Han, C.-K.; Zhuang, X.-J.; Lu, Y.; Li, X.-J.; et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J. Hepatol. 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Dianatinasab, A.; Koroni, R.; Bahramian, M.; Bagheri-Hosseinabadi, Z.; Vaismoradi, M.; Fararouei, M.; Amanat, S. The effects of aerobic, resistance, and combined exercises on the plasma irisin levels, HOMA-IR, and lipid profiles in women with metabolic syndrome: A randomized controlled trial. J. Exerc. Sci. Fit. 2020, 18, 168–176. [Google Scholar] [CrossRef]
- Amanat, S.; Sinaei, E.; Panji, M.; MohammadporHodki, R.; Bagheri-Hosseinabadi, Z.; Asadimehr, H.; Fararouei, M.; Dianatinasab, A. A Randomized Controlled Trial on the Effects of 12 Weeks of Aerobic, Resistance, and Combined Exercises Training on the Serum Levels of Nesfatin-1, Irisin-1 and HOMA-IR. Front. Physiol. 2020, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity–Correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Liu, S.; Wong, M.D.; Tan, C.S.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J. Diabetes Complicat. 2014, 28, 208–213. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome Division of Endocrinology, Diabetes, and Metabolism (K). J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Shields, K.; Mantzoros, C.S. Irisin: A renaissance in metabolism? Metabolism 2013, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alokail, M.S.; Rahman, S.; Amer, O.E.; Al-Attas, O.S.; Alfawaz, H.; Tripathi, G.; Sabico, S.; Chrousos, G.P.; McTernan, P.G.; et al. Habitual physical activity is associated with circulating irisin in healthy controls but not in subjects with diabetes mellitus type 2. Eur. J. Clin. Investig. 2015, 45, 775–781. [Google Scholar] [CrossRef]
- Türk, Y.; Theel, W.; Kasteleyn, M.J.; Franssen, F.M.E.; Hiemstra, P.S.; Rudolphus, A.; Taube, C.; Braunstahl, G. High intensity training in obesity: A Meta-analysis. Obes. Sci. Pract. 2017, 3, 258–271. [Google Scholar] [CrossRef] [Green Version]
- Hansen, D.; Niebauer, J.; Cornelissen, V.; Barna, O.; Neunhäuserer, D.; Stettler, C.; Tonoli, C.; Greco, E.; Fagard, R.; Coninx, K.; et al. Exercise Prescription in Patients with Different Combinations of Cardiovascular Disease Risk Factors: A Consensus Statement from the EXPERT Working Group. Sports Med. 2018, 48, 1781–1797. [Google Scholar] [CrossRef]
- Battista, F.; Belligoli, A.; Neunhaeuserer, D.; Gasperetti, A.; Bettini, S.; Compagnin, C.; Marchese, R.; Quinto, G.; Bergamin, M.; Vettor, R.; et al. Metabolic Response to Submaximal and Maximal Exercise in People with Severe Obesity, Prediabetes, and Diabetes. Obes. Facts 2021, 14, 415–424. [Google Scholar] [CrossRef]
- Vecchiato, M.; Neunhaeuserer, D.; Quinto, G.; Bettini, S.; Gasperetti, A.; Battista, F.; Vianello, A.; Vettor, R.; Busetto, L.; Ermolao, A. Cardiopulmonary exercise testing in patients with moderate-severe obesity: A clinical evaluation tool for OSA? Sleep Breath. 2021, 26, 1115–1123. [Google Scholar] [CrossRef] [PubMed]


| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Community-dwelling adult patients with T2D | Exercise training | 1. Exercise training vs. controls 2. AT vs. RT 3. HIT vs. MIT | Irisin secretion compared with baseline |
| Author and Year | Population | Age and Gender | T2D Duration (Years) | Number of Participants (Group Distribution) | Study Intervention | Type of Study | Intensity | Supervised | Training Duration (weeks) | Training Volume (min/week) | Antidiabetic Drugs Treatment | Blood Sample Collection | Δ Irisin Concentration | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banitalebi et al., 2019 [16] | T2D; Overweight or obese; sedentary. | 30–65 y; only women | nr | n = 42 (HIIT = 14; RT + AT = 14; controls = 14) | HIIT vs. RT + AT vs. controls | RCT | RT + AT: 1-RM + 60%HRmax; HIIT: 100% of perceived exertion | y | 10 | 150 | Oral (n = 27) +Insulin (n = 20) +Combined (n = 5) | 48 h after intervention period | HIIT: +13.1% RT + AT: +23.6% Controls: +6.5% | No significant differences in serum irisin between groups |
| Dünnwald et al., 2019 [14] | T2D | 50–65 y; both genders | HIIT: 10.7 ± 4.6; CMT: 6.9 ± 4.3 | n = 14 (HIIT = 8 vs. CMT = 6) | HIIT vs. CMT | nRCT | HIIT: 90–95% HRmax (85% VO2max); CMT: 70%HRmax (60% VO2max) | y | 4 | 126 | Oral (n = 11) | 1 day after intervention period | HIIT: +7% CMT: −1.7% | HIIT but not CMT increases the serum irisin concentration |
| Enteshary et al., 2019 [15] | T2D | 35–45 y; only women | nr | n = 26 divided in 3 groups (controls vs. CMT vs. CHT) | CMT vs. CHT vs. controls | RCT | CMT: 62%HRmax; CHT: 80%HRmax | nr | 8 | 150 CMT-375 CHT | Insulin (n not reported) | nr | CMT: +88% CHT: +367% Controls: +6% | CMT and CHT both increase serum irisin. CHT seems to be more effective. |
| Motahari Rad et al., 2020 [19] | T2D | 40–50 y; only men | nr | n = 43 (RT + AT = 15; AT + RT = 15; controls = 13) | RT + AT vs. AT + RT vs. controls | RCT | RT: from 40% to 80% of 1-RM; AT: 75–95%HRmax | y | 12 | 30 (3 times per week of 10 × 1 min HIIT-rest) | Oral (n = 43) | 48h after intervention period | RT + AT: +18.7% AT + RT: +27.2% Controls: −4.5% | Both RT + AT and AT + RT groups increase irisin compared with controls |
| Bonfante et al., 2022 [17] | T2D; Overweight; sedentary | 40–60 y; both genders | RT + AT: 5.5 ± 2.62; controls: 4.94 ± 3.05 | n = 34 (RT + AT = 17; controls = 17) | RT + AT vs. controls | RCT | RT: 1–3 sets of submaximal exercise, AT: 35min at 50–70% of VO2max) | nr | 16 | RT: 120; AT: 105 | Oral (n = 60) | nr | RT + AT: +27.5% Controls: −7.5% | Combined training increases serum irisin |
| Yang et al., 2020 [18] | T2D | Exercise group: 47.7 ± 7.4 y; Controls: 45.2 ± 8.8 y; both genders | nr | n = 60 (AT + RT = 30; controls = 30) | AT (+non enforced RT) vs. controls | RCT | Moderate AT: 60% VO2max; RT: nr | y | 12 | 150 | Oral (n = ?) | nr | AT(+RT): +97% Controls: −8% | Exercise group results in an improvement of the serum irisin compared with controls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchiato, M.; Zanardo, E.; Battista, F.; Quinto, G.; Bergia, C.; Palermi, S.; Duregon, F.; Ermolao, A.; Neunhaeuserer, D. The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review. J. Clin. Med. 2023, 12, 62. https://doi.org/10.3390/jcm12010062
Vecchiato M, Zanardo E, Battista F, Quinto G, Bergia C, Palermi S, Duregon F, Ermolao A, Neunhaeuserer D. The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review. Journal of Clinical Medicine. 2023; 12(1):62. https://doi.org/10.3390/jcm12010062
Chicago/Turabian StyleVecchiato, Marco, Emanuele Zanardo, Francesca Battista, Giulia Quinto, Chiara Bergia, Stefano Palermi, Federica Duregon, Andrea Ermolao, and Daniel Neunhaeuserer. 2023. "The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review" Journal of Clinical Medicine 12, no. 1: 62. https://doi.org/10.3390/jcm12010062
APA StyleVecchiato, M., Zanardo, E., Battista, F., Quinto, G., Bergia, C., Palermi, S., Duregon, F., Ermolao, A., & Neunhaeuserer, D. (2023). The Effect of Exercise Training on Irisin Secretion in Patients with Type 2 Diabetes: A Systematic Review. Journal of Clinical Medicine, 12(1), 62. https://doi.org/10.3390/jcm12010062








