Local Gentamicin Fixation with Sprayed Fibrin—An In Vivo Animal Study Reveals New Options to Treat Soft Tissue Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Care
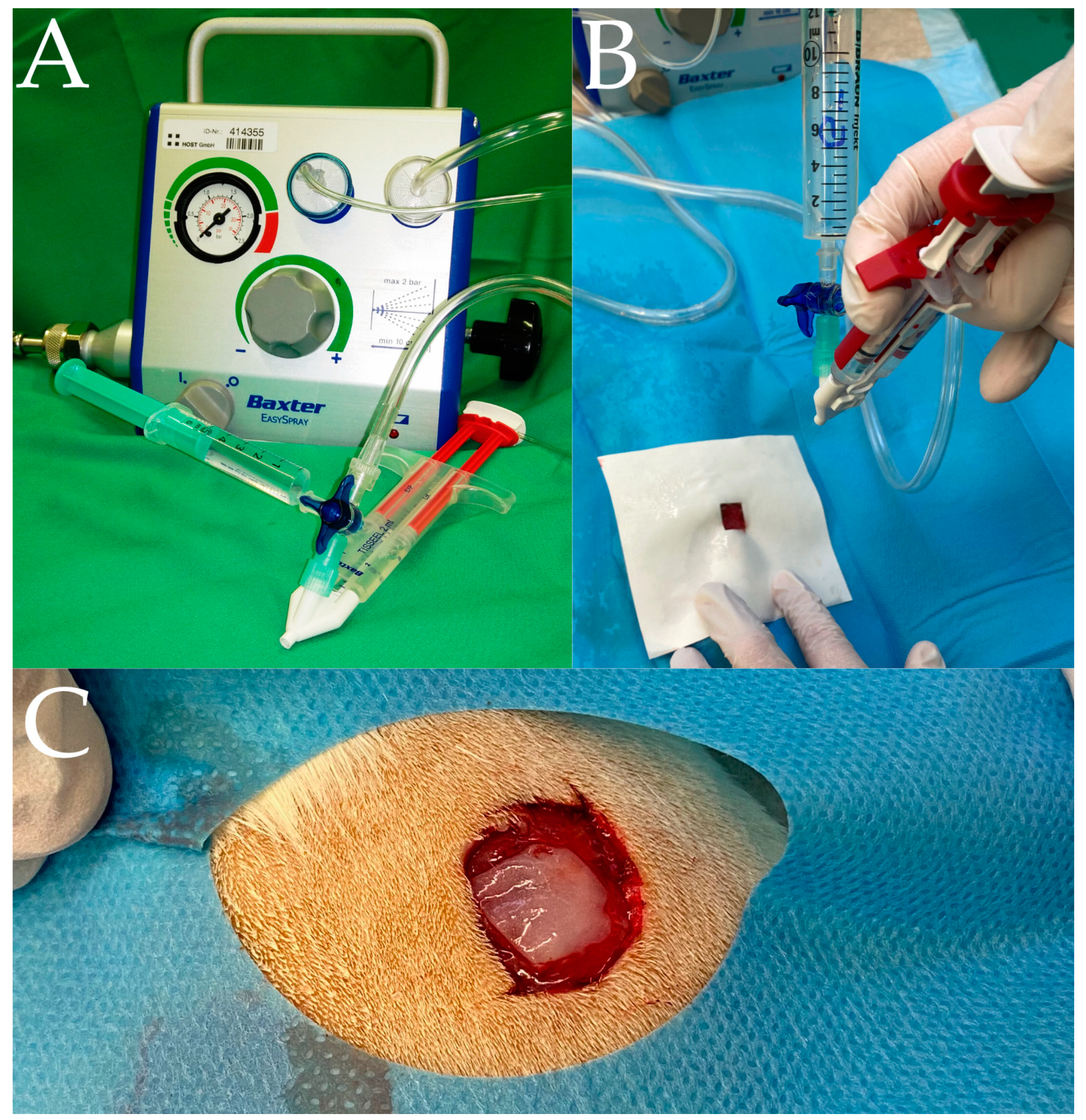
2.2. Preparation of the Gentamicin/Fibrin Mixture
2.3. Surgery and Wound Spraying
2.4. LC-MS/MS-Analysis
2.5. Statistical Analysis
3. Results
3.1. One Hour in Tissue Time
3.2. Two Hours in Tissue Time
3.3. Four Hours in Tissue Time
3.4. Group Comparison over Time
3.5. Higher Blood Levels of Gentamicin in G and GF− Groups after 4 h
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walter, G.; Kemmerer, M.; Kappler, C.; Hoffmann, R. Treatment algorithms for chronic osteomyelitis. Dtsch. Arztebl. Int. 2012, 109, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Patzakis, M.J.; Zalavras, C.G. Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: Current management concepts. J. Am. Acad. Orthop. Surg. 2005, 13, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Logters, T.; Hakimi, M.; Windolf, J.; Schadel-Hopfner, M. Surgical reality and prognostic criteria of severe infections of the hand. Handchir. Mikrochir. Plast. Chir. 2009, 41, 271–276. [Google Scholar] [CrossRef] [PubMed]
- La Padula, S.; Hersant, B.; Helynck, P.; Mezi SidAhmed, M.; Meningaud, J.P. Proposal of a Noninvasive Method to Reduce Injection-Related Bruising in Aesthetic Medicine: Transillumination. Aesthetic Plast. Surg. 2020, 44, 530–534. [Google Scholar] [CrossRef]
- Hersant, B.; Werkoff, G.; Sawan, D.; Sidahmed-Mezi, M.; Bosc, R.; La Padula, S.; Kalsoum, S.; Ouidir, N.; Meningaud, J.P.; Belkacemi, Y. Carbon dioxide laser treatment for vulvovaginal atrophy in women treated for breast cancer: Preliminary results of the feasibility EPIONE trial. Ann. Chir. Plast. Esthet. 2020, 65, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Hersant, B.; La Padula, S.; Meningaud, J.P. Facelifts: Improving the long-term outcomes of lower face and neck rejuvenation surgery: The lower face and neck rejuvenation combined method. J. Craniomaxillofac. Surg. 2018, 46, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Chiummariello, S.; Guarro, G.; Pica, A.; Alfano, C. Evaluation of negative pressure vacuum-assisted system in acute and chronic wounds closure: Our experience. Il Giornale di Chirurgia 2012, 33, 358–362. [Google Scholar]
- Nather, A.; Chionh, S.B.; Han, A.Y.; Chan, P.P.; Nambiar, A. Effectiveness of vacuum-assisted closure (VAC) therapy in the healing of chronic diabetic foot ulcers. Ann. Acad. Med. 2010, 39, 353–358. [Google Scholar] [CrossRef]
- Varga, M.; Sixta, B.; Bem, R.; Matia, I.; Jirkovska, A.; Adamec, M. Application of gentamicin-collagen sponge shortened wound healing time after minor amputations in diabetic patients—A prospective, randomised trial. Arch. Med. Sci. 2014, 10, 283–287. [Google Scholar] [CrossRef]
- von Stechow, D.; Rauschmann, M.A. Effectiveness of combination use of antibiotic-loaded PerOssal with spinal surgery in patients with spondylodiscitis. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 2009, 43, 298–305. [Google Scholar] [CrossRef]
- Buchholz, H.W.; Engelbrecht, H. Depot effects of various antibiotics mixed with Palacos resins. Chirurg 1970, 41, 511–515. [Google Scholar] [PubMed]
- Klemm, K. Gentamicin-PMMA-beads in treating bone and soft tissue infections (author’s transl). Zentralbl. Chir. 1979, 104, 934–942. [Google Scholar]
- Penn-Barwell, J.G.; Murray, C.K.; Wenke, J.C. Local antibiotic delivery by a bioabsorbable gel is superior to PMMA bead depot in reducing infection in an open fracture model. J. Orthop. Trauma 2014, 28, 370–375. [Google Scholar] [CrossRef]
- Linz, M.S.; Mattappallil, A.; Finkel, D.; Parker, D. Clinical Impact of Staphylococcus aureus Skin and Soft Tissue Infections. Antibiotics 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Khoury, H.; Girgenti, D.; Welner, S.; Yu, H. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg. Infect. 2017, 18, 461–473. [Google Scholar] [CrossRef]
- An, R.; Ou, Y.; Pang, L.; Yuan, Y.; Li, Q.; Xu, H.; Sheng, B. Epidemiology and Risk Factors of Community-Associated Bloodstream Infections in Zhejiang Province, China, 2017–2020. Infect. Drug Resist. 2023, 16, 1579–1590. [Google Scholar] [CrossRef]
- Garrine, M.; Quinto, L.; Costa, S.S.; Messa, A., Jr.; Massinga, A.J.; Vubil, D.; Nhampossa, T.; Massora, S.; Acacio, S.; Cossa, A.; et al. Epidemiology and clinical presentation of community-acquired Staphylococcus aureus bacteraemia in children under 5 years of age admitted to the Manhica District Hospital, Mozambique, 2001–2019. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 653–659. [Google Scholar] [CrossRef]
- Vollinger, M.; Partecke, B.D. Infections of flexor tendon sheath—Treatment and results. Handchir. Mikrochir. Plast. Chir. 2003, 35, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Harle, A.; van Ende, R. Management of wound sepsis after spinal fusion surgery. Acta Orthop. Belg. 1991, 57 (Suppl. S1), 242–246. [Google Scholar]
- Sendi, P.; Zimmerli, W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Cross, J.D.; Brown, K.V.; Murray, C.K.; Wenke, J.C. Effect of various concentrations of antibiotics on osteogenic cell viability and activity. J. Orthop. Res. 2011, 29, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Dust, F.; Wagner, P.V.; Gurke, R.; Frank, J.; Henrich, D.; Marzi, I.; Verboket, R.D. Local Fixation of Colistin with Fibrin Spray: An in vivo Animal Study for the Therapy of Skin and Soft Tissue Infections. Front. Surg. 2022, 9, 749600. [Google Scholar] [CrossRef]
- Janko, M.; Nau, C.; Marzi, I.; Frank, J. Local fixation of antibiotics by fibrin spray: In bone defects with soft tissue involvement. Chirurg 2017, 88, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.; Marzi, I.; Fleck, S.C.; Frank, J.; Janko, M. Local fixation of antibiotics with fibrin spray on soft tissues: Experimental study on the relevance of the application techniques. Eur. J. Trauma Emerg. Surg. 2020, 46, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.A.; Hönig, J.F.; Giesen, K.; Halling, F. Application of the Fibrin Glue Spray System in Surgery of Maxillary Sinuses. In Fibrin Sealing in Surgical and Nonsurgical Fields; Springer: Berlin/Heidelberg, Germany, 1994; pp. 152–157. [Google Scholar]
- Köckerling, F.; Scheele, J.; Gall, F.P. Fibrin sealing in liver surgery. Eur. Surg. 1991, 23, 100–103. [Google Scholar] [CrossRef]
- Negron, O.; Hur, W.S.; Prasad, J.; Paul, D.S.; Rowe, S.E.; Degen, J.L.; Abrahams, S.R.; Antoniak, S.; Conlon, B.P.; Bergmeier, W.; et al. Fibrin(ogen) engagement of S. aureus promotes the host antimicrobial response and suppression of microbe dissemination following peritoneal infection. PLoS Pathog. 2022, 18, e1010227. [Google Scholar] [CrossRef]
- Klasinc, R.; Augustin, L.A.; Below, H.; Baguhl, R.; Assadian, O.; Presterl, E.; Kramer, A. Evaluation of three experimental in vitro models for the assessment of the mechanical cleansing efficacy of wound irrigation solutions. Int. Wound J. 2018, 15, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, Y.-C.; Hsu, Y.-M.; Chang, C.-H. Enhancing Drug Release From Antibiotic-loaded Bone Cement Using Porogens. J. Am. Acad. Orthop. Surg. 2016, 24, 188–195. [Google Scholar] [CrossRef]
- Pibalpakdee, P.; Wongratanacheewin, S.; Taweechaisupapong, S.; Niumsup, P.R. Diffusion and activity of antibiotics against Burkholderia pseudomallei biofilms. Int. J. Antimicrob. Agents 2012, 39, 356–359. [Google Scholar] [CrossRef]
- Barza, M.; Weinstein, L. Penetration of antibiotics into fibrin loci in vivo. I. Comparison of penetration of ampicillin into fibrin clots, abscesses, and “interstitial fluid”. J. Infect. Dis. 1974, 129, 59–65. [Google Scholar] [CrossRef]
- Yim, H.; Yang, H.T.; Cho, Y.S.; Seo, C.H.; Lee, B.C.; Ko, J.H.; Kwak, I.S.; Kim, D.; Hur, J.; Kim, J.H.; et al. Clinical study of cultured epithelial autografts in liquid suspension in severe burn patients. Burn. J. Int. Soc. Burn. Inj. 2011, 37, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Harkin, D.G.; Dawson, R.A.; Upton, Z. Optimized delivery of skin keratinocytes by aerosolization and suspension in fibrin tissue adhesive. Wound Repair Regen. 2006, 14, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, A.; Verboket, R.D.; Kainz, D.M.; von Stetten, F.; Fruh, S.M. Rapid Detection of Pathogens in Wound Exudate via Nucleic Acid Lateral Flow Immunoassay. Biosensors 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Verboket, R.D.; Muhlenfeld, N.; Woschek, M.; Marzi, I.; Pieper, M.; Zollner, J.P.; Strzelczyk, A.; Willems, L.M. Inpatient treatment costs, cost-driving factors and potential reimbursement problems due to fall-related fractures in patients with Parkinson’s disease. Chirurg 2020, 91, 421–427. [Google Scholar] [CrossRef]
- Verboket, R.D.; Muhlenfeld, N.; Sterz, J.; Stormann, P.; Marzi, I.; Balcik, Y.; Rosenow, F.; Strzelczyk, A.; Willems, L.M. Inpatient treatment costs, cost-driving factors and potential reimbursement problems due to epileptic seizure-related injuries and fractures. Chirurg 2021, 92, 361–368. [Google Scholar] [CrossRef]


| Group | 1 h | 2 h | 4 h |
|---|---|---|---|
| Gentamicin without fixation (G) | n = 12 | n = 12 | n = 12 |
| Consecutive spraying of gentamicin and fibrin glue (GF−) | n = 12 | n = 12 | n = 12 |
| Simultaneous spraying of gentamicin and fibrin glue (GF+) | n = 12 | n = 12 | n = 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kejwal, M.B.; Verboket, R.D.; Sommer, K.; Dust, F.; Thomas, D.; Störmann, P.; Frank, J.; Henrich, D.; Marzi, I.; Janko, M.C. Local Gentamicin Fixation with Sprayed Fibrin—An In Vivo Animal Study Reveals New Options to Treat Soft Tissue Infections. J. Clin. Med. 2023, 12, 3390. https://doi.org/10.3390/jcm12103390
Kejwal MB, Verboket RD, Sommer K, Dust F, Thomas D, Störmann P, Frank J, Henrich D, Marzi I, Janko MC. Local Gentamicin Fixation with Sprayed Fibrin—An In Vivo Animal Study Reveals New Options to Treat Soft Tissue Infections. Journal of Clinical Medicine. 2023; 12(10):3390. https://doi.org/10.3390/jcm12103390
Chicago/Turabian StyleKejwal, Meike B., René D. Verboket, Katharina Sommer, Fabian Dust, Dominique Thomas, Philipp Störmann, Johannes Frank, Dirk Henrich, Ingo Marzi, and Maren C. Janko. 2023. "Local Gentamicin Fixation with Sprayed Fibrin—An In Vivo Animal Study Reveals New Options to Treat Soft Tissue Infections" Journal of Clinical Medicine 12, no. 10: 3390. https://doi.org/10.3390/jcm12103390







