The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD
Abstract
1. Introduction
2. COPD
3. Multimorbidity
4. RAGE
4.1. RAGE Signaling Leads to Chronic Inflammation
4.2. RAGE Structure and Variants
4.3. Pulmonary RAGE
4.4. Genetics as a Common Cause of Altered RAGE Signaling
4.5. Early Origins of Altered RAGE Signaling across Diseases
5. Common Causes Leading to AGE Accumulation
5.1. Conditions Leading to Enhanced Endogenous AGE Formation in Chronic Inflammatory Disease and Aging
5.2. Enhanced Intake through a Western Diet
5.3. Enhanced AGE Formation through Inhalation of Cigarette Smoke
5.4. Limitations in Physiological Detoxification
6. Common Mechanisms Driven by AGE-RAGE
6.1. Local RAGE-Driven Inflammatory Signaling and Aging
6.2. Local RAGE-Independent Effects of AGEs
6.3. Systemic Effects—Interorgan Crosstalk
7. The AGE-RAGE Axis in Age-Related Chronic Inflammatory Diseases
7.1. AGE-RAGE in COPD
7.2. AGE-RAGE in Insulin Resistance, Diabetes, and Diabetic Complications Diabetes
7.3. AGE-RAGE in Cardiovascular Diseases
7.4. AGE-RAGE in Osteoporosis
7.5. AGE-RAGE in Sarcopenia
7.6. AGE-RAGE in Renal Disease
7.7. AGE-RAGE in Depression and Anxiety
8. Therapeutic Implications
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Pahal, P.; Hashmi, M.F.; Sharma, S. Chronic Obstructive Pulmonary Disease Compensatory Measures; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- dos Santos, N.C.; Miravitlles, M.; Camelier, A.A.; de Almeida, V.D.C.; Maciel, R.R.B.T.; Camelier, F.W.R. Prevalence and Impact of Comorbidities in Individuals with Chronic Obstructive Pulmonary Disease: A Systematic Review. Tuberc. Respir. Dis. 2022, 85, 205–220. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, N.L.; Gopal, P.; Rutten, E.P.; Wouters, E.F.; Schalkwijk, C.G. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; Overview of clinical evidence and potential contributions to disease. Int. J. Biochem. Cell Biol. 2016, 81, 403–418. [Google Scholar] [CrossRef]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.E.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Syed, A.; Zhu, Q.; Smith, E.A. Ligand binding affinity and changes in the lateral diffusion of receptor for advanced glycation endproducts (RAGE). Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 3141–3149. [Google Scholar] [CrossRef]
- Díez, R.L.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 2244–2252. [Google Scholar] [CrossRef]
- Tobon-Velasco, J.C.; Cuevas, E.; Torres-Ramos, M.A. Receptor for AGEs (RAGE) as Mediator of NF-kB Pathway Activation in Neuroinflammation and Oxidative Stress. CNS Neurol. Disord. Drug Targets 2014, 13, 1615–1626. [Google Scholar] [CrossRef]
- Oczypok, E.A.; Perkins, T.N.; Oury, T.D. All the “RAGE” in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 28 March 2023).
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004, 364, 709–721. [Google Scholar]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. BMJ 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Thun, M.J.; Apicella, L.F.; Henley, S.J. Smoking vs other risk factors as the cause of smoking-attributable deaths: Confounding in the courtroom. JAMA 2000, 284, 706–712. [Google Scholar] [CrossRef]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Salvi, S.S.; Brashier, B.B.; Londhe, J.; Pyasi, K.; Vincent, V.; Kajale, S.S.; Tambe, S.; Mandani, K.; Nair, A.; Mak, S.M.; et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir. Res. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Lange, P.; Celli, B.R.; Agustí, A.; Jensen, G.B.; Divo, M.; Faner, R.; Guerra, S.; Marott, J.L.; Martinez, F.D.; Martinez-Camblor, P.; et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2015, 373, 111–122. [Google Scholar] [CrossRef]
- Meiners, S.; Eickelberg, O.; Königshoff, M. Hallmarks of the ageing lung. Eur. Respir. J. 2015, 45, 807–827. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Triest, F.J.J.; Franssen, F.M.E.; Reynaert, N.; Gaffron, S.; Spruit, M.A.; Janssen, D.J.A.; Rutten, E.P.A.; Wouters, E.F.M.; Vanfleteren, L.E.G.W. Disease-Specific Comorbidity Clusters in COPD and Accelerated Aging. J. Clin. Med. 2019, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Triest, F.J.; Franssen, F.M.; Spruit, M.A.; Groenen, M.T.; Wouters, E.F.; Vanfleteren, L.E. Poor agreement between chart-based and objectively identified comorbidities of COPD. Eur. Respir. J. 2015, 46, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Alter, P.; Kahnert, K.; Trudzinski, F.C.; Bals, R.; Watz, H.; Speicher, T.; Söhler, S.; Andreas, S.; Welte, T.; Rabe, K.F.; et al. Disease Progression and Age as Factors Underlying Multimorbidity in Patients with COPD: Results from COSYCONET. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Young, R.P.; Hopkins, R.; Eaton, T.E. Forced expiratory volume in one second: Not just a lung function test but a marker of premature death from all causes. Eur. Respir. J. 2007, 30, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; van den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Roodt, J.O.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef]
- Vikjord, S.A.A.; Brumpton, B.M.; Mai, X.; Romundstad, S.; Langhammer, A.; Vanfleteren, L. TheHUNTstudy: Association of comorbidity clusters with long-term survival and incidence of exacerbation in a population-based NorwegianCOPDcohort. Respirology 2022, 27, 277–285. [Google Scholar] [CrossRef]
- Grosdidier, S.; Ferrer, A.; Faner, R.; Piñero, J.; Roca, J.; Cosío, B.; Agustí, A.; Gea, J.; Sanz, F.; Furlong, L.I. Network medicine analysis of COPD multimorbidities. Respir. Res. 2014, 15, 111. [Google Scholar] [CrossRef]
- Divo, M.J.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.M.; De-Torres, J.P.; Zulueta, J.J.; Cabrera, C.; Zagaceta, J.; Sanchez-Salcedo, P.; Berto, J.; et al. COPD comorbidities network. Eur. Respir. J. 2015, 46, 640–650. [Google Scholar] [CrossRef]
- Figueira-Gonçalves, J.M.; Golpe, R.; Esteban, C.; García-Bello, M.; Blanco-Cid, N.; Aramburu, A.; García-Talavera, I.; Martín-Martínez, M.D.; Baeza-Ruiz, A.; Expósito-Marrero, A. Evaluation of the multimorbidity network and its relationship with clinical phenotypes in chronic obstructive pulmonary disease: The GALAXIA study. Clin. Respir. J. 2022, 16, 504–512. [Google Scholar] [CrossRef]
- Agustí, A.; Melén, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene–environment interactions across the lifespan. Lancet Respir. Med. 2022, 10, 512–524. [Google Scholar] [CrossRef]
- Hudson, B.I.; Carter, A.M.; Harja, E.; Kalea, A.Z.; Arriero, M.; Yang, H.; Grant, P.J.; Schmidt, A.M. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2007, 22, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Vianna, M.; Gerlach, M.; Brett, J.; Ryan, J.; Kao, J.; Esposito, C.; Hegarty, H.; Hurley, W.; Clauss, M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 1992, 267, 14987–14997. [Google Scholar] [CrossRef] [PubMed]
- Hori, O.; Brett, J.; Slattery, T.; Cao, R.; Zhang, J.; Chen, J.X.; Nagashima, M.; Lundh, E.R.; Vijay, S.; Nitecki, D.; et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995, 270, 25752–25761. [Google Scholar] [CrossRef]
- Du Yan, S.; Zhu, H.; Fu, J.; Yan, S.F.; Roher, A.; Tourtellotte, W.W.; Rajavashisth, T.; Chen, X.; Godman, G.C.; Stern, D.; et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: A proinflammatory pathway in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1997, 94, 5296–5301. [Google Scholar] [CrossRef]
- Ruan, B.H.; Li, X.; Winkler, A.R.; Cunningham, K.M.; Kuai, J.; Greco, R.M.; Nocka, K.H.; Fitz, L.J.; Wright, J.F.; Pittman, D.D.; et al. Complement C3a, CpG Oligos, and DNA/C3a Complex Stimulate IFN-α Production in a Receptor for Advanced Glycation End Product-Dependent Manner. J. Immunol. 2010, 185, 4213–4222. [Google Scholar] [CrossRef]
- Tian, J.; Avalos, A.M.; Mao, S.-Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.T.; Sirois, C.M.; et al. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Sirois, C.M.; Jin, T.; Miller, A.L.; Bertheloot, D.; Nakamura, H.; Horvath, G.L.; Mian, A.; Jiang, J.; Schrum, J.; Bossaller, L.; et al. RAGE is a nucleic acid receptor that promotes inflammatory responses to DNA. J. Exp. Med. 2013, 210, 2447–2463. [Google Scholar] [CrossRef]
- Li, J.; Schmidt, A.M. Characterization and Functional Analysis of the Promoter of RAGE, the Receptor for Advanced Glycation End Products. J. Biol. Chem. 1997, 272, 16498–16506. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Kloting, I.; et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Armour, C.L.; Phipps, S.; Sukkar, M.B. RAGE and TLRs: Relatives, friends or neighbours? Mol. Immunol. 2013, 56, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Stopper, H.; Schinzel, R.; Sebekova, K.; Heidland, A. Genotoxicity of advanced glycation end products in mammalian cells. Cancer Lett. 2002, 190, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mallidis, C.; Agbaje, I.; Rogers, D.; Glenn, J.; McCullough, S.; Atkinson, A.B.; Steger, K.; Stitt, A.; McClure, N. Distribution of the receptor for advanced glycation end products in the human male reproductive tract: Prevalence in men with diabetes mellitus. Hum. Reprod. 2007, 22, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Fleming, T.; Terjung, S.; Gorzelanny, C.; Gebhardt, C.; Agrawal, R.; Mall, M.A.; Ranzinger, J.; Zeier, M.; Madhusudhan, T.; et al. Homeostatic nuclear RAGE–ATM interaction is essential for efficient DNA repair. Nucleic Acids Res. 2017, 45, 10595–10613. [Google Scholar] [CrossRef] [PubMed]
- Kutikhin, A.G.; Yuzhalin, A.E.; Tsitko, E.A.; Brusina, E.B. Pattern Recognition Receptors and DNA Repair: Starting to Put a Jigsaw Puzzle Together. Front. Immunol. 2014, 5, 343. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Sugihara, T.; Munesue, S.; Yamamoto, Y.; Sakurai, S.; Akhter, N.; Kitamura, Y.; Shiba, K.; Watanabe, T.; Yonekura, H.; Hayashi, Y.; et al. Endogenous Secretory Receptor for Advanced Glycation End-Products Inhibits Amyloid-β1-42 Uptake into Mouse Brain. J. Alzheimer’s Dis. 2012, 28, 709–720. [Google Scholar] [CrossRef]
- Schlueter, C.; Hauke, S.; Flohr, A.M.; Rogalla, P.; Bullerdiek, J. Tissue-specific expression patterns of the RAGE receptor and its soluble forms--a result of regulated alternative splicing? Biochim. Biophys. Acta 2003, 1630, 1–6. [Google Scholar] [CrossRef]
- Grossin, N.; Wautier, M.-P.S.; Picot, J.; Stern, D.; Wautier, J.-L.T. Differential effect of plasma or erythrocyte AGE-ligands of RAGE on expression of transcripts for receptor isoforms. Diabetes Metab. 2009, 35, 410–417. [Google Scholar] [CrossRef]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for Advanced Glycation End Products Is Subjected to Protein Ectodomain Shedding by Metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516. [Google Scholar] [CrossRef] [PubMed]
- Galichet, A.; Weibel, M.; Heizmann, C.W. Calcium-regulated intramembrane proteolysis of the RAGE receptor. Biochem. Biophys. Res. Commun. 2008, 370, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Uchida, T.; Matthay, M.A.; Makita, K. Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells. Am. J. Physiol. Cell Mol. Physiol. 2011, 300, L516–L525. [Google Scholar] [CrossRef]
- Goova, M.T.; Li, J.; Kislinger, T.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Nowygrod, S.; Wolf, B.M.; Caliste, X.; Yan, S.F.; et al. Blockade of Receptor for Advanced Glycation End-Products Restores Effective Wound Healing in Diabetic Mice. Am. J. Pathol. 2001, 159, 513–525. [Google Scholar] [CrossRef]
- Park, L.; Raman, K.G.; Lee, K.J.; Lu, Y.; Ferran, L.J., Jr.; Chow, W.S.; Stern, D.; Schmidt, A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998, 4, 1025–1031. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar] [CrossRef]
- Santos, I.; Daga, D.; Frigeri, H.; Ra, R.; Almeida, A.; Souza, E.; Pedrosa, F.; Fadel-Picheth, C.; Picheth, G. Short Communication The functional polymorphisms -429T>C and -374T>A of the RAGE gene promoter are not associated with gestational diabetes in Euro-Brazilians. Genet. Mol. Res. 2010, 9, 1130–1135. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Schmidt, A.M.; Hudson, B.I. RAGE: A novel biological and genetic marker for vascular disease. Clin. Sci. 2009, 116, 621–637. [Google Scholar] [CrossRef]
- Osawa, M.; Yamamoto, Y.; Munesue, S.; Murakami, N.; Sakurai, S.; Watanabe, T.; Yonekura, H.; Uchigata, Y.; Iwamoto, Y.; Yamamoto, H. De-N-glycosylation or G82S mutation of RAGE sensitizes its interaction with advanced glycation endproducts. Biochim. Biophys. Acta (BBA) Gen. Subj. 2007, 1770, 1468–1474. [Google Scholar] [CrossRef]
- Park, S.J.; Kleffmann, T.; Hessian, P.A. The G82S Polymorphism Promotes Glycosylation of the Receptor for Advanced Glycation End Products (RAGE) at Asparagine 81. J. Biol. Chem. 2011, 286, 21384–21392. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, J.Y.; Kang, S.-M.; Kim, J.-S.; Chae, J.S.; Kim, O.Y.; Koh, S.J.; Lee, H.C.; Ahn, C.W.; Song, Y.D.; et al. Association of the Gly82Ser polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating levels of soluble RAGE and inflammatory markers in nondiabetic and nonobese Koreans. Metabolism 2007, 56, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Henry, A.P.; Hodge, E.; Kheirallah, A.K.; Billington, C.K.; Rimington, T.L.; Bhaker, S.K.; Obeidat, M.; Melén, E.; Merid, S.K.; et al. The Ser82 RAGE Variant Affects Lung Function and Serum RAGE in Smokers and sRAGE Production In Vitro. PLoS ONE 2016, 11, e0164041. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Li, M.; Halushka, M.K.; Astor, B.C.; Pankow, J.S.; Boerwinkle, E.; Coresh, J.; Selvin, E.; Kao, W.H.L. Genetics of Plasma Soluble Receptor for Advanced Glycation End-Products and Cardiovascular Outcomes in a Community-based Population: Results from the Atherosclerosis Risk in Communities Study. PLoS ONE 2015, 10, e0128452. [Google Scholar] [CrossRef]
- Cheng, D.T.; Kim, D.K.; Cockayne, D.A.; Belousov, A.; Bitter, H.; Cho, M.H.; Duvoix, A.; Edwards, L.D.; Lomas, D.A.; Miller, B.E.; et al. Systemic Soluble Receptor for Advanced Glycation Endproducts Is a Biomarker of Emphysema and Associated with AGER Genetic Variants in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 948–957. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Investig. 2001, 108, 949–955. [Google Scholar] [CrossRef]
- Demling, N.; Ehrhardt, C.; Kasper, M.; Laue, M.; Knels, L.; Rieber, E.P. Promotion of cell adherence and spreading: A novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2005, 323, 475–488. [Google Scholar] [CrossRef]
- Dahlin, K.; Mager, E.M.; Allen, L.; Tigue, Z.; Goodglick, L.; Wadehra, M.; Dobbs, L. Identification of Genes Differentially Expressed in Rat Alveolar Type I Cells. Am. J. Respir. Cell Mol. Biol. 2004, 31, 309–316. [Google Scholar] [CrossRef]
- Shirasawa, M.; Fujiwara, N.; Hirabayashi, S.; Ohno, H.; Iida, J.; Makita, K.; Hata, Y. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 2004, 9, 165–174. [Google Scholar] [CrossRef]
- Fehrenbach, H.; Kasper, M.; Tschernig, T.; Shearman, M.S.; Schuh, D.; Müller, M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol. Biol. 1998, 44, 1147–1157. [Google Scholar]
- Katsuoka, F.; Kawakami, Y.; Arai, T.; Imuta, H.; Fujiwara, M.; Kanma, H.; Yamashita, K. Type II Alveolar Epithelial Cells in Lung Express Receptor for Advanced Glycation End Products (RAGE) Gene. Biochem. Biophys. Res. Commun. 1997, 238, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Sambamurthy, N.; Leme, A.S.; Oury, T.D.; Shapiro, S.D. The Receptor for Advanced Glycation End Products (RAGE) Contributes to the Progression of Emphysema in Mice. PLoS ONE 2015, 10, e0118979. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, A.; Baier, J.; Simm, A.; Haase, R.; Bartling, B. Receptor for advanced glycation end-products modulates lung development and lung sensitivity to hyperoxic injury in newborn mice. Pflugers Arch. 2019, 471, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.; Herr, C.; Niederstraßer, J.; Beisswenger, C.; Bals, R. Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS ONE 2017, 12, e0180092. [Google Scholar] [CrossRef] [PubMed]
- Englert, J.M.; Hanford, L.E.; Kaminski, N.; Tobolewski, J.M.; Tan, R.J.; Fattman, C.L.; Ramsgaard, L.; Richards, T.J.; Loutaev, I.; Nawroth, P.P.; et al. A Role for the Receptor for Advanced Glycation End Products in Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2008, 172, 583–591. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Kubo, H.; Ishizawa, K.; Hegab, A.E.; Yamamoto, Y.; Yamamoto, H.; Yamaya, M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am. J. Physiol. Cell Mol. Physiol. 2007, 293, L1427–L1436. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Stogsdill, J.A.; Stogsdill, M.P.; Heimann, N.B. Up-Regulation of Receptors for Advanced Glycation End-Products by Alveolar Epithelium Influences Cytodifferentiation and Causes Severe Lung Hypoplasia. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1195–1202. [Google Scholar] [CrossRef]
- Stogsdill, J.A.; Stogsdill, M.P.; Porter, J.L.; Hancock, J.M.; Robinson, A.B.; Reynolds, P.R. Embryonic Overexpression of Receptors for Advanced Glycation End-Products by Alveolar Epithelium Induces an Imbalance between Proliferation and Apoptosis. Am. J. Respir. Cell Mol. Biol. 2012, 47, 60–66. [Google Scholar] [CrossRef]
- Fineschi, S.; De Cunto, G.; Facchinetti, F.; Civelli, M.; Imbimbo, B.P.; Carnini, C.; Villetti, G.; Lunghi, B.; Stochino, S.; Gibbons, D.L.; et al. Receptor for Advanced Glycation End Products Contributes to Postnatal Pulmonary Development and Adult Lung Maintenance Program in Mice. Am. J. Respir. Cell Mol. Biol. 2013, 48, 164–171. [Google Scholar] [CrossRef]
- Mukherjee, T.K.; Mukhopadhyay, S.; Hoidal, J.R. Implication of receptor for advanced glycation end product (RAGE) in pulmonary health and pathophysiology. Respir. Physiol. Neurobiol. 2008, 162, 210–215. [Google Scholar] [CrossRef]
- Morbini, P.; Villa, C.; Campo, I.; Zorzetto, M.; Inghilleri, S.; Luisetti, M. The receptor for advanced glycation end products and its ligands: A new inflammatory pathway in lung disease? Mod. Pathol. 2006, 19, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Serveaux-Dancer, M.; Jabaudon, M.; Creveaux, I.; Belville, C.; Blondonnet, R.; Gross, C.; Constantin, J.-M.; Blanchon, L.; Sapin, V. Pathological Implications of Receptor for Advanced Glycation End-Product (AGER) Gene Polymorphism. Dis. Markers 2019, 2019, 2067353. [Google Scholar] [CrossRef] [PubMed]
- Hancock, D.B.; Eijgelsheim, M.; Wilk, J.B.; Gharib, S.A.; Loehr, L.R.; Marciante, K.D.; Franceschini, N.; van Durme, Y.M.T.A.; Chen, T.-H.; Barr, R.G.; et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat. Genet. 2009, 42, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Repapi, E.; Sayers, I.; Wain, L.V.; Burton, P.R.; Johnson, T.; Obeidat, M.; Zhao, J.H.; Ramasamy, A.; Zhai, G.; Vitart, V.; et al. Genome-wide association study identifies five loci associated with lung function. Nat. Genet. 2010, 42, 36–44. [Google Scholar] [CrossRef]
- Manichaikul, A.; Hoffman, E.A.; Smolonska, J.; Gao, W.; Cho, M.H.; Baumhauer, H.; Budoff, M.; Austin, J.H.M.; Washko, G.R.; Carr, J.J.; et al. Genome-Wide Study of Percent Emphysema on Computed Tomography in the General Population. The Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am. J. Respir. Crit. Care Med. 2014, 189, 408–418. [Google Scholar] [CrossRef]
- Young, R.P.; Hay, B.A.; Hopkins, R.J. Does RAGE protect smokers from COPD? Eur. Respir. J. 2011, 38, 743–744. [Google Scholar] [CrossRef]
- Gibson, A.-M.; Doyle, L.W. Respiratory outcomes for the tiniest or most immature infants. Semin. Fetal Neonatal Med. 2014, 19, 105–111. [Google Scholar] [CrossRef]
- Agustí, A.; Noell, G.; Brugada, J.; Faner, R. Lung function in early adulthood and health in later life: A transgenerational cohort analysis. Lancet Respir. Med. 2017, 5, 935–945. [Google Scholar] [CrossRef]
- Starr, M.C.; Hingorani, S.R. Prematurity and future kidney health. Curr. Opin. Pediatr. 2018, 30, 228–235. [Google Scholar] [CrossRef]
- Carr, H.; Cnattingius, S.; Granath, F.; Ludvigsson, J.F.; Bonamy, A.-K.E. Preterm Birth and Risk of Heart Failure Up to Early Adulthood. J. Am. Coll. Cardiol. 2017, 69, 2634–2642. [Google Scholar] [CrossRef]
- Parkinson, J.R.; Hyde, M.J.; Gale, C.; Santhakumaran, S.; Modi, N. Preterm Birth and the Metabolic Syndrome in Adult Life: A Systematic Review and Meta-analysis. Pediatrics 2013, 131, e1240–e1263. [Google Scholar] [CrossRef] [PubMed]
- de Jong, F.; Monuteaux, M.C.; van Elburg, R.M.; Gillman, M.W.; Belfort, M.B. Systematic Review and Meta-Analysis of Preterm Birth and Later Systolic Blood Pressure. Hypertension 2012, 59, 226–234. [Google Scholar] [CrossRef]
- Kajantie, E.; Strang-Karlsson, S.; Evensen, K.A.I.; Haaramo, P. Adult outcomes of being born late preterm or early term—What do we know? Semin. Fetal Neonatal. Med. 2019, 24, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.T.; Van Der Meer, R.; Slaughter, J.C.; Steele, S.; Plosa, E.J.; Sucre, J.M.; Moore, P.E.; Aschner, J.L.; Blackwell, T.S.; Young, L.R. Inverse Relationship between Soluble RAGE and Risk for Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2018, 197, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Ohto, H.; Nollet, K.E.; Sato, K.; Miyazaki, K.; Maeda, H.; Ichikawa, H.; Chishiki, M.; Kashiwabara, N.; Kume, Y.; et al. Biomarker Potential of the Soluble Receptor for Advanced Glycation End Products to Predict Bronchopulmonary Dysplasia in Premature Newborns. Front. Pediatr. 2021, 9, 649526. [Google Scholar] [CrossRef] [PubMed]
- Röhl, A.; Baek, S.H.; Kachroo, P.; Morrow, J.D.; Tantisira, K.; Silverman, E.K.; Weiss, S.T.; Sharma, A.; Glass, K.; DeMeo, D.L. Protein interaction networks provide insight into fetal origins of chronic obstructive pulmonary disease. Respir. Res. 2022, 23, 69. [Google Scholar] [CrossRef]
- Winden, D.R.; Barton, D.B.; Betteridge, B.C.; Bodine, J.S.; Jones, C.M.; Rogers, G.D.; Chavarria, M.; Wright, A.J.; Jergensen, Z.R.; Jimenez, F.R.; et al. Antenatal exposure of maternal secondhand smoke (SHS) increases fetal lung expression of RAGE and induces RAGE-mediated pulmonary inflammation. Respir. Res. 2014, 15, 129. [Google Scholar] [CrossRef]
- Sukjamnong, S.; Chan, Y.L.; Zakarya, R.; Saad, S.; Sharma, P.; Santiyanont, R.; Chen, H.; Oliver, B.G. Effect of long-term maternal smoking on the offspring’s lung health. Am. J. Physiol. Cell Mol. Physiol. 2017, 313, L416–L423. [Google Scholar] [CrossRef]
- Tsai, C.; Chou, H.; Chen, C. Perinatal nicotine exposure alters lung development and induces HMGB1-RAGE expression in neonatal mice. Birth Defects Res. 2020, 113, 570–578. [Google Scholar] [CrossRef]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. 1), 3–27. [Google Scholar] [CrossRef]
- Ohashi, K.; Takahashi, H.K.; Mori, S.; Liu, K.; Wake, H.; Sadamori, H.; Matsuda, H.; Yagi, T.; Yoshino, T.; Nishibori, M.; et al. Advanced glycation end products enhance monocyte activation during human mixed lymphocyte reaction. Clin. Immunol. 2010, 134, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hallam, K.M.; Li, Q.; Ananthakrishnan, R.; Kalea, A.; Zou, Y.S.; Vedantham, S.; Schmidt, A.M.; Yan, S.F.; Ramasamy, R. Aldose Reductase and AGE-RAGE pathways: Central roles in the pathogenesis of vascular dysfunction in aging rats. Aging Cell 2010, 9, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef] [PubMed]
- Scheijen, J.L.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.; Schalkwijk, C.G. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. Int. Rev. J. 2017, 8, 54–62. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C.; Tessier, F.J.; Niquet-Léridon, C.; Strauch, C.; Monnier, V.M.; Navarro, M.P. Metabolic transit of Nε-carboxymethyl-lysine after consumption of AGEs from bread crust. Food Funct. 2013, 4, 1032–1039. [Google Scholar] [CrossRef]
- Ramos, I.R.; Niquet-Léridon, C.; Strauch, C.; Monnier, V.M.; Tessier, F.; Navarro, M.P.; Delgado-Andrade, C. An Advanced Glycation End Product (AGE)-Rich Diet Promotes Nε-Carboxymethyl-lysine Accumulation in the Cardiac Tissue and Tendons of Rats. J. Agric. Food Chem. 2014, 62, 6001–6006. [Google Scholar] [CrossRef]
- Li, M.; Zeng, M.; He, Z.; Zheng, Z.; Qin, F.; Tao, G.; Zhang, S.; Chen, J. Effects of Long-Term Exposure to Free Nε-(Carboxymethyl)lysine on Rats Fed a High-Fat Diet. J. Agric. Food Chem. 2015, 63, 10995–11001. [Google Scholar] [CrossRef]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Wallenstein, S.; Striker, G.E.; Vlassara, H. Reduced Oxidant Stress and Extended Lifespan in Mice Exposed to a Low Glycotoxin Diet: Association with Increased AGER1 Expression. Am. J. Pathol. 2007, 170, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Waqas, K.; Chen, J.; van der Eerden, B.C.J.; Ikram, M.A.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Dietary Advanced Glycation End-Products (dAGEs) Intake and Bone Health: A Cross-Sectional Analysis in the Rotterdam Study. Nutrients 2020, 12, 2377. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Tsuda, S.; Goto, A.; Ohno, Y.; Yokoyama, S.; Goto, K.; Hayashi, T. Potential involvement of dietary advanced glycation end products in impairment of skeletal muscle growth and muscle contractile function in mice. Br. J. Nutr. 2017, 117, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.A.; Davidson, B.A.; Ottosen, J.; Ohtake, P.J.; Raghavendran, K.; Mullan, B.A.; Dayton, M.T.; Knight, P.R. Effect of High Advanced Glycation End-Product Diet on Pulmonary Inflammatory Response and Pulmonary Function Following Gastric Aspiration. Shock 2012, 38, 677–684. [Google Scholar] [CrossRef]
- Grossin, N.; Auger, F.; Niquet-Leridon, C.; Durieux, N.; Montaigne, D.; Schmidt, A.M.; Susen, S.; Jacolot, P.; Beuscart, J.-B.; Tessier, F.J.; et al. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol. Nutr. Food Res. 2015, 59, 927–938. [Google Scholar] [CrossRef]
- Lin, R.-Y.; Choudhury, R.P.; Cai, W.; Lu, M.; Fallon, J.T.; Fisher, E.A.; Vlassara, H. Dietary glycotoxins promote diabetic atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2003, 168, 213–220. [Google Scholar] [CrossRef]
- Cai, W.; Ramdas, M.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA 2012, 109, 15888–15893. [Google Scholar] [CrossRef]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine Prevents Diabetes-Induced Arterial Wall Protein Cross-Linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef]
- Morcos, M.; Du, X.; Pfisterer, F.; Hutter, H.; Sayed, A.A.R.; Thornalley, P.; Ahmed, N.; Baynes, J.; Thorpe, S.; Kukudov, G.; et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell 2008, 7, 260–269. [Google Scholar] [CrossRef]
- West, R.K.; Moshier, E.; Lubitz, I.; Schmeidler, J.; Godbold, J.; Cai, W.; Uribarri, J.; Vlassara, H.; Silverman, J.M.; Beeri, M.S. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mech. Ageing Dev. 2014, 140, 10–12. [Google Scholar] [CrossRef]
- Peppa, M.; Mavroeidi, I. Experimental Animal Studies Support the Role of Dietary Advanced Glycation End Products in Health and Disease. Nutrients 2021, 13, 3467. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Stirban, A.; Sander, D.; Cai, W.; Negrean, M.; Buenting, C.E.; Koschinsky, T.; Vlassara, H. Single Oral Challenge by Advanced Glycation End Products Acutely Impairs Endothelial Function in Diabetic and Nondiabetic Subjects. Diabetes Care 2007, 30, 2579–2582. [Google Scholar] [CrossRef] [PubMed]
- Iersel, L.E.J.V.; Beijers, R.J.H.C.G.; Gosker, H.R.; Schols, A.M.W.J. Nutrition as a modifiable factor in the onset and progression of pulmonary function impairment in COPD: A systematic review. Nutr. Rev. 2022, 80, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Shaheen, S.O.; Ntani, G.; Jameson, K.A.; Syddall, H.E.; Sayer, A.A.; Dennison, E.M.; Cooper, C.; Robinson, S.M. Hertfordshire Cohort Study Group Processed meat consumption and lung function: Modification by antioxidants and smoking. Eur. Respir. J. 2013, 43, 972–982. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Palimeri, S.; Palioura, E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 415–426. [Google Scholar] [CrossRef]
- Unoki-Kubota, H.; Yamagishi, S.-I.; Takeuchi, M.; Bujo, H.; Saito, Y. Pyridoxamine, an inhibitor of advanced glycation end product (AGE) formation ameliorates insulin resistance in obese, type 2 diabetic mice. Protein Pept. Lett. 2010, 17, 1177–1181. [Google Scholar] [CrossRef]
- Snelson, M.; Coughlan, M. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef]
- Snelson, M.; Tan, S.M.; Clarke, R.E.; de Pasquale, C.; Thallas-Bonke, V.; Nguyen, T.-V.; Penfold, S.A.; Harcourt, B.E.; Sourris, K.C.; Lindblom, R.S.; et al. Processed foods drive intestinal barrier permeability and microvascular diseases. Sci. Adv. 2021, 7, eabe4841. [Google Scholar] [CrossRef]
- Ramsheh, M.Y.; Haldar, K.; Esteve-Codina, A.; Purser, L.F.; Richardson, M.; Müller-Quernheim, J.; Greulich, T.; Nowinski, A.; Barta, I.; Stendardo, M.; et al. Lung microbiome composition and bronchial epithelial gene expression in patients with COPD versus healthy individuals: A bacterial 16S rRNA gene sequencing and host transcriptomic analysis. Lancet Microbe 2021, 2, e300–e310. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Gopal, P.; Reynaert, N.L.; Scheijen, J.L.J.M.; Engelen, L.; Schalkwijk, C.G.; Franssen, F.M.; Wouters, E.F.; Rutten, E.P. Plasma advanced glycation end-products and skin autofluorescence are increased in COPD. Eur. Respir. J. 2013, 43, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Hoonhorst, S.J.; Loi, A.T.L.T.; Hartman, J.E.; Telenga, E.D.; Berge, M.V.D.; Koenderman, L.; Lammers, J.W.J.; Boezen, H.M.; Postma, D.S.; Hacken, N.H.T. Advanced glycation end products in the skin are enhanced in COPD. Metabolism 2014, 63, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-P.; Wu, Y.-W.; Wu, Z.-Z.; Liu, H.-Y.; Nie, J.-H.; Tong, J. Up-regulation of RAGE and S100A6 in rats exposed to cigarette smoke. Environ. Toxicol. Pharmacol. 2009, 28, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ferhani, N.; Letuve, S.; Kozhich, A.; Thibaudeau, O.; Grandsaigne, M.; Maret, M.; Dombret, M.-C.; Sims, G.P.; Kolbeck, R.; Coyle, A.J.; et al. Expression of High-Mobility Group Box 1 and of Receptor for Advanced Glycation End Products in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 181, 917–927. [Google Scholar] [CrossRef]
- Nicholl, I.D.; Stitt, A.W.; Moore, J.E.; Ritchie, A.J.; Archer, D.B.; Bucala, R. Increased Levels of Advanced Glycation Endproducts in the Lenses and Blood Vessels of Cigarette Smokers. Mol. Med. 1998, 4, 594–601. [Google Scholar] [CrossRef]
- Mullick, A.E.; McDonald, J.M.; Melkonian, G.; Talbot, P.; Pinkerton, K.E.; Rutledge, J.C. Reactive carbonyls from tobacco smoke increase arterial endothelial layer injury. Am. J. Physiol. Circ. Physiol. 2002, 283, H591–H597. [Google Scholar] [CrossRef]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, A. Urinary biomarkers of oxidative/nitrosative stress in healthy smokers. Inhal. Toxicol. 2011, 23, 148–156. [Google Scholar] [CrossRef]
- Roemer, E.; Schorp, M.K.; Piadé, J.-J.; Seeman, J.I.; Leyden, D.E.; Haussmann, H.-J. Scientific assessment of the use of sugars as cigarette tobacco ingredients: A review of published and other publicly available studies. Crit. Rev. Toxicol. 2012, 42, 244–278. [Google Scholar] [CrossRef]
- Kaur, G.; Gaurav, A.; Lamb, T.; Perkins, M.; Muthumalage, T.; Rahman, I. Current Perspectives on Characteristics, Compositions, and Toxicological Effects of E-Cigarettes Containing Tobacco and Menthol/Mint Flavors. Front. Physiol. 2020, 11, 613948. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of Emissions from Air Pollution Sources. 3. C1−C29 Organic Compounds from Fireplace Combustion of Wood. Environ. Sci. Technol. 2001, 35, 1716–1728. [Google Scholar] [CrossRef]
- Rowan, S.; Bejarano, E.; Taylor, A. Mechanistic targeting of advanced glycation end-products in age-related diseases. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 3631–3643. [Google Scholar] [CrossRef] [PubMed]
- Pasupulati, A.K.; Chitra, P.S.; Reddy, G.B. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol. Concepts 2016, 7, 293–309. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Glyoxalase in diabetes, obesity and related disorders. Semin. Cell Dev. Biol. 2011, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, C.; Huang, M.; Tang, C.; Liu, X.; Yue, Y.; Diao, Q.; Zheng, Z.; Liu, D. Glyoxalase system: A systematic review of its biological activity, related-diseases, screening methods and small molecule regulators. Biomed. Pharmacother. 2020, 131, 110663. [Google Scholar] [CrossRef]
- Xue, J.; Ray, R.; Singer, D.; Böhme, D.; Burz, D.S.; Rai, V.; Hoffmann, R.; Shekhtman, A. The Receptor for Advanced Glycation End Products (RAGE) Specifically Recognizes Methylglyoxal-Derived AGEs. Biochemistry 2014, 53, 3327–3335. [Google Scholar] [CrossRef]
- Herold, K.; Moser, B.; Chen, Y.; Zeng, S.; Yan, S.F.; Ramasamy, R.; Emond, J.; Clynes, R.; Schmidt, A.M. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: Inflammatory signals gone awry in the primal response to stress. J. Leukoc. Biol. 2007, 82, 204–212. [Google Scholar] [CrossRef]
- Bansal, S.; Siddarth, M.; Chawla, D.; Banerjee, B.D.; Madhu, S.V.; Tripathi, A.K. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol. Cell Biochem. 2012, 361, 289–296. [Google Scholar] [CrossRef]
- Sick, E.; Brehin, S.; André, P.; Coupin, G.; Landry, Y.; Takeda, K.; Gies, J. Advanced glycation end products (AGEs) activate mast cells. Br. J. Pharmacol. 2010, 161, 442–455. [Google Scholar] [CrossRef]
- Collison, K.S.; Parhar, R.S.; Saleh, S.S.; Meyer, B.F.; Kwaasi, A.A.; Hammami, M.M.; Schmidt, A.M.; Stern, D.M.; Al-Mohanna, F.A. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs). J. Leukoc. Biol. 2002, 71, 433–444. [Google Scholar] [CrossRef]
- Li, Y.M.; Tan, A.X.; Vlassara, H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation–modified proteins to a conserved motif. Nat. Med. 1995, 1, 1057–1061. [Google Scholar] [CrossRef]
- Van Zoelen, M.A.; Achouiti, A.; Van Der Poll, T. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit. Care 2011, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Monboisse, J.C.; Rittie, L.; Lamfarraj, H.; Garnotel, R.; Gillery, P. In vitro glycoxidation alters the interactions between collagens and human polymorphonuclear leucocytes. Biochem. J. 2000, 350 Pt 3, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Touré, F.; Zahm, J.-M.; Garnotel, R.; Lambert, E.; Bonnet, N.; Schmidt, A.M.; Vitry, F.; Chanard, J.; Gillery, P.; Rieu, P. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem. J. 2008, 416, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Akirav, E.M.; Chen, W.; Henegariu, O.; Moser, B.; Desai, D.; Shen, J.M.; Webster, J.C.; Andrews, R.C.; Mjalli, A.M.; et al. RAGE Ligation Affects T Cell Activation and Controls T Cell Differentiation. J. Immunol. 2008, 181, 4272–4278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, S.S.; Colgan, J.; Zhang, H.-P.; Luban, J.; Schmidt, A.M.; Stern, D.; Herold, K.C. Blockade of Late Stages of Autoimmune Diabetes by Inhibition of the Receptor for Advanced Glycation End Products. J. Immunol. 2004, 173, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Stavropoulos, F.; Bhattacharyya, I.; Stewart, C.; Perez, F.M.; Caudle, R.M. Receptor of advanced glycation end product (RAGE) expression in the minor salivary glands of patients with Sjögren’s syndrome: A preliminary study. Scand. J. Rheumatol. 2004, 33, 174–178. [Google Scholar] [CrossRef]
- Goury, A.; Meghraoui-Kheddar, A.; Belmokhtar, K.; Vuiblet, V.; Ortillon, J.; Jaisson, S.; Devy, J.; Le Naour, R.; Tabary, T.; Cohen, J.H.M.; et al. Deletion of Receptor for Advanced Glycation End Products Exacerbates Lymphoproliferative Syndrome and Lupus Nephritis in B6-MRL Fas lpr/j Mice. J. Immunol. 2015, 194, 3612–3622. [Google Scholar] [CrossRef]
- Kume, S.; Kato, S.; Yamagishi, S.-I.; Inagaki, Y.; Ueda, S.; Arima, N.; Okawa, T.; Kojiro, M.; Nagata, K. Advanced Glycation End-Products Attenuate Human Mesenchymal Stem Cells and Prevent Cognate Differentiation into Adipose Tissue, Cartilage, and Bone. J. Bone Miner. Res. 2005, 20, 1647–1658. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, K.-A.; Shin, Y.-J.; Kim, H.; Majid, A.; Bae, O.-N. Methylglyoxal induced advanced glycation end products (AGE)/receptor for AGE (RAGE)-mediated angiogenic impairment in bone marrow-derived endothelial progenitor cells. J. Toxicol. Environ. Heath Part A 2018, 81, 266–277. [Google Scholar] [CrossRef]
- Chen, J.; Brodsky, S.V.; Goligorsky, D.M.; Hampel, D.J.; Li, H.; Gross, S.S.; Goligorsky, M.S. Glycated Collagen I Induces Premature Senescence-Like Phenotypic Changes in Endothelial Cells. Circ. Res. 2002, 90, 1290–1298. [Google Scholar] [CrossRef]
- Pun, P.B.L.; Murphy, M.P. Pathological Significance of Mitochondrial Glycation. Int. J. Cell Biol. 2012, 2012, 843505. [Google Scholar] [CrossRef] [PubMed]
- Queisser, M.A.; Yao, D.; Geisler, S.; Hammes, H.-P.; Lochnit, G.; Schleicher, E.D.; Brownlee, M.; Preissner, K.T. Hyperglycemia Impairs Proteasome Function by Methylglyoxal. Diabetes 2010, 59, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Nokin, M.-J.; Durieux, F.; Peixoto, P.; Chiavarina, B.; Peulen, O.; Blomme, A.; Turtoi, A.; Costanza, B.; Smargiasso, N.; Baiwir, D.; et al. Methylglyoxal, a glycolysis side-product, induces Hsp90 glycation and YAP-mediated tumor growth and metastasis. Elife 2016, 5, e19375. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A.; Bendayan, M. Histones from Diabetic Rats Contain Increased Levels of Advanced Glycation End Products. Biochem. Biophys. Res. Commun. 1995, 212, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, J.M.; Rabbani, G.; Ahmad, S.; Hasan, Q.; Khan, R.H.; Alam, K.; Choi, I. Glycation of H1 Histone by 3-Deoxyglucosone: Effects on Protein Structure and Generation of Different Advanced Glycation End Products. PLoS ONE 2015, 10, e0130630. [Google Scholar] [CrossRef]
- Sugimoto, K.; Yasujima, M.; Yagihashi, S. Role of Advanced Glycation End Products in Diabetic Neuropathy. Curr. Pharm. Des. 2008, 14, 953–961. [Google Scholar] [CrossRef]
- Bjorksten, J.; Tenhu, H. The crosslinking theory of aging—Added evidence. Exp. Gerontol. 1990, 25, 91–95. [Google Scholar] [CrossRef]
- Yuen, A.; Laschinger, C.; Talior, I.; Lee, W.; Chan, M.; Birek, J.; Young, E.W.; Sivagurunathan, K.; Won, E.; Simmons, C.A.; et al. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010, 29, 537–548. [Google Scholar] [CrossRef]
- Vicens-Zygmunt, V.; Estany, S.; Colom, A.; Montes-Worboys, A.; Machahua, C.; Sanabria, A.J.; Llatjos, R.; Escobar, I.; Manresa, F.; Dorca, J.; et al. Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir. Res. 2015, 16, 82. [Google Scholar] [CrossRef]
- Brownlee, M.; Cerami, A.; Vlassara, H. Advanced Glycosylation End Products in Tissue and the Biochemical Basis of Diabetic Complications. N. Engl. J. Med. 1988, 318, 1315–1321. [Google Scholar] [CrossRef]
- Dobler, D.; Ahmed, N.; Song, L.; Eboigbodin, K.E.; Thornalley, P.J. Increased Dicarbonyl Metabolism in Endothelial Cells in Hyperglycemia Induces Anoikis and Impairs Angiogenesis by RGD and GFOGER Motif Modification. Diabetes 2006, 55, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Pedchenko, V.K.; Chetyrkin, S.V.; Chuang, P.; Ham, A.-J.L.; Saleem, M.A.; Mathieson, P.W.; Hudson, B.G.; Voziyan, P.A. Mechanism of Perturbation of Integrin-Mediated Cell-Matrix Interactions by Reactive Carbonyl Compounds and Its Implication for Pathogenesis of Diabetic Nephropathy. Diabetes 2005, 54, 2952–2960. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.P.; Laxton, R.; Patel, K.; Ye, S. Advanced Glycation End-Product of Low Density Lipoprotein Activates the Toll-Like 4 Receptor Pathway Implications for Diabetic Atherosclerosis. Arter. Thromb. Vasc. Biol. 2008, 28, 2275–2281. [Google Scholar] [CrossRef]
- Sobenin, I.; Tertov, V.; Koschinsky, T.; Bünting, C.; Slavina, E.; Dedovc, I.; Orekhov, A. Modified low density lipoprotein from diabetic patients causes cholesterol accumulation in human intimal aortic cells. Atherosclerosis 1993, 100, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.L.; Laimins, M.; Lopes-Virella, M.F. Isolation, characterization, and metabolism of the glycated and nonglycated subfractions of low-density lipoproteins isolated from type I diabetic patients and nondiabetic subjects. Diabetes 1995, 44, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Glycosylated low density lipoprotein is more sensitive to oxidation: Implications for the diabetic patient? Atherosclerosis 1993, 102, 63–67. [Google Scholar] [CrossRef]
- Godfrey, L.; Yamada-Fowler, N.; Smith, J.; Thornalley, P.J.; Rabbani, N. Arginine-directed glycation and decreased HDL plasma concentration and functionality. Nutr. Diabetes 2014, 4, e134. [Google Scholar] [CrossRef]
- Bacchetti, T.; Masciangelo, S.; Armeni, T.; Bicchiega, V.; Ferretti, G. Glycation of human high density lipoprotein by methylglyoxal: Effect on HDL-paraoxonase activity. Metabolism 2013, 63, 307–311. [Google Scholar] [CrossRef]
- Naitoh, T.; Kitahara, M.; Tsuruzoe, N. Tumor necrosis factor-α is induced through phorbol ester- and glycated human albumin-dependent pathway in THP-1 cells. Cell Signal. 2001, 13, 331–334. [Google Scholar] [CrossRef]
- Miele, C.; Riboulet, A.; Maitan, M.A.; Oriente, F.; Romano, C.; Formisano, P.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Human Glycated Albumin Affects Glucose Metabolism in L6 Skeletal Muscle Cells by Impairing Insulin-induced Insulin Receptor Substrate (IRS) Signaling through a Protein Kinase Cα-mediated Mechanism. J. Biol. Chem. 2003, 278, 47376–47387. [Google Scholar] [CrossRef]
- Turpin, C.; Catan, A.; Guerin-Dubourg, A.; Debussche, X.; Bravo, S.B.; Álvarez, E.; Elsen, J.V.D.; Meilhac, O.; Rondeau, P.; Bourdon, E. Enhanced oxidative stress and damage in glycated erythrocytes. PLoS ONE 2020, 15, e0235335. [Google Scholar] [CrossRef]
- Wautier, M.-P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.-L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Iwata, H.; Ukeda, H.; Maruyama, T.; Fujino, T.; Sawamura, M. Effect of carbonyl compounds on red blood cells deformability. Biochem. Biophys. Res. Commun. 2004, 321, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.S.; Chatterjee, S.; Cheng, G.; Andersen, E.; Mohammed, A.; Siegel, D.L.; Schmidt, A.M.; Albelda, S.M.; Lee, J.S. Advanced glycation end products on stored red blood cells increase endothelial reactive oxygen species generation through interaction with receptor for advanced glycation end products. Transfusion 2010, 50, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Mangalmurti, N.S.; Friedman, J.L.; Wang, L.-C.; Stolz, N.; Muthukumaran, G.; Siegel, N.L.; Schmidt, A.M.; Lee, J.; Albelda, S.M. The receptor for advanced glycation end products mediates lung endothelial activation by RBCs. Am. J. Physiol. Cell Mol. Physiol. 2013, 304, L250–L263. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.; Ermis, C.; Altunbas, H.; Balci, M.K. Serum HbA1c Levels and Exercise Capacity in Diabetic Patients. Jpn. Heart J. 2001, 42, 607–616. [Google Scholar] [CrossRef]
- Biao, X.; Chibber, R.; Ruggiero, D.; Kohner, E.; Ritter, J.; Ferro, A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003, 17, 1289–1291. [Google Scholar] [CrossRef]
- Ritthaler, U.; Deng, Y.; Zhang, Y.; Greten, J.; Abel, M.; Sido, B.; Allenberg, J.; Otto, G.; Roth, H.; Bierhaus, A. Expression of receptors for advanced glycation end products in peripheral occlusive vascular disease. Am. J. Pathol. 1995, 146, 688–694. [Google Scholar]
- Tan, K.C.B.; Shiu, S.W.M.; Chow, W.S.; Leng, L.; Bucala, R.; Betteridge, D.J. Association between serum levels of soluble receptor for advanced glycation end products and circulating advanced glycation end products in type 2 diabetes. Diabetologia 2006, 49, 2756–2762. [Google Scholar] [CrossRef]
- Li, J.-T.; Hou, F.-F.; Guo, Z.-J.; Shan, Y.-X.; Zhang, X.; Liu, Z.-Q. Advanced Glycation End Products Upregulate C-reactive Protein Synthesis by Human Hepatocytes Through Stimulation of Monocyte IL-6 and IL-1β Production. Scand. J. Immunol. 2007, 66, 555–562. [Google Scholar] [CrossRef]
- Gawlowski, T.; Stratmann, B.; Ruetter, R.; Buenting, C.E.; Menart, B.; Weiss, J.; Vlassara, H.; Koschinsky, T.; Tschoepe, D. Advanced glycation end products strongly activate platelets. Eur. J. Nutr. 2009, 48, 475–481. [Google Scholar] [CrossRef]
- Hangaishia, M.; Taguchia, J.; Miyatab, T.; Ikaria, Y.; Togoa, M.; Hashimotoa, Y.; Watanabea, T.; Kimuraa, S.; Kurokawab, K.; Ohno, M. Increased Aggregation of Human Platelets Produced by Advanced Glycation End Productsin Vitro. Biochem. Biophys. Res. Commun. 1998, 248, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.X.; Dayal, S. Inflammation mediated platelet hyperactivity in aging. Ann. Blood 2020, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated Serum Advanced Glycation Endproducts in Obese Indicate Risk for the Metabolic Syndrome: A Link Between Healthy and Unhealthy Obesity? J. Clin. Endocrinol. Metab. 2015, 100, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Fujiya, A.; Nagasaki, H.; Seino, Y.; Okawa, T.; Kato, J.; Fukami, A.; Himeno, T.; Uenishi, E.; Tsunekawa, S.; Kamiya, H.; et al. The role of S100B in the interaction between adipocytes and macrophages. Obesity 2013, 22, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gaens, K.H.; Goossens, G.H.; Niessen, P.M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Blaak, E.E.; et al. Nε -(Carboxymethyl)lysine-Receptor for Advanced Glycation End Product Axis Is a Key Modulator of Obesity-Induced Dysregulation of Adipokine Expression and Insulin Resistance. Arter. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; del Pozo, C.H.; Rosario, R.; Zou, Y.S.; Ananthakrishnan, R.; Xu, X.; Patel, P.R.; Benoit, V.M.; Yan, S.F.; Li, H.; et al. RAGE Regulates the Metabolic and Inflammatory Response to High-Fat Feeding in Mice. Diabetes 2014, 63, 1948–1965. [Google Scholar] [CrossRef]
- Wilson, A.F.; Elston, R.C.; Tran, L.D.; Siervogel, R.M. Use of the robust sib-pair method to screen for single-locus, multiple-locus, and pleiotropic effects: Application to traits related to hypertension. Am. J. Hum. Genet. 1991, 48, 862–872. [Google Scholar]
- Wuschke, S.; Dahm, S.; Schmidt, C.; Joost, H.-G.; Al-Hasani, H. A meta-analysis of quantitative trait loci associated with body weight and adiposity in mice. Int. J. Obes. 2006, 31, 829–841. [Google Scholar] [CrossRef]
- Maessen, D.B.; Brouwers, O.; Miyata, T.; Stehouwer, C.; Schalkwijk, C. Glyoxalase-1 overexpression reduces body weight and adipokine expression and improves insulin sensitivity in high-fat diet-induced obese mice. Diabetologia 2014, 57, 713. [Google Scholar]
- Chen, L.; Wang, T.; Guo, L.; Shen, Y.; Yang, T.; Wan, C.; Liao, Z.; Xu, D.; Wen, F. Overexpression of RAGE Contributes to Cigarette Smoke-Induced Nitric Oxide Generation in COPD. Lung 2014, 192, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, L.; Nicholson, L.F.; Black, P.N. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir. Med. 2011, 105, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, J.-R.; Kim, W.J.; Sundar, I.K.; Rahman, I.; Park, S.-M.; Yang, S.-R. Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signaling. FASEB J. 2017, 31, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Sukkar, M.; Wood, L.; Tooze, M.; Simpson, J.L.; McDonald, V.M.; Gibson, P.; Wark, P. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur. Respir. J. 2011, 39, 721–729. [Google Scholar] [CrossRef]
- Kanazawa, H.; Kodama, T.; Asai, K.; Matsumura, S.; Hirata, K. Increased levels of Nε-(carboxymethyl)lysine in epithelial lining fluid from peripheral airways in patients with chronic obstructive pulmonary disease: A pilot study. Clin. Sci. 2010, 119, 143–149. [Google Scholar] [CrossRef]
- Hoonhorst, S.J.M.; Loi, A.T.L.T.; Pouwels, S.D.; Faiz, A.; Telenga, E.D.; Berge, M.V.D.; Koenderman, L.; Lammers, J.-W.J.; Boezen, H.M.; van Oosterhout, A.J.M.; et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir. Res. 2016, 17, 46. [Google Scholar] [CrossRef]
- Stogsdill, M.P.; Stogsdill, J.A.; Bodine, B.G.; Fredrickson, A.C.; Sefcik, T.L.; Wood, T.T.; Kasteler, S.D.; Reynolds, P.R. Conditional Overexpression of Receptors for Advanced Glycation End-Products in the Adult Murine Lung Causes Airspace Enlargement and Induces Inflammation. Am. J. Respir. Cell Mol. Biol. 2013, 49, 128–134. [Google Scholar] [CrossRef]
- Sanders, K.A.; Delker, D.A.; Huecksteadt, T.; Beck, E.; Wuren, T.; Chen, Y.; Zhang, Y.; Hazel, M.W.; Hoidal, J.R. RAGE is a Critical Mediator of Pulmonary Oxidative Stress, Alveolar Macrophage Activation and Emphysema in Response to Cigarette Smoke. Sci. Rep. 2019, 9, 231. [Google Scholar] [CrossRef]
- Waseda, K.; Miyahara, N.; Taniguchi, A.; Kurimoto, E.; Ikeda, G.; Koga, H.; Fujii, U.; Yamamoto, Y.; Gelfand, E.W.; Yamamoto, H.; et al. Emphysema Requires the Receptor for Advanced Glycation End-Products Triggering on Structural Cells. Am. J. Respir. Cell Mol. Biol. 2015, 52, 482–491. [Google Scholar] [CrossRef]
- Allam, V.S.R.R.; Faiz, A.; Lam, M.; Rathnayake, S.N.H.; Ditz, B.; Pouwels, S.D.; Brandsma, C.; Timens, W.; Hiemstra, P.S.; Tew, G.W.; et al. RAGE and TLR4 differentially regulate airway hyperresponsiveness: Implications for COPD. Allergy 2020, 76, 1123–1135. [Google Scholar] [CrossRef]
- Haider, S.H.; Veerappan, A.; Crowley, G.; Caraher, E.J.; Ostrofsky, D.; Mikhail, M.; Lam, R.; Wang, Y.; Sunseri, M.; Kwon, S.; et al. Multiomics of World Trade Center Particulate Matter–induced Persistent Airway Hyperreactivity. Role of Receptor for Advanced Glycation End Products. Am. J. Respir. Cell Mol. Biol. 2020, 63, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.R.; Wasley, K.M.; Allison, C.H. Diesel Particulate Matter Induces Receptor for Advanced Glycation End-Products (RAGE) Expression in Pulmonary Epithelial Cells, and RAGE Signaling Influences NF-κB–Mediated Inflammation. Environ. Health Perspect. 2011, 119, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Yerkovich, S.T.; Towers, M.A.; Carroll, M.L.; Thomas, R.; Upham, J.W. Reduced soluble receptor for advanced glycation end-products in COPD. Eur. Respir. J. 2010, 37, 516–522. [Google Scholar] [CrossRef]
- Miniati, M.; Monti, S.; Basta, G.; Cocci, F.; Fornai, E.; Bottai, M. Soluble receptor for advanced glycation end products in COPD: Relationship with emphysema and chronic cor pulmonale: A case-control study. Respir. Res. 2011, 12, 37. [Google Scholar] [CrossRef]
- Gopal, P.; Reynaert, N.L.; Scheijen, J.L.J.M.; Schalkwijk, C.G.; Franssen, F.M.E.; Wouters, E.F.M.; Rutten, E.P.A. Association of plasma sRAGE, but not esRAGE with lung function impairment in COPD. Respir. Res. 2014, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Rutten, E.P.A.; Dentener, M.A.; Wouters, E.F.M.; Reynaert, N.L. Decreased plasma sRAGE levels in COPD: Influence of oxygen therapy. Eur. J. Clin. Investig. 2012, 42, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Cockayne, D.A.; Cheng, D.T.; Waschki, B.; Sridhar, S.; Ravindran, P.; Hilton, H.; Kourteva, G.; Bitter, H.; Pillai, S.G.; Visvanathan, S.; et al. Systemic Biomarkers of Neutrophilic Inflammation, Tissue Injury and Repair in COPD Patients with Differing Levels of Disease Severity. PLoS ONE 2012, 7, e38629. [Google Scholar] [CrossRef]
- Iwamoto, H.; Gao, J.; Pulkkinen, V.; Toljamo, T.; Nieminen, P.; Mazur, W. Soluble receptor for advanced glycation end-products and progression of airway disease. BMC Pulm. Med. 2014, 14, 68. [Google Scholar] [CrossRef]
- Coxson, H.O.; Dirksen, A.; Edwards, L.D.; Yates, J.C.; Agusti, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Crim, C.; Duvoix, A.; et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: A prospective analysis from the ECLIPSE study. Lancet Respir. Med. 2013, 1, 129–136. [Google Scholar] [CrossRef]
- Malik, P.; Hoidal, J.R.; Mukherjee, T.K. Implication of RAGE Polymorphic Variants in COPD Complication and Anti-COPD Therapeutic Potential of sRAGE. COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 737–748. [Google Scholar] [CrossRef]
- Perkins, T.N.; Oczypok, E.A.; Dutz, R.E.; Donnell, M.L.; Myerburg, M.M.; Oury, T.D. The receptor for advanced glycation end products is a critical mediator of type 2 cytokine signaling in the lungs. J. Allergy Clin. Immunol. 2019, 144, 796–808.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, J.; Sun, H.; Wei, Q.; Nong, G. sRAGE Inhibits the Mucus Hypersecretion in a Mouse Model with Neutrophilic Asthma. Immunol. Investig. 2021, 51, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Perkins, T.N.; Donnell, M.L.; Oury, T.D. The axis of the receptor for advanced glycation endproducts in asthma and allergic airway disease. Allergy 2020, 76, 1350–1366. [Google Scholar] [CrossRef]
- Kang, J.H.; Hwang, S.M.; Chung, I.Y. S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology 2014, 144, 79–90. [Google Scholar] [CrossRef] [PubMed]
- John, M.; McKeever, T.; Al Haddad, M.; Hall, I.; Sayers, I.; Cockcroft, J.R.; Bolton, C.E. Traditional and emerging indicators of cardiovascular risk in chronic obstructive pulmonary disease. Chronic Respir. Dis. 2016, 13, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.W.; Weidner, J.; Franssen, F.; Reynaert, N.L.; Gaffon, S.; Wouters, E.F.M.; Spruit, M.A. Biomarker-based clustering of patients with chronic obstructive pulmonary disease. ERJ Open 2023, 9, 00301–02022. [Google Scholar] [CrossRef]
- Biemel, K.M.; Friedl, D.A.; Lederer, M.O. Identification and Quantification of Major Maillard Cross-links in Human Serum Albumin and Lens Protein. J. Biol. Chem. 2002, 277, 24907–24915. [Google Scholar] [CrossRef]
- Kilhovd, B.; Giardino, I.; Torjesen, P.; Birkeland, K.; Berg, T.; Thornalley, P.; Brownlee, M.; Hanssen, K. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 2003, 52, 163–167. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Berg, T.J.; I Birkeland, K.; Thorsby, P.; Hanssen, K.F. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care 1999, 22, 1543–1548. [Google Scholar] [CrossRef]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Beulens, J.W.; van Dieren, S.; Scheijen, J.L.; van der Bend, A.D.L.; Spijkerman, A.M.; van der Schouw, Y.T.; Stehouwer, C.D.; Schalkwijk, C.G. Plasma Advanced Glycation End Products Are Associated with Incident Cardiovascular Events in Individuals With Type 2 Diabetes: A Case-Cohort Study With a Median Follow-up of 10 Years (EPIC-NL). Diabetes 2014, 64, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.B.; Shiu, S.W.M.; Wong, Y.; Tam, X. Serum advanced glycation end products (AGEs) are associated with insulin resistance. Diabetes/Metab. Res. Rev. 2011, 27, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.-I.; Matsui, T.; Takeuchi, M.; Nitta, Y.; Kodama, N.; Mizoguchi, M.; Imaizumi, T. Serum Levels of Advanced Glycation End Products (AGEs) are Independent Correlates of Insulin Resistance in Nondiabetic Subjects. Cardiovasc. Ther. 2010, 30, 42–48. [Google Scholar] [CrossRef]
- Lim, M.; Park, L.; Shin, G.; Hong, H.; Kang, I.; Park, Y. Induction of Apoptosis of β Cells of the Pancreas by Advanced Glycation End-Products, Important Mediators of Chronic Complications of Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2008, 1150, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.S.; Sheu, M.L.; Yang, R.S.; Chan, D.C.; Wu, C.T.; Yang, T.H.; Chiang, C.K.; Liu, S.H. The pathological role of advanced glycation end products-downregulated heat shock protein 60 in islet beta-cell hypertrophy and dysfunction. Oncotarget 2016, 7, 23072–23087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Tsuneyama, K.; Kominami, R.; Shinohara, H.; Sakurai, S.; Yonekura, H.; Watanabe, T.; Takano, Y.; Yamamoto, H.; Yamamoto, Y. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod. Pathol. 2005, 18, 1385–1396. [Google Scholar] [CrossRef]
- Monnier, V.M.; Sell, D.R.; Genuth, S. Glycation Products as Markers and Predictors of the Progression of Diabetic Complications. Ann. N. Y. Acad. Sci. 2005, 1043, 567–581. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef]
- Bos, D.C.; De Ranitz-Greven, W.L.; De Valk, H.W. Advanced Glycation End Products, Measured as Skin Autofluorescence and Diabetes Complications: A Systematic Review. Diabetes Technol. Ther. 2011, 13, 773–779. [Google Scholar] [CrossRef]
- Yamanaka, M.; Matsumura, T.; Ohno, R.-I.; Fujiwara, Y.; Shinagawa, M.; Sugawa, H.; Hatano, K.; Shirakawa, J.-I.; Kinoshita, H.; Ito, K.; et al. Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 2016, 58, 135–140. [Google Scholar] [CrossRef]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chida, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of Advanced Glycation End Products with coronary Artery Calcification in Japanese Subjects with Type 2 Diabetes as Assessed by Skin Autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Sell, D.R.; Carlson, E.C.; Monnier, V.M. Differential effects of type 2 (non-insulin-dependent) diabetes mellitus on pentosidine formation in skin and glomerular basement membrane. Diabetologia 1993, 36, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.-P.; Alt, A.; Niwa, T.; Clausen, J.T.; Bretzel, R.G.; Brownlee, M.; Schleicher, E.D. Differential accumulation of advanced glycation end products in the course of diabetic retinopathy. Diabetologia 1999, 42, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Tanji, N.; Markowitz, G.S.; Fu, C.; Kislinger, T.; Taguchi, A.; Pischetsrieder, M.; Stern, D.; Schmidt, A.M.; D’Agati, V.D. Expression of Advanced Glycation End Products and Their Cellular Receptor RAGE in Diabetic Nephropathy and Nondiabetic Renal Disease. J. Am. Soc. Nephrol. 2000, 11, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Toyoda, M.; Yamamoto, N.; Miyauchi, M.; Katoh, M.; Kimura, M.; Maruyama, M.; Honma, M.; Umezono, T.; Yagame, M. Relationship between the Expression of Advanced Glycation End-Products (AGE) and the Receptor for AGE (RAGE) mRNA in Diabetic Nephropathy. Intern. Med. 2006, 45, 435–441. [Google Scholar] [CrossRef]
- Cipollone, F.; Iezzi, A.; Fazia, M.; Zucchelli, M.; Pini, B.; Cuccurullo, C.; De Cesare, D.; De Blasis, G.; Muraro, R.; Bei, R.; et al. The Receptor RAGE as a Progression Factor Amplifying Arachidonate-Dependent Inflammatory and Proteolytic Response in Human Atherosclerotic Plaques. Circulation 2003, 108, 1070–1077. [Google Scholar] [CrossRef]
- Grossin, N.; Wautier, M.-P.; Meas, T.; Guillausseau, P.-J.; Massin, P.; Wautier, J.-L. Severity of diabetic microvascular complications is associated with a low soluble RAGE level. Diabetes Metab. 2008, 34, 392–395. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.; Matsui, T.; Adachi, H.; Takeuchi, M.; Imaizumi, T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are correlated with AGEs in both diabetic and non-diabetic subjects. Clin. Exp. Med. 2007, 7, 188–190. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, L.; Zhang, F.R.; Chen, Q.J.; De Caterina, R.; Shen, W.F. Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. Eur. J. Heart Fail. 2011, 13, 440–449. [Google Scholar] [CrossRef]
- Kerkeni, M.; Saïdi, A.; Bouzidi, H.; Ben Yahya, S.; Hammami, M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc. Res. 2012, 84, 378–383. [Google Scholar] [CrossRef]
- Basta, G.; Sironi, A.M.; Lazzerini, G.; Del Turco, S.; Buzzigoli, E.; Casolaro, A.; Natali, A.; Ferrannini, E.; Gastaldelli, A. Circulating Soluble Receptor for Advanced Glycation End Products Is Inversely Associated with Glycemic Control and S100A12 Protein. J. Clin. Endocrinol. Metab. 2006, 91, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamagishi, S.-I.; Adachi, H.; Matsui, T.; Kurita-Nakamura, Y.; Takeuchi, M.; Inoue, H.; Imaizumi, T. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes/Metab. Res. Rev. 2008, 24, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamagishi, S.-I.; Adachi, H.; Matsui, T.; Kurita-Nakamura, Y.; Takeuchi, M.; Inoue, H.; Imaizumi, T. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvasc. Res. 2008, 76, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamagishi, S.-I.; Adachi, H.; Kurita-Nakamura, Y.; Matsui, T.; Yoshida, T.; Imaizumi, T. Serum Levels of sRAGE, the Soluble Form of Receptor for Advanced Glycation End Products, Are Associated with Inflammatory Markers in Patients with Type 2 Diabetes. Mol. Med. 2007, 13, 185–189. [Google Scholar] [CrossRef]
- Fujisawa, K.; Katakami, N.; Kaneto, H.; Naka, T.; Takahara, M.; Sakamoto, F.; Irie, Y.; Miyashita, K.; Kubo, F.; Yasuda, T.; et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis 2013, 227, 425–428. [Google Scholar] [CrossRef]
- González, I.; Romero, J.; Rodríguez, B.L.; Pérez-Castro, R.; Rojas, A. The immunobiology of the receptor of advanced glycation end-products: Trends and challenges. Immunobiology 2013, 218, 790–797. [Google Scholar] [CrossRef]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma Level of Endogenous Secretory RAGE Is Associated with Components of the Metabolic Syndrome and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Katakami, N.; Matsuhisa, M.; Kaneto, H.; Yamasaki, Y. Serum endogenous secretory RAGE levels are inversely associated with carotid IMT in type 2 diabetic patients. Atherosclerosis 2007, 190, 22–23. [Google Scholar] [CrossRef]
- Di Pino, A.; Urbano, F.; Zagami, R.M.; Filippello, A.; Di Mauro, S.; Piro, S.; Purrello, F.; Rabuazzo, A.M. Low Endogenous Secretory Receptor for Advanced Glycation End-Products Levels Are Associated with Inflammation and Carotid Atherosclerosis in Prediabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1701–1709. [Google Scholar] [CrossRef]
- Choi, K.; Yoo, H.; Kim, H.; Lee, K.; Seo, J.; Kim, S.; Kim, N.; Choi, D.; Baik, S. Association between endogenous secretory RAGE, inflammatory markers and arterial stiffness. Int. J. Cardiol. 2009, 132, 96–101. [Google Scholar] [CrossRef]
- Katakami, N.; Matsuhisa, M.; Kaneto, H.; Matsuoka, T.-A.; Sakamoto, K.; Yasuda, T.; Yamasaki, Y. Endogenous secretory RAGE but not soluble RAGE is associated with carotid atherosclerosis in type 1 diabetes patients. Diabetes Vasc. Dis. Res. 2008, 5, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Matsuhisa, M.; Kaneto, H.; Matsuoka, T.-A.; Sakamoto, K.; Yasuda, T.; Umayahara, Y.; Kosugi, K.; Yamasaki, Y. Serum endogenous secretory RAGE level is an independent risk factor for the progression of carotid atherosclerosis in type 1 diabetes. Atherosclerosis 2009, 204, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, F.; Liu, L.; Zhang, Q. Prevalence of osteoporosis in patients with diabetes mellitus: A systematic review and meta-analysis of observational studies. BMC Endocr. Disord. 2023, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Anık, A.; Anık, A.; Uysal, P. Assessment of pulmonary function by impulse oscillometry and spirometry in children with type 1 diabetes mellitus. Pediatr. Pulmonol. 2020, 55, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.M.; Hasu, M.; Popov, D.; Zhang, J.H.; Chen, J.; Yan, S.D.; Brett, J.; Cao, R.; Kuwabara, K.; Costache, G. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8807–8811. [Google Scholar] [CrossRef]
- Simm, A.; Caßelmann, C.; Schubert, A.; Hofmann, S.; Reimann, A.; Silber, R.-E. Age associated changes of AGE-receptor expression: RAGE upregulation is associated with human heart dysfunction. Exp. Gerontol. 2004, 39, 407–413. [Google Scholar] [CrossRef]
- Nerlich, A.G.; Schleicher, E.D. N(epsilon)-(carboxymethyl)lysine in atherosclerotic vascular lesions as a marker for local oxidative stress. Atherosclerosis 1999, 144, 41–47. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Wouters, K.; Huijberts, M.S.; Gijbels, M.J.; Sluimer, J.C.; Scheijen, J.L.; Heeneman, S.; Biessen, E.A.; Daemen, M.J.; Brownlee, M.; et al. Higher levels of advanced glycation endproducts in human carotid atherosclerotic plaques are associated with a rupture-prone phenotype. Eur. Heart J. 2013, 35, 1137–1146. [Google Scholar] [CrossRef]
- Kume, S.; Takeya, M.; Mori, T.; Araki, N.; Suzuki, H.; Horiuchi, S.; Kodama, T.; Miyauchi, Y.; Takahashi, K. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am. J. Pathol. 1995, 147, 654–667. [Google Scholar]
- Baidoshvili, A.; Krijnen, P.; Kupreishvili, K.; Ciurana, C.; Bleeker, W.; Nijmeijer, R.; Visser, C.; Visser, F.; Meijer, C.; Stooker, W.; et al. Nε -(Carboxymethyl)lysine Depositions in Intramyocardial Blood Vessels in Human and Rat Acute Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2006, 26, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Begieneman, M.P.; Rijvers, L.; Kubat, B.; Paulus, W.J.; Vonk, A.B.; van Rossum, A.C.; Schalkwijk, C.G.; Stooker, W.; Niessen, H.W.; Krijnen, P.A. Atrial Fibrillation Coincides with the Advanced Glycation End Product Nε-(Carboxymethyl)Lysine in the Atrium. Am. J. Pathol. 2015, 185, 2096–2104. [Google Scholar] [CrossRef]
- Sun, L.; Ishida, T.; Yasuda, T.; Kojima, Y.; Honjo, T.; Yamamoto, Y.; Yamamoto, H.; Ishibashi, S.; Hirata, K.-I.; Hayashi, Y. RAGE mediates oxidized LDL-induced pro-inflammatory effects and atherosclerosis in non-diabetic LDL receptor-deficient mice. Cardiovasc. Res. 2008, 82, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, L.G.; Wendt, T.; Qu, W.; Lu, Y.; Lalla, E.; Rong, L.L.; Goova, M.T.; Moser, B.; Kislinger, T.; Lee, D.C.; et al. RAGE Blockade Stabilizes Established Atherosclerosis in Diabetic Apolipoprotein E–Null Mice. Circulation 2002, 106, 2827–2835. [Google Scholar] [CrossRef]
- Wendt, T.; Harja, E.; Bucciarelli, L.; Qu, W.; Lu, Y.; Rong, L.L.; Jenkins, D.G.; Stein, G.; Schmidt, A.M.; Yan, S.F. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis 2006, 185, 70–77. [Google Scholar] [CrossRef]
- Soro-Paavonen, A.; Watson, A.M.D.; Li, J.; Paavonen, K.; Koitka, A.; Calkin, A.C.; Barit, D.; Coughlan, M.T.; Drew, B.G.; Lancaster, G.I.; et al. Receptor for Advanced Glycation End Products (RAGE) Deficiency Attenuates the Development of Atherosclerosis in Diabetes. Diabetes 2008, 57, 2461–2469. [Google Scholar] [CrossRef]
- Brodeur, M.R.; Bouvet, C.; Bouchard, S.; Moreau, S.; Leblond, J.; Deblois, D.; Moreau, P. Reduction of Advanced-Glycation End Products Levels and Inhibition of RAGE Signaling Decreases Rat Vascular Calcification Induced by Diabetes. PLoS ONE 2014, 9, e85922. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, M.; Zhang, Z.; Yu, Y.; Chen, Q.; Zhang, W.; Zhao, X. Blockade of receptor for advanced glycation end products protects against systolic overload-induced heart failure after transverse aortic constriction in mice. Eur. J. Pharmacol. 2016, 791, 535–543. [Google Scholar] [CrossRef]
- Semba, R.D.; Najjar, S.S.; Sun, K.; Lakatta, E.G.; Ferrucci, L. Serum Carboxymethyl-Lysine, an Advanced Glycation End Product, Is Associated with Increased Aortic Pulse Wave Velocity in Adults. Am. J. Hypertens. 2009, 22, 74–79. [Google Scholar] [CrossRef]
- Semba, R.D.; Sun, K.; Schwartz, A.V.; Varadhan, R.; Harris, T.B.; Satterfield, S.; Garcia, M.; Ferrucci, L.; Newman, A.B. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J. Hypertens. 2015, 33, 797–803. [Google Scholar] [CrossRef]
- McNulty, M.; Mahmud, A.; Feely, J. Advanced Glycation End-Products and Arterial Stiffness in Hypertension. Am. J. Hypertens. 2007, 20, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Inukai, T.; Tayama, K.; Takemura, Y. Serum concentrations of advanced glycation endproducts are associated with the development of atherosclerosis as well as diabetic microangiopathy in patients with type 2 diabetes. Acta Diabetol. 2000, 37, 87–92. [Google Scholar] [CrossRef]
- Kanauchi, M.; Tsujimoto, N.; Hashimoto, T. Advanced Glycation End Products in Nondiabetic Patients with Coronary Artery Disease. Diabetes Care 2001, 24, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Raposeiras-Roubín, S.; Rodiño-Janeiro, B.K.; Grigorian-Shamagian, L.; Seoane-Blanco, A.; Moure-González, M.; Varela-Román, A.; Álvarez, E.; González-Juanatey, J.R. Evidence for a role of advanced glycation end products in atrial fibrillation. Int. J. Cardiol. 2011, 157, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Takeishi, Y.; Arimoto, T.; Niizeki, T.; Shishido, T.; Takahashi, H.; Nozaki, N.; Hirono, O.; Tsunoda, Y.; Nitobe, J.; et al. High Serum Level of Pentosidine, an Advanced Glycation End Product (AGE), is a Risk Factor of Patients with Heart Failure. J. Card. Fail. 2007, 13, 199–206. [Google Scholar] [CrossRef]
- Falcone, C.; Emanuele, E.; D’angelo, A.; Buzzi, M.P.; Belvito, C.; Cuccia, M.; Geroldi, D. Plasma Levels of Soluble Receptor for Advanced Glycation End Products and Coronary Artery Disease in Nondiabetic Men. Arter. Thromb. Vasc. Biol. 2005, 25, 1032–1037. [Google Scholar] [CrossRef]
- Falcone, C.; Buzzi, M.P.; Bozzini, S.; Boiocchi, C.; D’angelo, A.; Schirinzi, S.; Choi, J.; Kilama, M.O.; Esposito, C.; Torreggiani, M.; et al. Relationship between sRAGE and eotaxin-3 with CRP in hypertensive patients at high cardiovascular risk. J. Nephrol. 2012, 26, 144–151. [Google Scholar] [CrossRef]
- Geroldi, D.; Falcone, C.; Emanuele, E.; D’Angelo, A.; Calcagnino, M.; Buzzi, M.P.; A Scioli, G.; Fogari, R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J. Hypertens. 2005, 23, 1725–1729. [Google Scholar] [CrossRef]
- Mayer, O.; Seidlerová, J.; Filipovský, J.; Vágovičová, P.; Wohlfahrt, P.; Cífková, R.; Windrichová, J.; Topolčan, O. Soluble receptor for advanced glycation end products and increased aortic stiffness in the general population. Hypertens. Res. 2015, 39, 266–271. [Google Scholar] [CrossRef]
- Lazo, M.; Halushka, M.; Shen, L.; Maruthur, N.; Rebholz, C.M.; Rawlings, A.; Hoogeveen, R.; Brinkley, T.E.; Ballantyne, C.M.; Astor, B.C.; et al. Soluble receptor for advanced glycation end products and the risk for incident heart failure: The Atherosclerosis Risk in Communities Study. Am. Heart J. 2015, 170, 961–967. [Google Scholar] [CrossRef]
- Koyama, Y.; Takeishi, Y.; Niizeki, T.; Suzuki, S.; Kitahara, T.; Sasaki, T.; Kubota, I. Soluble Receptor for Advanced Glycation End Products (RAGE) is a Prognostic Factor for Heart Failure. J. Card. Fail. 2008, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Baek, J.-Y.; Shin, W.S.; Kim, D.-B.; Jang, S.W.; Shin, D.I.; Koh, Y.S.; Seo, S.M.; Uhm, J.-S.; Kim, T.H.; et al. Soluble Receptor of Advanced Glycated Endproducts Is Associated with Plaque Vulnerability in Patients with Acute Myocardial Infarction. Circ. J. 2011, 75, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Bangert, A.; Andrassy, M.; Müller, A.-M.; Bockstahler, M.; Fischer, A.; Volz, C.H.; Leib, C.; Göser, S.; Korkmaz-Icöz, S.; Zittrich, S.; et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc. Natl. Acad. Sci. USA 2016, 113, E155–E164. [Google Scholar] [CrossRef] [PubMed]
- Cuccurullo, C.; Iezzi, A.; Fazia, M.L.; De Cesare, D.; Di Francesco, A.; Muraro, R.; Bei, R.; Ucchino, S.; Spigonardo, F.; Chiarelli, F.; et al. Suppression of Rage as a Basis of Simvastatin-Dependent Plaque Stabilization in Type 2 Diabetes. Arter. Thromb. Vasc. Biol. 2006, 26, 2716–2723. [Google Scholar] [CrossRef]
- Santilli, F.; Bucciarelli, L.; Noto, D.; Cefalù, A.B.; Davì, V.; Ferrante, E.; Pettinella, C.; Averna, M.R.; Ciabattoni, G.; Davì, G. Decreased plasma soluble RAGE in patients with hypercholesterolemia: Effects of statins. Free Radic. Biol. Med. 2007, 43, 1255–1262. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorpe, S.R.; Thallas-Bonke, V.; Pete, J.; Thomas, M.C.; Deemer, E.R.; Bassal, S.; El-Osta, A.; Long, D.M.; Panagiotopoulos, S.; et al. Modulation of Soluble Receptor for Advanced Glycation End Products by Angiotensin-Converting Enzyme-1 Inhibition in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2005, 16, 2363–2372. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamagishi, S.-I.; Nakamura, Y.; Takenaka, K.; Matsui, T.; Jinnouchi, Y.; Imaizumi, T. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc. Res. 2005, 70, 137–141. [Google Scholar] [CrossRef]
- Odetti, P.; Rossi, S.; Monacelli, F.; Poggi, A.; Cirnigliaro, M.; Federici, M.; Federici, A. Advanced Glycation End Products and Bone Loss during Aging. Ann. N. Y. Acad. Sci. 2005, 1043, 710–717. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Tang, S.Y.; Baumbach, B.M.; Hwu, P.B.; Sakkee, A.N.; van der Ham, F.; DeGroot, J.; Bank, R.A.; Keaveny, T.M. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 2005, 37, 825–832. [Google Scholar] [CrossRef]
- Hein, G.; Weiss, C.; Lehmann, G.; Niwa, T.; Stein, G.; Franke, S. Advanced glycation end product modification of bone proteins and bone remodelling: Hypothesis and preliminary immunohistochemical findings. Ann. Rheum. Dis. 2006, 65, 101–104. [Google Scholar] [CrossRef]
- Cecil, D.L.; Johnson, K.; Rediske, J.; Lotz, M.; Schmidt, A.M.; Terkeltaub, R. Inflammation-Induced Chondrocyte Hypertrophy Is Driven by Receptor for Advanced Glycation End Products. J. Immunol. 2005, 175, 8296–8302. [Google Scholar] [CrossRef] [PubMed]
- Yammani, R.R.; Carlson, C.S.; Bresnick, A.R.; Loeser, R.F. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: Role of the receptor for advanced glycation end products. Arthritis Rheum. 2006, 54, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Immel, D.; Xi, C.-X.; Bierhaus, A.; Feng, X.; Mei, L.; Nawroth, P.; Stern, D.M.; Xiong, W.-C. Regulation of osteoclast function and bone mass by RAGE. J. Exp. Med. 2006, 203, 1067–1080. [Google Scholar] [CrossRef]
- Philip, B.K.; Childress, P.J.; Robling, A.G.; Heller, A.; Nawroth, P.P.; Bierhaus, A.; Bidwell, J.P. RAGE supports parathyroid hormone-induced gains in femoral trabecular bone. Am. J. Physiol. Metab. 2010, 298, E714–E725. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, T.; Bierhaus, A.; Nawroth, P.P. RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes Infect. 2004, 6, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, J.; Li, Z. Receptor for advanced glycation end products inhibits proliferation in osteoblast through suppression of Wnt, PI3K and ERK signaling. Biochem. Biophys. Res. Commun. 2012, 423, 684–689. [Google Scholar] [CrossRef]
- Yang, D.-H.; Chiang, T.-I.; Chang, I.-C.; Lin, F.-H.; Wei, J.C.-C.; Cheng, Y.-W. Increased Levels of Circulating Advanced Glycation End-Products in Menopausal Women with Osteoporosis. Int. J. Med. Sci. 2014, 11, 453–460. [Google Scholar] [CrossRef]
- Hein, G.; Wiegand, R.; Lehmann, G.; Stein, G.; Franke, S. Advanced glycation end-products pentosidine and N -carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology 2003, 42, 1242–1246. [Google Scholar] [CrossRef]
- Yamamoto, M.; Yamaguchi, T.; Yamauchi, M.; Yano, S.; Sugimoto, T. Serum Pentosidine Levels Are Positively Associated with the Presence of Vertebral Fractures in Postmenopausal Women with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1013–1019. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Garnero, P.; Hillier, T.A.; Sellmeyer, D.E.; Strotmeyer, E.S.; Feingold, K.R.; Resnick, H.E.; Tylavsky, F.A.; Black, D.M.; Cummings, S.R.; et al. Pentosidine and Increased Fracture Risk in Older Adults with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2380–2386. [Google Scholar] [CrossRef]
- Gineyts, E.; Munoz, F.; Bertholon, C.; Sornay-Rendu, E.; Chapurlat, R. Urinary levels of pentosidine and the risk of fracture in postmenopausal women: The OFELY study. Osteoporos. Int. 2009, 21, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kuroda, T.; Saito, M.; Shiraki, M. Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. J. Bone Miner. Res. 2011, 26, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.R.; Iwata, K.; Phipps, R.; Burr, D.B. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone 2006, 39, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Donato, R. HMGB1/RAGE regulates muscle satellite cell homeostasis via p38 MAPK/myogenin-dependent repression of Pax7 transcription. J. Cell Sci. 2012, 125, 1440–1454. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Yang, R.-S.; Sheu, M.-L.; Chan, D.-C.; Yang, T.-H.; Tsai, K.-S.; Chiang, C.-K.; Liu, S.-H. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 2015, 238, 470–482. [Google Scholar] [CrossRef]
- Davis, H.M.; Essex, A.L.; Valdez, S.; Deosthale, P.J.; Aref, M.W.; Allen, M.R.; Bonetto, A.; Plotkin, L.I. Short-term pharmacologic RAGE inhibition differentially affects bone and skeletal muscle in middle-aged mice. Bone 2019, 124, 89–102. [Google Scholar] [CrossRef]
- Egawa, T.; Kido, K.; Yokokawa, T.; Fujibayashi, M.; Goto, K.; Hayashi, T. Involvement of receptor for advanced glycation end products in microgravity-induced skeletal muscle atrophy in mice. Acta Astronaut. 2020, 176, 332–340. [Google Scholar] [CrossRef]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Waqas, K.; Chen, J.; Trajanoska, K.; Ikram, M.A.; Uitterlinden, A.G.; Rivadeneira, F.; Zillikens, M.C. Skin Autofluorescence, a Noninvasive Biomarker for Advanced Glycation End-products, Is Associated with Sarcopenia. J. Clin. Endocrinol. Metab. 2021, 107, e793–e803. [Google Scholar] [CrossRef]
- Tabara, Y.; Ikezoe, T.; Yamanaka, M.; Setoh, K.; Segawa, H.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Ichihashi, N.; Tsuboyama, T.; et al. Advanced Glycation End Product Accumulation Is Associated with Low Skeletal Muscle Mass, Weak Muscle Strength, and Reduced Bone Density: The Nagahama Study. J. Gerontol. Ser. A 2018, 74, 1446–1453. [Google Scholar] [CrossRef]
- Mori, H.; Kuroda, A.; Ishizu, M.; Ohishi, M.; Takashi, Y.; Otsuka, Y.; Taniguchi, S.; Tamaki, M.; Kurahashi, K.; Yoshida, S.; et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in patients with type 2 diabetes. J. Diabetes Investig. 2019, 10, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does Accumulation of Advanced Glycation End Products Contribute to the Aging Phenotype? J. Gerontol. Ser. A 2010, 65A, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Dalal, M.; Ferrucci, L.; Sun, K.; Beck, J.; Fried, L.P.; Semba, R.D. Elevated Serum Advanced Glycation End Products and Poor Grip Strength in Older Community-Dwelling Women. J. Gerontol. Ser. A 2009, 64, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Toyoguchi, T.; Inage, K.; Fujimoto, K.; Orita, S.; Suzuki, M.; Kanamoto, H.; Abe, K.; Norimoto, M.; Umimura, T.; et al. Advanced glycation end products are associated with sarcopenia in older women: Aging marker dynamics. J. Women Aging 2019, 33, 328–340. [Google Scholar] [CrossRef]
- Tanaka, K.; Kanazawa, I.; Sugimoto, T. Elevated Serum Pentosidine and Decreased Serum IGF-I Levels are Associated with Loss of Muscle Mass in Postmenopausal Women with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2015, 124, 163–166. [Google Scholar] [CrossRef]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.-I.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 2020, 10, 17647. [Google Scholar] [CrossRef]
- Whitson, H.E.; Arnold, A.M.; Yee, L.M.; Mukamal, K.J.; Kizer, J.R.; Djousse, L.; Ix, J.H.; Siscovick, D.; Tracy, R.P.; Thielke, S.M.; et al. Serum Carboxymethyl-Lysine, Disability, and Frailty in Older Persons: The Cardiovascular Health Study. J. Gerontol. Ser. A 2013, 69, 710–716. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lee, E.J.; Chung, H.S.; Yoo, H.J.; Kang, H.J.; Song, W.; Baik, S.H.; Choi, K.M. The association of low muscle mass with soluble receptor for advanced glycation end products (sRAGE): The Korean Sarcopenic Obesity Study (KSOS). Diabetes/Metab. Res. Rev. 2018, 34, e2974. [Google Scholar] [CrossRef]
- Wu, S.-E.; Chiu, Y.-L.; Kao, T.-W.; Chen, W.-L. Elevated level of the soluble receptor for advanced glycation end-products involved in sarcopenia: An observational study. BMC Geriatr. 2021, 21, 531. [Google Scholar] [CrossRef]
- Gomez-Cabrero, D.; Walter, S.; Abugessaisa, I.; Miñambres-Herraiz, R.; Palomares, L.B.; Butcher, L.; Erusalimsky, J.D.; Garcia-Garcia, F.J.; Carnicero, J.; Hardman, T.C.; et al. A robust machine learning framework to identify signatures for frailty: A nested case-control study in four aging European cohorts. Geroscience 2021, 43, 1317–1329. [Google Scholar] [CrossRef]
- Butcher, L.; Carnicero, J.A.; Cabrero, D.G.; Dartigues, J.-F.; Peres, K.; Garcia-Garcia, F.J.; Rodriguez-Mañas, L.; Erusalimsky, J.D. FRAILOMIC Consortium Increased levels of soluble Receptor for Advanced Glycation End-products (RAGE) are associated with a higher risk of mortality in frail older adults. Age Ageing 2019, 48, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, G.; Rouleau, A.-J.; Couture, V.; Söllrald, T.; Drouin, G.; Veillette, N.; Grandbois, M.; Grenier, G. Increased Stiffness in Aged Skeletal Muscle Impairs Muscle Progenitor Cell Proliferative Activity. PLoS ONE 2015, 10, e0136217. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.K.; Kayupov, E.; Gumucio, J.P.; Mendias, C.; Claflin, D.; Brooks, S.V. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 2014, 117, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Snow, L.M.; Fugere, N.A.; Thompson, L.V. Advanced Glycation End-Product Accumulation and Associated Protein Modification in Type II Skeletal Muscle with Aging. J. Gerontol. Ser. A 2007, 62, 1204–1210. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev. Mol. Med. 2009, 11, e9. [Google Scholar] [CrossRef]
- Agalou, S.; Ahmed, N.; Babaei-Jadidi, R.; Dawnay, A.; Thornalley, P.J. Profound Mishandling of Protein Glycation Degradation Products in Uremia and Dialysis. J. Am. Soc. Nephrol. 2005, 16, 1471–1485. [Google Scholar] [CrossRef]
- Horie, K.; Miyata, T.; Maeda, K.; Miyata, S.; Sugiyama, S.; Sakai, H.; Strihou, C.V.Y.D.; Monnier, V.M.; Witztum, J.L.; Kurokawa, K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Investig. 1997, 100, 2995–3004. [Google Scholar] [CrossRef]
- Papanastasiou, P.; Grass, L.; Rodela, H.; Patrikarea, A.; Oreopoulos, D.; Diamandis, E.P. Immunological quantification of advanced glycosylation end-products in the serum of patients on hemodialysis or CAPD. Kidney Int. 1994, 46, 216–222. [Google Scholar] [CrossRef]
- Semba, R.D.; Fink, J.C.; Sun, K.; Windham, B.G.; Ferrucci, L. Serum Carboxymethyl-Lysine, a Dominant Advanced Glycation End Product, Is Associated with Chronic Kidney Disease: The Baltimore Longitudinal Study of Aging. J. Ren. Nutr. 2010, 20, 74–81. [Google Scholar] [CrossRef]
- Hou, F.F.; Ren, H.; Owen, W.F.; Guo, Z.J.; Chen, P.Y.; Schmidt, A.M.; Miyata, T.; Zhang, X. Enhanced Expression of Receptor for Advanced Glycation End Products in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2004, 15, 1889–1896. [Google Scholar] [CrossRef]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial Dysfunction in Patients with Chronic Kidney Disease Results from Advanced Glycation End Products (AGE)-Mediated Inhibition of Endothelial Nitric Oxide Synthase through RAGE Activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Ansell, D.; Kent, D.M.; Griffith, J.L.; Naimark, D.; Wanner, C.; Tangri, N. Predicting Mortality in Incident Dialysis Patients: An Analysis of the United Kingdom Renal Registry. Am. J. Kidney Dis. 2011, 57, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.; Thomas, M.C.; Fernando, D.; Macmillan, N.; Power, D.A.; Ierino, F.L. Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrol. Dial. Transplant. 2006, 21, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Sun, J.; Qureshi, A.R.; Isoyama, N.; Leurs, P.; Anderstam, B.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Lindholm, B. Plasma Pentosidine and Its Association with Mortality in Patients with Chronic Kidney Disease. PLoS ONE 2016, 11, e0163826. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Nakayama, M.; Kanno, M.; Kimura, H.; Watanabe, K.; Tani, Y.; Kusano, Y.; Suzuki, H.; Hayashi, Y.; Asahi, K.; et al. Skin Autofluorescence Is Associated with the Progression of Chronic Kidney Disease: A Prospective Observational Study. PLoS ONE 2013, 8, e83799. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Hartog, J.W.; Graaff, R.; Huisman, R.J.; Links, T.P.; Hollander, N.C.D.; Thorpe, S.R.; Baynes, J.W.; Navis, G.; Gans, R.O.; et al. Skin Autofluorescence, a Measure of Cumulative Metabolic Stress and Advanced Glycation End Products, Predicts Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 3687–3693. [Google Scholar] [CrossRef]
- Bouma, B.; Kroon-Batenburg, L.M.; Wu, Y.-P.; Brünjes, B.; Posthuma, G.; Kranenburg, O.; de Groot, P.G.; Voest, E.E.; Gebbink, M.F. Glycation Induces Formation of Amyloid Cross-β Structure in Albumin. J. Biol. Chem. 2003, 278, 41810–41819. [Google Scholar] [CrossRef]
- Brancaccio, D.; Gallieni, M.; Niwa, T.; Braidotti, P.; Coggi, G. Ultrastructural localization of advanced glycation end products and beta2-microglobulin in dialysis amyloidosis. J. Nephrol. 2000, 13, 129–136. [Google Scholar]
- Rebholz, C.M.; Astor, B.C.; Grams, M.; Halushka, M.; Lazo, M.; Hoogeveen, R.; Ballantyne, C.M.; Coresh, J.; Selvin, E. Association of plasma levels of soluble receptor for advanced glycation end products and risk of kidney disease: The Atherosclerosis Risk in Communities study. Nephrol. Dial. Transplant. 2014, 30, 77–83. [Google Scholar] [CrossRef]
- Basta, G.; Leonardis, D.; Mallamaci, F.; Cutrupi, S.; Pizzini, P.; Gaetano, L.; Tripepi, R.; Tripepi, G.; De Caterina, R.; Zoccali, C. Circulating soluble receptor of advanced glycation end product inversely correlates with atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010, 77, 225–231. [Google Scholar] [CrossRef]
- Dozio, E.; Vettoretti, S.; Caldiroli, L.; Nerini-Molteni, S.; Tacchini, L.; Ambrogi, F.; Messa, P.; Romanelli, M.M.C. Advanced Glycation End Products (AGE) and Soluble Forms of AGE Receptor: Emerging Role as Mortality Risk Factors in CKD. Biomedicines 2020, 8, 638. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kato, I.; Doi, T.; Yonekura, H.; Ohashi, S.; Takeuchi, M.; Watanabe, T.; Yamagishi, S.-I.; Sakurai, S.; Takasawa, S.; et al. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J. Clin. Investig. 2001, 108, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Morley, A.L.; Koitka, A.; Samuel, P.; Coughlan, M.T.; Penfold, S.A.; Thomas, M.C.; Bierhaus, A.; Nawroth, P.P.; Yamamoto, H.; et al. Receptor for AGEs (RAGE) blockade may exert its renoprotective effects in patients with diabetic nephropathy via induction of the angiotensin II type 2 (AT2) receptor. Diabetologia 2010, 53, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Teissier, T.; Quersin, V.; Gnemmi, V.; Daroux, M.; Howsam, M.; Delguste, F.; Lemoine, C.; Fradin, C.; Schmidt, A.-M.; Cauffiez, C.; et al. Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions. Aging Cell 2019, 18, e12850. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, L.J.; Teichberg, S.; Fuh, H.; Li, Y.M.; Steffes, M. Advanced glycation end products induce glomerular sclerosis and albuminuria in normal rats. Proc. Natl. Acad. Sci. USA 1994, 91, 11704–11708. [Google Scholar] [CrossRef] [PubMed]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Rong, L.L.; Moser, B.; Markowitz, G.S.; et al. RAGE Drives the Development of Glomerulosclerosis and Implicates Podocyte Activation in the Pathogenesis of Diabetic Nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.C.; Xu, C.; Duman, R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain, Behav. Immun. 2017, 72, 2–13. [Google Scholar] [CrossRef]
- Katon, W.; Von Korff, M.; Ciechanowski, P.; Russo, J.; Lin, E.; Simon, G.; Ludman, E.; Walker, E.; Bush, T.; Young, B. Behavioral and Clinical Factors Associated with Depression Among Individuals with Diabetes. Diabetes Care 2004, 27, 914–920. [Google Scholar] [CrossRef]
- van Dooren, F.E.P.; Pouwer, F.; Schalkwijk, C.G.; Sep, S.J.S.; Stehouwer, C.D.A.; Henry, R.M.A.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.H.; Koster, A.; et al. Advanced Glycation End Product (AGE) Accumulation in the Skin is Associated with Depression: The Maastricht Study. Depress. Anxiety 2016, 34, 59–67. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Wang, T.; Liang, J.; Lin, W.; Li, L.; Wen, J.; Lin, L.; Huang, H. Association Between Serum Endogenous Secretory Receptor for Advanced Glycation End Products and Risk of Type 2 Diabetes Mellitus with Combined Depression in the Chinese Population. Diabetes Technol. Ther. 2012, 14, 936–942. [Google Scholar] [CrossRef]
- Emanuele, E.; Martinelli, V.; Carlin, M.V.; Fugazza, E.; Barale, F.; Politi, P. Serum levels of soluble receptor for advanced glycation endproducts (sRAGE) in patients with different psychiatric disorders. Neurosci. Lett. 2011, 487, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Saavedra, G.; Zumpe, C.; Somoza, V.; Klenovicsová, K.; Birlouez-Aragon, I. Plasma Concentration and Urinary Excretion of Nε-(Carboxymethyl)lysine in Breast Milk- and Formula-fed Infants. Ann. N. Y. Acad. Sci. 2008, 1126, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Katsikis, I.; Piperi, C.; Alexandraki, K.; Panidis, D. Effect of long-term orlistat treatment on serum levels of advanced glycation end-products in women with polycystic ovary syndrome. Clin. Endocrinol. 2006, 66, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Miyazaki, A.; Fujimoto, S. Decrease in serum levels of advanced glycation end-products by short-term lifestyle modification in non-diabetic middle-aged females. Med. Sci. Monit. 2009, 15, PH-65-73. [Google Scholar]
- Gu, Q.; Wang, B.; Zhang, X.-F.; Ma, Y.-P.; Liu, J.-D.; Wang, X.-Z. Contribution of receptor for advanced glycation end products to vasculature-protecting effects of exercise training in aged rats. Eur. J. Pharmacol. 2014, 741, 186–194. [Google Scholar] [CrossRef]
- Bolton, W.K.; Cattran, D.C.; Williams, M.E.; Adler, S.G.; Appel, G.B.; Cartwright, K.; Foiles, P.G.; Freedman, B.I.; Raskin, P.; Ratner, R.E.; et al. Randomized Trial of an Inhibitor of Formation of Advanced Glycation End Products in Diabetic Nephropathy. Am. J. Nephrol. 2004, 24, 32–40. [Google Scholar] [CrossRef]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; McGill, J.B. Effects of Pyridoxamine in Combined Phase 2 Studies of Patients with Type 1 and Type 2 Diabetes and Overt Nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin Inhibits Advanced Glycation End Product Formation by Trapping Methylglyoxal and Glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Tan, K.C.B.; Chow, W.S.; Tso, A.W.K.; Xu, A.; Tse, H.F.; Hoo, R.L.C.; Betteridge, D.J.; Lam, K.S.L. Thiazolidinedione increases serum soluble receptor for advanced glycation end-products in type 2 diabetes. Diabetologia 2007, 50, 1819–1825. [Google Scholar] [CrossRef]
- Bewley, M.A.; Budd, R.C.; Ryan, E.; Cole, J.; Collini, P.; Marshall, J.; Kolsum, U.; Beech, G.; Emes, R.D.; Tcherniaeva, I.; et al. Opsonic Phagocytosis in Chronic Obstructive Pulmonary Disease Is Enhanced by Nrf2 Agonists. Am. J. Respir. Crit. Care Med. 2018, 198, 739–750. [Google Scholar] [CrossRef]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Singh, I.; Sagare, A.P.; Bell, R.D.; Ross, N.T.; LaRue, B.; Love, R.; Perry, S.; Paquette, N.; Deane, R.J.; et al. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Investig. 2012, 122, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Hong, S.-H.; Rahman, I.; Yang, S.-R. Inhibition of RAGE Attenuates Cigarette Smoke-Induced Lung Epithelial Cell Damage via RAGE-Mediated Nrf2/DAMP Signaling. Front. Pharmacol. 2018, 9, 684. [Google Scholar] [CrossRef]
- Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2018, 5, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Burstein, A.H.; Grimes, I.; Galasko, D.R.; Aisen, P.S.; Sabbagh, M.; Mjalli, A.M.M. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 2014, 14, 12. [Google Scholar] [CrossRef]
- Burstein, A.H.; Brantley, S.J.; Dunn, I.; Altstiel, L.D.; Schmith, V. Assessment of Azeliragon QTc Liability Through Integrated, Model-Based Concentration QTc Analysis. Clin. Pharmacol. Drug Dev. 2019, 8, 426–435. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Pan, J.; Rai, V.; Zhang, J.; Reverdatto, S.; Quadri, N.; DeVita, R.J.; Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci. Rep. 2016, 6, 22450. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Rabbani, P.; Egaña-Gorroño, L.; Quadri, N.; Frye, L.; Zhou, B.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.; et al. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci. Transl. Med. 2021, 13, eabf7084. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, H.; Xie, B.; Tian, T.; Li, S.; Chen, Z.; Liu, J.; Zhao, X.; Zhang, L.; Deng, Y.; et al. Targeting adaptor protein SLP76 of RAGE as a therapeutic approach for lethal sepsis. Nat. Commun. 2021, 12, 308. [Google Scholar] [CrossRef]
- von Bauer, R.; Oikonomou, D.; Sulaj, A.; Mohammed, S.; Hotz-Wagenblatt, A.; Gröne, H.-J.; Arnold, B.; Falk, C.; Luethje, D.; Erhardt, A.; et al. CD166/ALCAM Mediates Proinflammatory Effects of S100B in Delayed Type Hypersensitivity. J. Immunol. 2013, 191, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.-M.; Yamamoto, Y.; Doi, T.; Kato, I.; Harashima, A.; Yonekura, H.; Watanabe, T.; Shinohara, H.; Takeuchi, M.; Tsuneyama, K.; et al. RAGE Control of Diabetic Nephropathy in a Mouse Model. Diabetes 2006, 55, 2510–2522. [Google Scholar] [CrossRef] [PubMed]
- Flyvbjerg, A.; Denner, L.; Schrijvers, B.F.; Tilton, R.G.; Mogensen, T.H.; Paludan, S.R.; Rasch, R. Long-Term Renal Effects of a Neutralizing RAGE Antibody in Obese Type 2 Diabetic Mice. Diabetes 2004, 53, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Koyama, H.; Morioka, T.; Tanaka, S.; Kizu, A.; Motoyama, K.; Mori, K.; Fukumoto, S.; Shioi, A.; Shimogaito, N.; et al. Receptor for Advanced Glycation End Products Is Involved in Impaired Angiogenic Response in Diabetes. Diabetes 2006, 55, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.; Thallas-Bonke, V.; Pete, J.; Long, D.M.; Gasser, A.; Tong, D.C.K.; Arnstein, M.; Thorpe, S.R.; Cooper, M.E.; Forbes, J.M. Combination Therapy with the Advanced Glycation End Product Cross-Link Breaker, Alagebrium, and Angiotensin Converting Enzyme Inhibitors in Diabetes: Synergy or Redundancy? Endocrinology 2007, 148, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Loera, S.; Tessler, C.; Chu, P.; Weiss, L.; Hardy, J.; Rahbar, S.; Figarola, J. LR-90 a new advanced glycation endproduct inhibitor prevents progression of diabetic nephropathy in streptozotocin-diabetic rats. Diabetologia 2003, 46, 1140–1152. [Google Scholar] [CrossRef]
- Candido, R.; Forbes, J.; Thomas, M.; Thallas, V.; Dean, R.G.; Burns, W.C.; Tikellis, C.; Ritchie, R.; Twigg, S.M.; Cooper, M.E.; et al. A Breaker of Advanced Glycation End Products Attenuates Diabetes-Induced Myocardial Structural Changes. Circ. Res. 2003, 92, 785–792. [Google Scholar] [CrossRef]
- Kass, D.A.; Shapiro, E.P.; Kawaguchi, M.; Capriotti, A.R.; Scuteri, A.; Degroof, R.C.; Lakatta, E. Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker. Circulation 2001, 104, 1464–1470. [Google Scholar] [CrossRef]
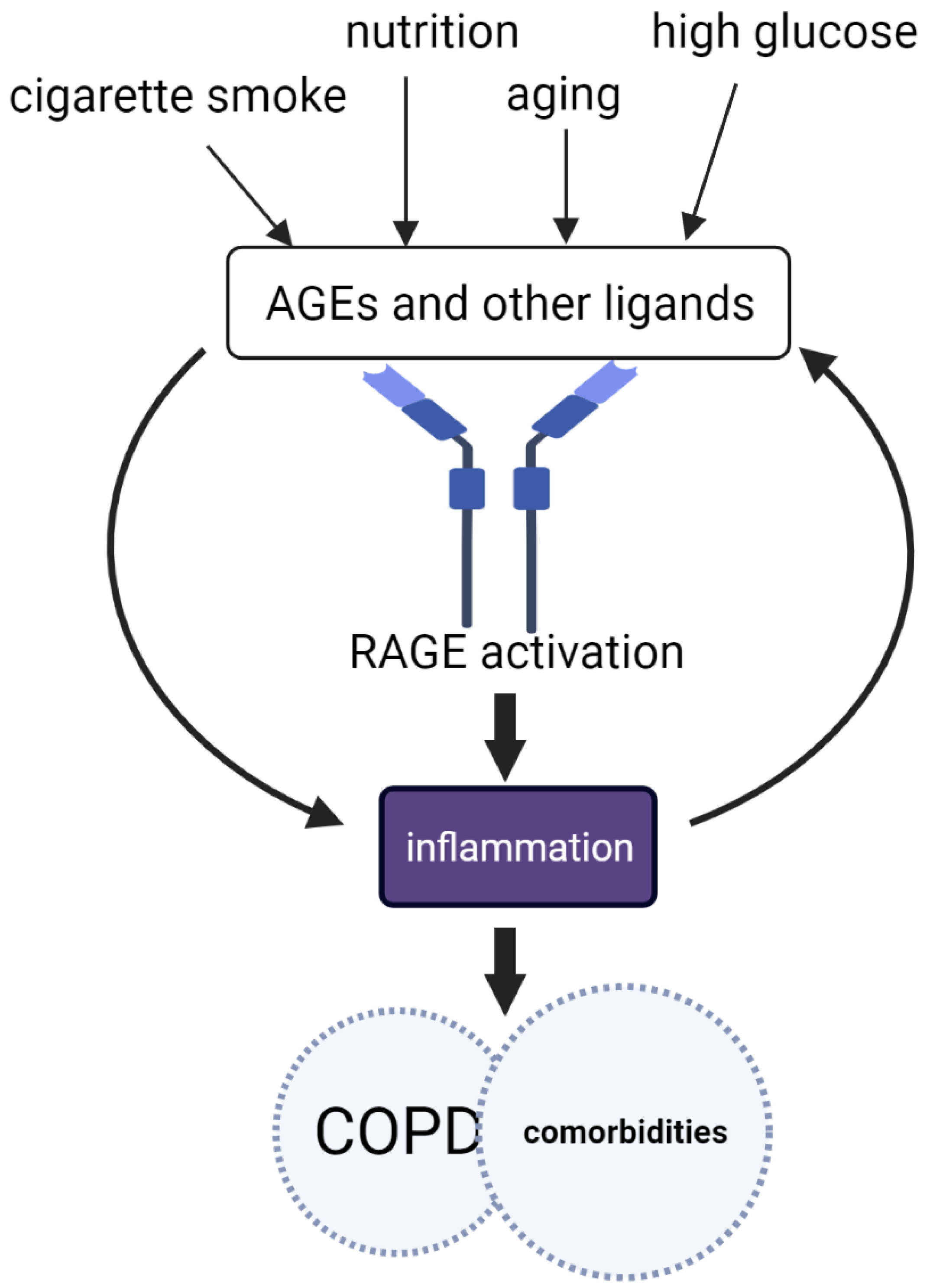



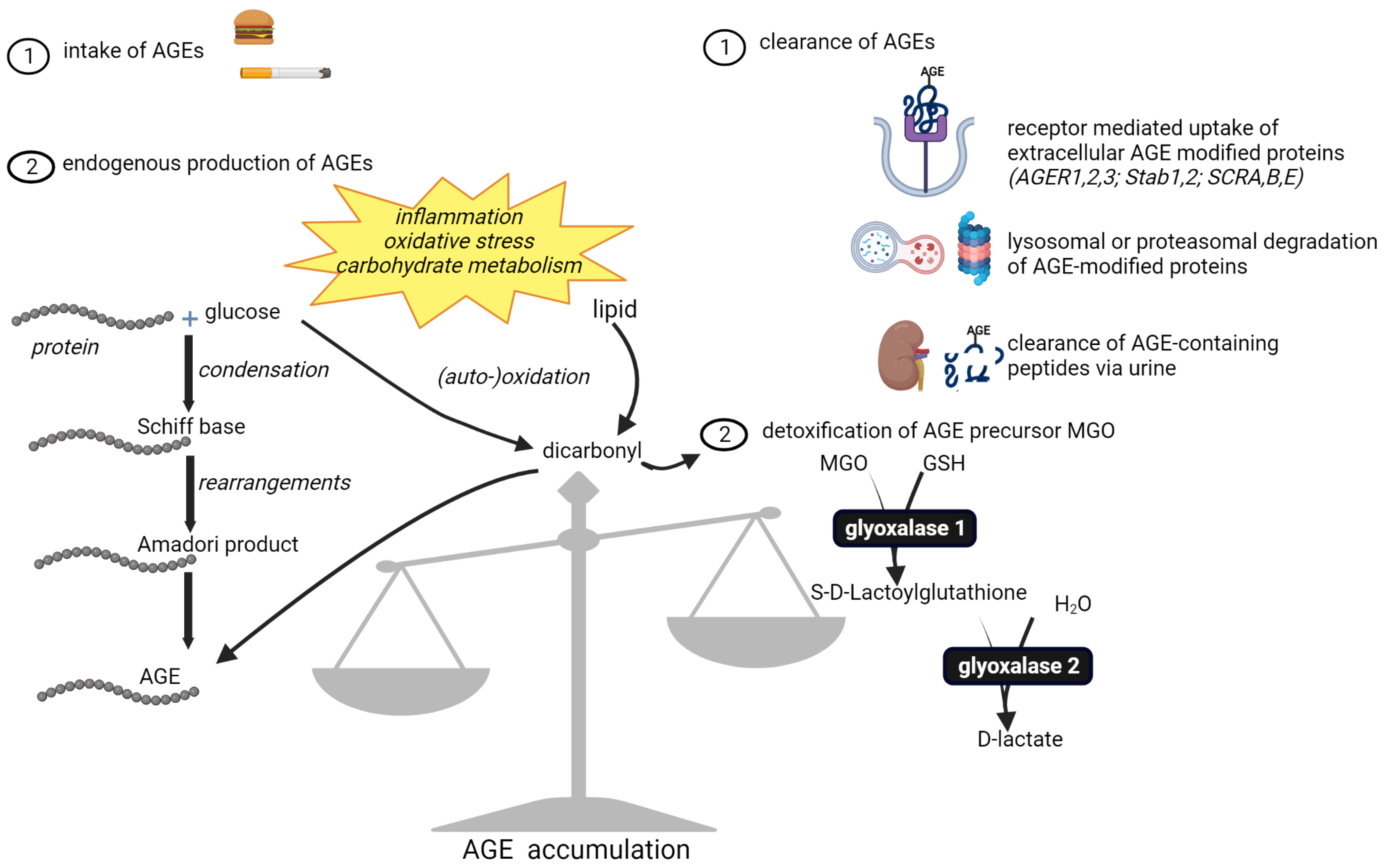




| Inhibition of AGEs | Cross-Link Breakers | Anti-RAGE |
|---|---|---|
| Limiting intake | Alagebrium (ALT-711) | Ligand binding inhibitors |
| Exercise | FPS-ZM1 | |
| Precursor scavengers | Azeliragon (TTP488) | |
| Aminoguanidine | Interaction with adapter | |
| Pyridoxamine | protein inhibitors | |
| Quercetin | DIAPH: RAGE299 | |
| Metformin | SLP76: TAT-SAM | |
| Rosiglitazone | sRAGE administration | |
| Inducers of glyoxalase | esRAGE upregulation | |
| Curcumin | ||
| Resveratrol | ||
| Sulforaphane | ||
| Hesperetin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynaert, N.L.; Vanfleteren, L.E.G.W.; Perkins, T.N. The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD. J. Clin. Med. 2023, 12, 3366. https://doi.org/10.3390/jcm12103366
Reynaert NL, Vanfleteren LEGW, Perkins TN. The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD. Journal of Clinical Medicine. 2023; 12(10):3366. https://doi.org/10.3390/jcm12103366
Chicago/Turabian StyleReynaert, Niki L., Lowie E. G. W. Vanfleteren, and Timothy N. Perkins. 2023. "The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD" Journal of Clinical Medicine 12, no. 10: 3366. https://doi.org/10.3390/jcm12103366
APA StyleReynaert, N. L., Vanfleteren, L. E. G. W., & Perkins, T. N. (2023). The AGE-RAGE Axis and the Pathophysiology of Multimorbidity in COPD. Journal of Clinical Medicine, 12(10), 3366. https://doi.org/10.3390/jcm12103366






