Hemophagocytic Lymphohistiocytosis Associated with Synergistic Defects of AP3B1 and ATM Genes: A Case Report and Literature Review
Abstract
1. Introduction
2. Case Presentation
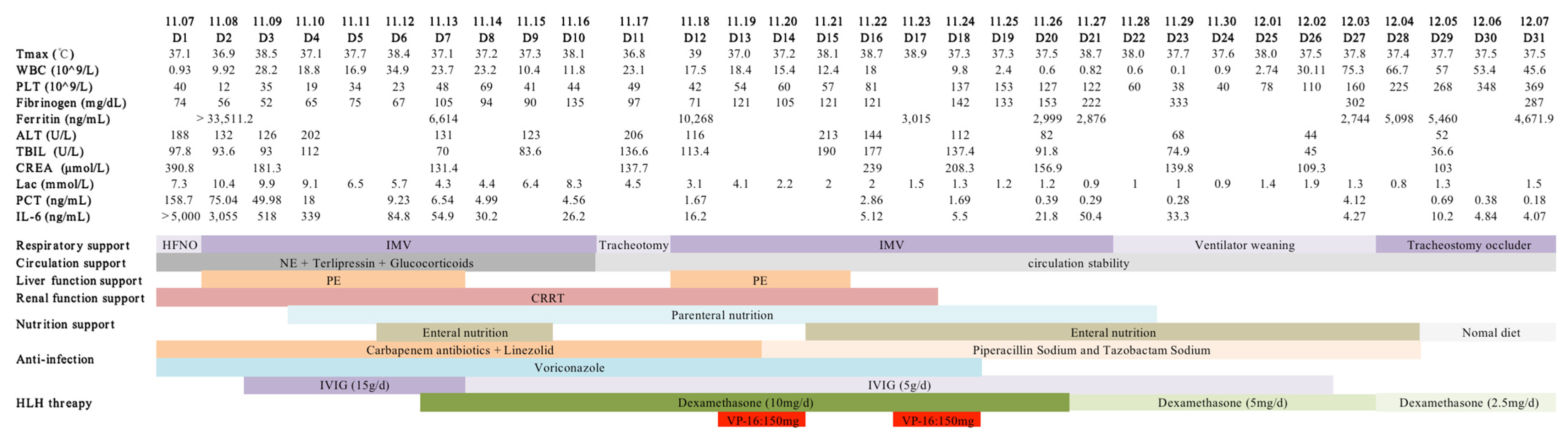
- White blood cell (WBC) and platelet (PLT) counts decreased dramatically. Hemoglobin (HGB) concentration was also reduced.
- Multiorgan dysfunction (including acute liver failure, acute kidney injury, and myocardial injury). Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (CREA), myocardial enzyme, and brain natriuretic peptide (BNP) were noticeably elevated.
- Respiratory failure with PaO2/FiO2 of 220 mmHg.
- Prolonged prothrombin time, markedly elevated D-dimer, and reduced fibrinogen levels.
- High inflammatory response dramatically increased procalcitonin (PCT), interleukin 6 (IL-6), C-reactive protein (CRP), and ferritin.
- High triglyceride (TG) (Table 1).
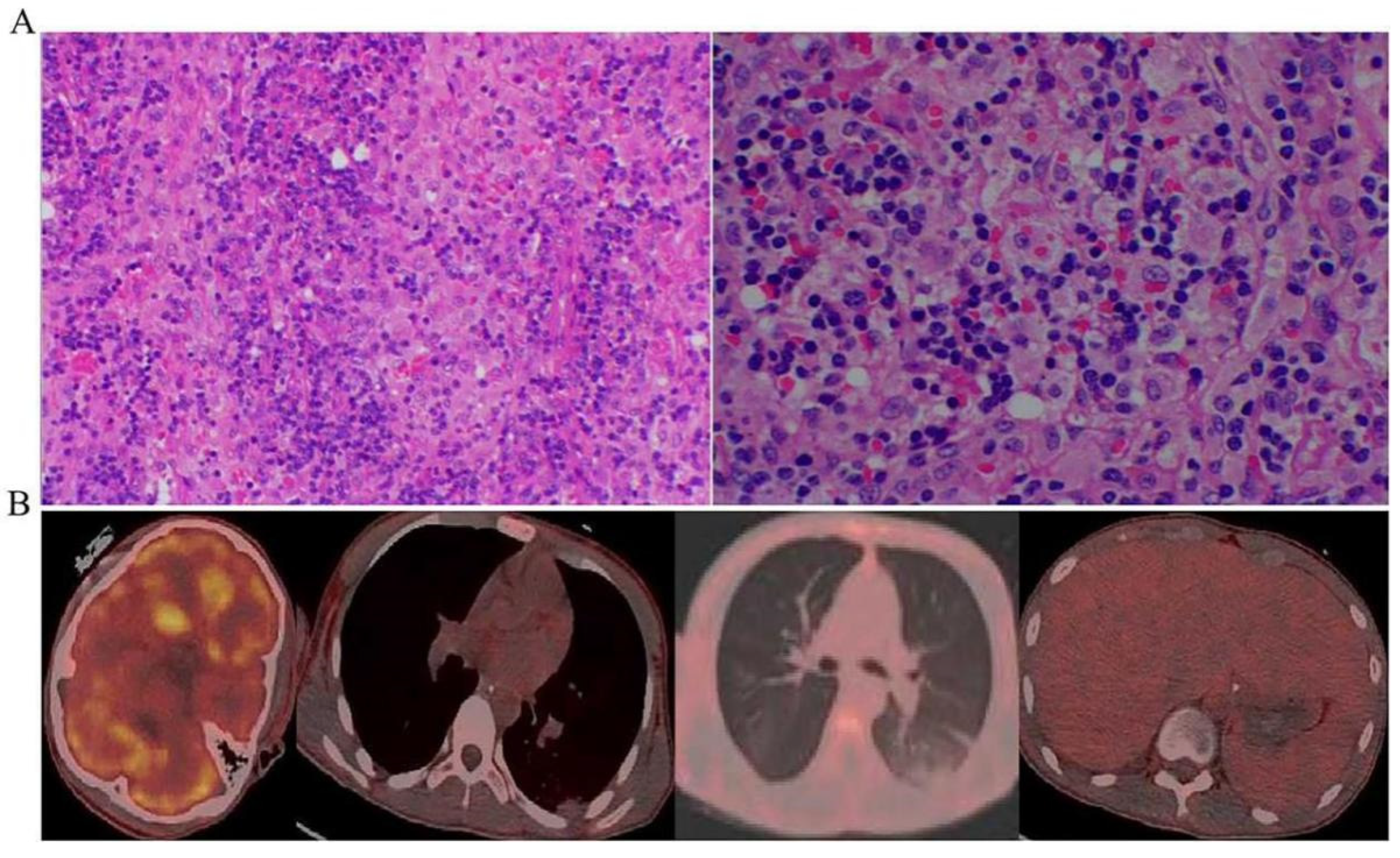
- The chest computed tomography (CT) scan showed inflammatory exudate and oedema in both lungs, bilateral pleural effusion, enlarged liver and spleen, and brain cell oedema (Figure 1).
- Within 24 h of admission to the ICU, the scores of acute physiology and chronic health evaluation-II (APACH II), sequential organ failure assessment (SOFA), and nutrition risk screening-2002 (NRS-2002) were 24, 15, and 5, respectively.
3. Materials and Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canna, S.W.; Marsh, R.A. Pediatric hemophagocytic lymphohistiocytosis. Blood 2020, 135, 1332–1343. [Google Scholar] [CrossRef]
- Griffin, G.; Shenoi, S.; Hughes, G.C. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101515. [Google Scholar] [CrossRef] [PubMed]
- Ishii, E. Hemophagocytic Lymphohistiocytosis in Children: Pathogenesis and Treatment. Front. Pediatr. 2016, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Ponnatt, T.S.; Lilley, C.M.; Mirza, K.M. Hemophagocytic Lymphohistiocytosis. Arch. Pathol. Lab. Med. 2022, 146, 507–519. [Google Scholar] [CrossRef]
- Hines, M.R.; von Bahr Greenwood, T.; Beutel, G.; Beutel, K.; Hays, J.A.; Horne, A.; Janka, G.; Jordan, M.B.; van Laar, J.A.M.; Lachmann, G.; et al. Consensus-Based Guidelines for the Recognition, Diagnosis, and Management of Hemophagocytic Lymphohistiocytosis in Critically Ill Children and Adults. Crit. Care Med. 2022, 50, 860–872. [Google Scholar] [CrossRef]
- Rajagopala, S.; Singh, N.; Agarwal, R.; Gupta, D.; Das, R. Severe hemophagocytic lymphohistiocytosis in adults-experience from an intensive care unit from North India. Indian J. Crit. Care Med. 2012, 16, 198–203. [Google Scholar] [PubMed]
- Al-Samkari, H.; Berliner, N. Hemophagocytic Lymphohistiocytosis. Annu. Rev. Pathol. 2018, 13, 27–49. [Google Scholar] [CrossRef]
- Henderson, L.A.; Cron, R.Q. Macrophage Activation Syndrome and Secondary Hemophagocytic Lymphohistiocytosis in Childhood Inflammatory Disorders: Diagnosis and Management. Paediatr. Drugs 2020, 22, 29–44. [Google Scholar] [CrossRef]
- Imashuku, S.; Morimoto, A.; Ishii, E. Virus-triggered secondary hemophagocytic lymphohistiocytosis. Acta Paediatr. 2021, 110, 2729–2736. [Google Scholar] [CrossRef]
- Janka, G.E. Familial and acquired hemophagocytic lymphohistiocytosis. Annu. Rev. Med. 2012, 63, 233–246. [Google Scholar] [CrossRef]
- Dell’Acqua, F.; Saettini, F.; Castelli, I.; Badolato, R.; Notarangelo, L.D.; Rizzari, C. Hermansky-Pudlak syndrome type II and lethal hemophagocytic lymphohistiocytosis: Case description and review of the literature. J. Allergy Clin. Immunol. Pract. 2019, 7, 2476–2478.e5. [Google Scholar] [CrossRef] [PubMed]
- Celiksoy, M.H.; Cubuk, P.O.; Guner, S.N.; Yildiran, A. A Case of Ataxia-telangiectasia Presented with Hemophagocytic Syndrome. J. Pediatr. Hematol. Oncol. 2018, 40, e547–e549. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Greenberg, J.; Henry, M.; Hermiston, M.L.; Kumar, A.; Hines, M.; Eckstein, O.; Ladisch, S.; Nichols, K.E.; et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr. Blood Cancer 2019, 66, e27929. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.B.; Allen, C.E.; Weitzman, S.; Filipovich, A.H.; McClain, K.L. How I treat hemophagocytic lymphohistiocytosis. Blood 2011, 118, 4041–4052. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.E.; McClain, K.L. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 177–1782. [Google Scholar] [CrossRef]
- Henter, J.I.; Horne, A.; Arico, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef]
- Allen, C.E.; Yu, X.; Kozinetz, C.A.; McClain, K.L. Highly elevated ferritin levels and the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 1227–1235. [Google Scholar] [CrossRef]
- Koperdanova, M.; Cullis, J.O. Interpreting raised serum ferritin levels. BMJ 2015, 351, h3692. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Lachmann, G.; Knaak, C.; Vorderwulbecke, G.; La Rosee, P.; Balzer, F.; Schenk, T.; Schuster, F.S.; Nyvlt, P.; Janka, G.; Brunkhorst, F.M.; et al. Hyperferritinemia in Critically Ill Patients. Crit. Care Med. 2020, 48, 459–465. [Google Scholar] [CrossRef]
- Otrock, Z.K.; Hock, K.G.; Riley, S.B.; de Witte, T.; Eby, C.S.; Scott, M.G. Elevated serum ferritin is not specific for hemophagocytic lymphohistiocytosis. Ann. Hematol. 2017, 96, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.; van Leeuwen, K.; Geissler, J.; van Alphen, F.; de Vries, E.; van der Kuip, M.; Terheggen, S.W.J.; Janssen, H.; van den Berg, T.K.; Meijer, A.B.; et al. Hermansky-Pudlak syndrome type 2: Aberrant pre-mRNA splicing and mislocalization of granule proteins in neutrophils. Hum. Mutat. 2017, 38, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.A.; Hermiston, M.L.; Nichols, K.E.; Meyer, L.K. Digenic Inheritance: Evidence and Gaps in Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2021, 12, 777851. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.L.; Bi-Karchin, J.; Le, L.; Marks, M.S. The road to lysosome-related organelles: Insights from Hermansky-Pudlak syndrome and other rare diseases. Traffic 2019, 20, 404–435. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Nabilou, S.; Mazinani, M.; Tajik, S.; Hamidieh, A.A.; Houshmand, M.; Fazlollahi, M.R.; Pourpak, Z. Partial albinism and immunodeficiency in patients with Hermansky-Pudlak Type II: Introducing 2 novel mutations. Scand. J. Immunol. 2021, 93, e12966. [Google Scholar] [CrossRef]
- Usmani, G.N.; Woda, B.A.; Newburger, P.E. Advances in understanding the pathogenesis of HLH. Br. J. Haematol. 2013, 161, 609–622. [Google Scholar] [CrossRef]
- Hasegawa, J.; Uchida, Y.; Mukai, K.; Lee, S.; Matsudaira, T.; Taguchi, T. A Role of Phosphatidylserine in the Function of Recycling Endosomes. Front. Cell Dev. Biol. 2021, 9, 783857. [Google Scholar] [CrossRef]
- Enders, A.; Zieger, B.; Schwarz, K.; Yoshimi, A.; Speckmann, C.; Knoepfle, E.M.; Kontny, U.; Muller, C.; Nurden, A.; Rohr, J.; et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood 2006, 108, 81–87. [Google Scholar] [CrossRef]
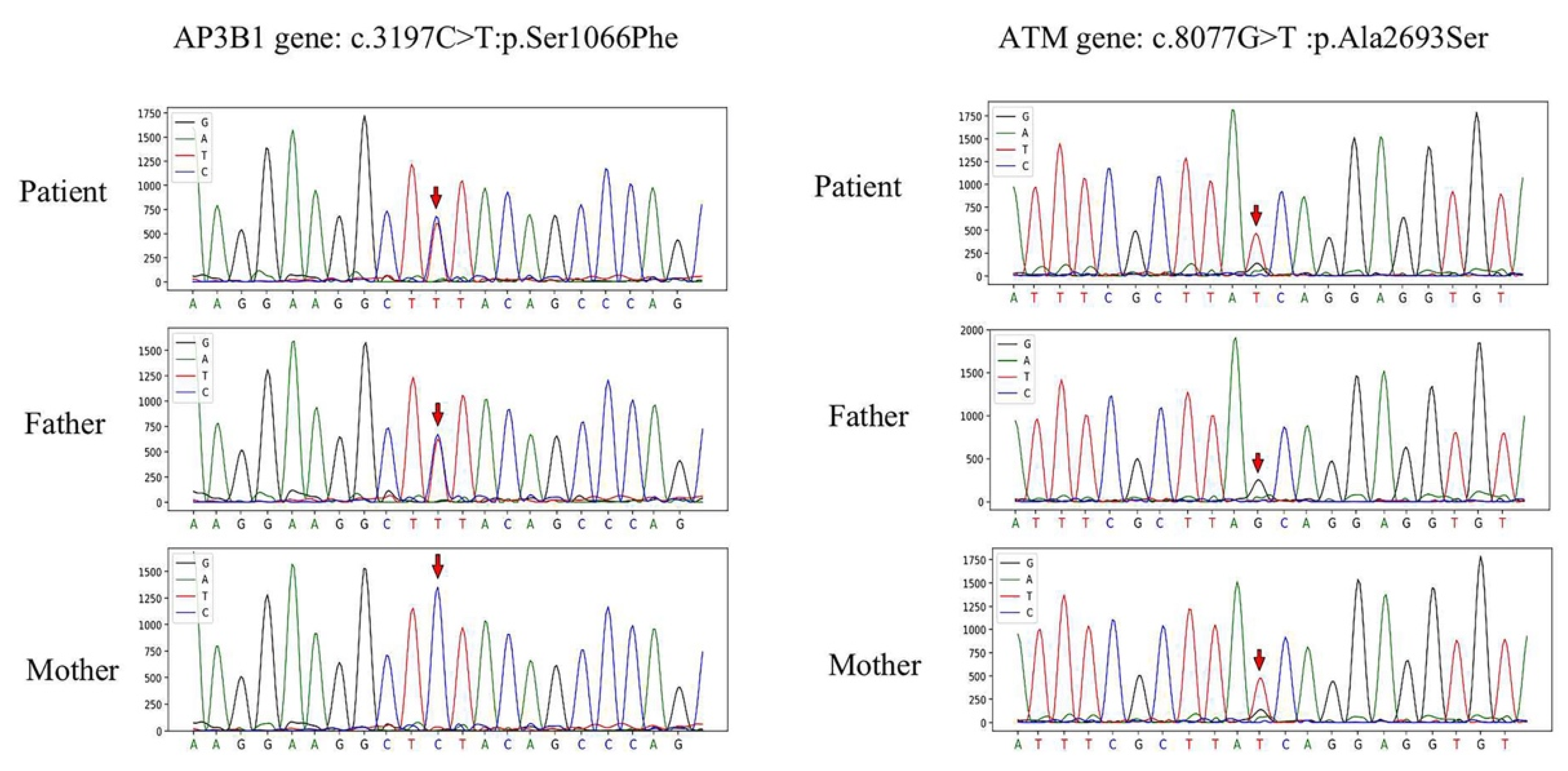
- Ma, D.; Rudd, E.; Edner, J.; Gavhed, S.; Ramme, K.G.; Fadeel, B.; Nordenskjold, M.; Henter, J.I.; Zheng, C. Sequence analysis of the SRGN, AP3B1, ARF6, and SH2D1A genes in familial hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 1067–1069. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, L.; Huang, L.; Zhou, J. Synergistic defects of UNC13D and AP3B1 leading to adult hemophagocytic lymphohistiocytosis. Int. J. Hematol. 2015, 102, 488–492. [Google Scholar] [CrossRef]
- Gumy-Pause, F.; Wacker, P.; Sappino, A.P. ATM gene and lymphoid malignancies. Leukemia 2004, 18, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kipps, T.; Kurzrock, R. ATM Mutations in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2016, 15, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M. Mechanisms Underlying the Suppression of Chromosome Rearrangements by Ataxia-Telangiectasia Mutated. Genes 2021, 12, 1232. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef]
- Shibata, A.; Jeggo, P.A. ATM’s Role in the Repair of DNA Double-Strand Breaks. Genes 2021, 12, 1370. [Google Scholar] [CrossRef]
- Macheret, M.; Halazonetis, T.D. DNA replication stress as a hallmark of cancer. Annu. Rev. Pathol. 2015, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Samuelsson-Horne, A.; Arico, M.; Egeler, R.M.; Elinder, G.; Filipovich, A.H.; Gadner, H.; Imashuku, S.; Komp, D.; Ladisch, S.; et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 2002, 100, 2367–2373. [Google Scholar] [CrossRef]
- Bergsten, E.; Horne, A.; Arico, M.; Astigarraga, I.; Egeler, R.M.; Filipovich, A.H.; Ishii, E.; Janka, G.; Ladisch, S.; Lehmberg, K.; et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: Long-term results of the cooperative HLH-2004 study. Blood 2017, 130, 2728–2738. [Google Scholar] [CrossRef]
- Jordan, M.B.; Filipovich, A.H. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: A journey of a thousand miles begins with a single (big) step. Bone Marrow Transplant. 2008, 42, 433–437. [Google Scholar] [CrossRef]
- Booth, C.; Gilmour, K.C.; Veys, P.; Gennery, A.R.; Slatter, M.A.; Chapel, H.; Heath, P.T.; Steward, C.G.; Smith, O.; O’Meara, A.; et al. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: A multicenter study on the manifestations, management and outcome of the disease. Blood 2011, 117, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ehl, S.; Astigarraga, I.; von Bahr Greenwood, T.; Hines, M.; Horne, A.; Ishii, E.; Janka, G.; Jordan, M.B.; La Rosee, P.; Lehmberg, K.; et al. Recommendations for the Use of Etoposide-Based Therapy and Bone Marrow Transplantation for the Treatment of HLH: Consensus Statements by the HLH Steering Committee of the Histiocyte Society. J. Allergy Clin. Immunol. Pract. 2018, 6, 1508–1517. [Google Scholar] [CrossRef] [PubMed]


| Measure | Normal Range | 11/07 Day 1 | 11/12 Day 5 | 11/17 Day 10 | 11/22 Day 15 | 11/27 Day 20 | 12/03 Day 25 | 12/08 Day 30 |
|---|---|---|---|---|---|---|---|---|
| WBC (109/L) | 3.5–9.5 | 0.93 | 22.36 | 23.1 | 18 | 0.82 | 75.27 | 33 |
| HGB (g/L) | 130–175 | 101 | 91 | 81 | 82 | 81 | 75 | 82 |
| PLT (109/L) | 125–350 | 40 | 23 | 49 | 81 | 122 | 160 | 409 |
| NEUT (109/L) | 1.8–6.3 | 0.42 | 11.62 | 16.1 | 11.2 | 12.9 | 13.4 | 11.9 |
| FIB (mg/dL) | 238–498 | 74 | 112 | 96 | 121 | 222 | 302 | 287 |
| PT (s) | 9.4–12.5 | 31.2 | 11.9 | 11.6 | 11.2 | 12.9 | 13.4 | 11.9 |
| APTT (s) | 25.1–36.5 | 80.6 | 37.6 | 33.6 | 25.8 | 24.7 | 29 | 31.6 |
| PTTA (%) | 80–130 | 26 | 79 | 78 | 96 | 88 | 84 | 87 |
| DD (ng/mL) | 0–500 | >35,000 | >35,000 | 58,879 | 28,946 | 15,324 | 3641 | 2903 |
| Ferritin (ng/mL) | 21.8–274.7 | >33,511 | 6614 | 10,268 | 3014 | 2876 | 2743 | 4671 |
| TG (mmol/L) | <1.7 | 2.83 | ||||||
| ALT (U/L) | 9–50 | 153 | 151 | 206 | 144 | 82 | 44 | |
| AST (U/L) | 15–40 | 661 | 994 | 658 | 199 | 57 | 38 | |
| TBIL (μmol/L) | 5–21 | 73.1 | 113.8 | 136.6 | 177 | 91.8 | 45 | |
| BUN (mmol/L) | 2.8–7.6 | 13.7 | 27.2 | 22.9 | 13 | |||
| CREA (μmol/L) | 64–104 | 390.8 | 239 | 156.9 | ||||
| PCT (ng/mL) | <0.05 | 158.68 | 9.23 | 2.86 | 0.29 | 4.12 | 0.18 | |
| IL-6 (ng/mL) | 0–7 | >5000 | 84.8 | 5.12 | 50.4 | 4.27 | ||
| BNP (pg/mL) | <100 | 177.1 | 196.7 | 23.9 | ||||
| CK-MB (ng/mL) | 0–6.6 | 21.1 | 9.3 | 1.4 | 1.8 | 4 | ||
| cTNI(pg/mL) | 0–26.2 | 4740.5 | 368.3 | 414.6 | 139.1 | 5.2 |
| At Least 5 of the Following 8 Findings | |
|---|---|
| Non-remitting fever ≥ 38.5 °C | Yes |
| Splenomegaly | Yes |
| Cytopenia (affects peripheral blood cells of lineage 2 or 3) | |
| HGB ≤ 9 g/dL, PLT ≤ 100 × 109/L NEUT ≤ 1 × 109/L | 10.1 40 × 109 0.42 × 109 |
| Hypofibrinogenemia (≤1.5 g/L) or | 0.74 |
| hypertriglyceridemia (≥3.0 mmol/L or 2.65 g/L) | 2.83 |
| Hyperferritinemia (≥500 ng/mL) Increased level of soluble CD25 (soluble interleukin (IL-2) receptor (sIL-2R)) (normal range: 458–1997 pg/mL) | >33,511 12,999.1 |
| Hemophagocytosis in bone marrow lymph nodes, spleen, or liver | Yes |
| Low or absent NK cell cytotoxicity (normal range > 15.11%) | 12.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, G.; Lu, Y.; Pan, H.; Deng, B.; Wu, S.; Peng, Z.; Ye, X. Hemophagocytic Lymphohistiocytosis Associated with Synergistic Defects of AP3B1 and ATM Genes: A Case Report and Literature Review. J. Clin. Med. 2023, 12, 95. https://doi.org/10.3390/jcm12010095
Yin G, Lu Y, Pan H, Deng B, Wu S, Peng Z, Ye X. Hemophagocytic Lymphohistiocytosis Associated with Synergistic Defects of AP3B1 and ATM Genes: A Case Report and Literature Review. Journal of Clinical Medicine. 2023; 12(1):95. https://doi.org/10.3390/jcm12010095
Chicago/Turabian StyleYin, Guangjiao, Yasu Lu, Huaqin Pan, Bin Deng, Sanyun Wu, Zhiyong Peng, and Xujun Ye. 2023. "Hemophagocytic Lymphohistiocytosis Associated with Synergistic Defects of AP3B1 and ATM Genes: A Case Report and Literature Review" Journal of Clinical Medicine 12, no. 1: 95. https://doi.org/10.3390/jcm12010095
APA StyleYin, G., Lu, Y., Pan, H., Deng, B., Wu, S., Peng, Z., & Ye, X. (2023). Hemophagocytic Lymphohistiocytosis Associated with Synergistic Defects of AP3B1 and ATM Genes: A Case Report and Literature Review. Journal of Clinical Medicine, 12(1), 95. https://doi.org/10.3390/jcm12010095






