Brain Hemispheric Asymmetry in Schizophrenia and Bipolar Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. Processing and Asymmetry Index
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characterization
3.2. Voxel-Based Morphometry—Asymmetry Index SCZ vs. BPD
3.3. Voxel-Based Morphometry—Asymmetry Index SCZ vs. HC and BPD vs. HC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Yeganeh-Doost, P.; Gruber, O.; Falkai, P.; Schmitt, A. The role of the cerebellum in schizophrenia: From cognition to molecular pathways. Clinics 2011, 66 (Suppl. S1), 71–77. [Google Scholar] [CrossRef]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Prim. 2018, 4, 18008. [Google Scholar] [CrossRef]
- Madeira, N.; Duarte, J.V.; Martins, R.; Costa, G.N.; Macedo, A.; Castelo-Branco, M. Morphometry and gyrification in bipolar disorder and schizophrenia: A comparative MRI study. NeuroImage Clin. 2020, 26, 102220. [Google Scholar] [CrossRef]
- Lee, D.-K.; Lee, H.; Park, K.; Joh, E.; Kim, C.-E.; Ryu, S. Common gray and white matter abnormalities in schizophrenia and bipolar disorder. PLoS ONE 2020, 15, e0232826. [Google Scholar] [CrossRef]
- Eken, A.; Akaslan, D.S.; Baskak, B.; Münir, K. Diagnostic classification of schizophrenia and bipolar disorder by using dynamic functional connectivity: An fNIRS study. J. Neurosci. Methods 2022, 376, 109596. [Google Scholar] [CrossRef]
- Nenadic, I.; Maitra, R.; Langbein, K.; Dietzek, M.; Lorenz, C.; Smesny, S.; Reichenbach, J.R.; Sauer, H.; Gaser, C. Brain structure in schizophrenia vs. psychotic bipolar I disorder: A VBM study. Schizophr. Res. 2015, 165, 212–219. [Google Scholar] [CrossRef]
- Sha, Z.; Schijven, D.; Francks, C. Patterns of brain asymmetry associated with polygenic risks for autism and schizophrenia implicate language and executive functions but not brain masculinization. Mol. Psychiatry 2021, 26, 7652–7660. [Google Scholar] [CrossRef]
- Núñez, C.; Paipa, N.; Senior, C.; Coromina, M.; Siddi, S.; Ochoa, S.; Brébion, G.; Stephan-Otto, C. Global brain asymmetry is increased in schizophrenia and related to avolition. Acta Psychiatr. Scand. 2017, 135, 448–459. [Google Scholar] [CrossRef]
- Okada, N.; Fukunaga, M.; Yamashita, F.; Koshiyama, D.; Yamamori, H.; Ohi, K.; Yasuda, Y.; Fujimoto, M.; Watanabe, Y.; Ya-hata, N.; et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 2016, 21, 1460–1466. [Google Scholar] [CrossRef]
- Dobri, M.L.; Diaz, A.P.; Selvaraj, S.; Quevedo, J.; Walss-Bass, C.; Soares, J.C.; Sanches, M. The Limits between Schizophrenia and Bipolar Disorder: What Do Magnetic Resonance Findings Tell Us? Behav. Sci. 2022, 12, 78. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Collinson, S.L.; Bezerianos, A.; Sim, K. Reduced hemispheric asymmetry of brain anatomical networks is linked to schizophrenia: A connectome study. Cereb. Cortex 2017, 27, 602–615. [Google Scholar] [CrossRef]
- Ellison-Wright, I.; Bullmore, E. Anatomy of bipolar disorder and schizophrenia: A meta-analysis. Schizophr. Res. 2010, 117, 1–12. [Google Scholar] [CrossRef]
- Madre, M.; Canales-Rodríguez, E.J.; Fuentes-Claramonte, P.; Alonso-Lana, S.; Salgado-Pineda, P.; Guerrero-Pedraza, A.; Moro, N.; Bosque, C.; Gomar, J.J.; Ortíz-Gil, J.; et al. Structural abnormality in schizophrenia versus bipolar disorder: A whole brain cortical thickness, surface area, volume and gyrification analyses. NeuroImage Clin. 2020, 25, 102131. [Google Scholar] [CrossRef]
- Hibar, D.P.; Westlye, L.T.; Doan, N.T.; Jahanshad, N.; Cheung, J.W.; Ching, C.R.K.; Versace, A.; Bilderbeck, A.C.; Uhlmann, A.; Mwangi, B.; et al. Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 2018, 23, 932–942. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Su, W.; Hu, H.; Xia, M.; Zhang, T.; Xu, L.; Zhang, X.; Taylor, H.; Osipowicz, K.; et al. Symptom-circuit mappings of the schizophrenia connectome. Psychiatry Res. 2023, 323, 115122. [Google Scholar] [CrossRef]
- Andreasen, N.C.; Pierson, R. The Role of the Cerebellum in Schizophrenia. Biol. Psychiatry 2008, 64, 81–88. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, J.; Gong, J.; Su, T.; Fu, S.; Huang, L.; Wang, Y. Common and specific patterns of functional and structural brain alterations in schizophrenia and bipolar disorder: A multimodal voxel-based meta-analysis. J. Psychiatry Neurosci. 2022, 47, E32–E47. [Google Scholar] [CrossRef]
- Kim, G.-W.; Kim, Y.-H.; Jeong, G.-W. Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: A DARTEL-based VBM study. PLoS ONE 2017, 12, e0177251. [Google Scholar] [CrossRef]
- Espírito-Santo, H.; Pires, C.F.; Garcia, I.Q.; Daniel, F.; Da Silva, A.G.; Fazio, R.L. Preliminary validation of the Portuguese Edinburgh Handedness Inventory in an adult sample. Appl. Neuropsychol. Adult 2017, 24, 275–287. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Morris, D.W.; Sweeney, J.A.; Pearlson, G.; Thaker, G.; Seidman, L.J.; Eack, S.M.; Tamminga, C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophr. Res. 2011, 133, 250–254. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.P.; Apperson, L.J.; Appelbaum, P.S.; Ortlip, P.; Brecosky, J.; Hammill, K.; Geller, J.L.; Roth, L. Insight in schizophrenia. its relationship to acute psychopathology. J. Nerv. Ment. Dis. 1989, 177, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Brissos, S.; Palhavã, F.; Marques, J.G.; Mexia, S.; Carmo, A.L.; Carvalho, M.; Dias, C.; Franco, J.D.; Mendes, R.; Zuzarte, P.; et al. The Portuguese version of the Personal and Social Performance Scale (PSP): Reliability, validity, and relationship with cognitive measures in hospitalized and community schizophrenia patients. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 1077–1086. [Google Scholar] [CrossRef]
- Lukoff, D.; Liberman, R.P.; Nuechterlein, K.H. Symptom Monitoring in the Rehabilitation of Schizophrenic Patients. Schizophr. Bull. 1986, 12, 578–603. [Google Scholar] [CrossRef]
- Atkins, M.; Burgess, A.P.; Bottomley, C.; Riccio, M. Chlorpromazine equivalents: A consensus of opinion for both clinical and research applications. Psychiatr. Bull. 1997, 21, 224–226. [Google Scholar] [CrossRef]
- Kurth, F.; Gaser, C.; Luders, E. A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nat. Protoc. 2015, 10, 293–304. [Google Scholar] [CrossRef]
- Luders, E.; Gaser, C.; Jancke, L.; Schlaug, G. A voxel-based approach to gray matter asymmetries. NeuroImage 2004, 22, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Andersson, J.; Ardekani, B.A.; Ashburner, J.; Avants, B.; Chiang, M.-C.; Christensen, G.E.; Collins, D.L.; Gee, J.; Hellier, P.; et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 2009, 46, 786–802. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Why Voxel-Based Morphometry Should Be Used. Neuroimage 2001, 14, 1238–1243. [Google Scholar] [CrossRef]
- Chrobak, A.A.; Siuda-Krzywicka, K.; Siwek, G.P.; Tereszko, A.; Janeczko, W.; Starowicz-Filip, A.; Siwek, M.; Dudek, D. Disrupted implicit motor sequence learning in schizophrenia and bipolar disorder revealed with ambidextrous Serial Reaction Time Task. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 169–175. [Google Scholar] [CrossRef]
- Picard, H.; Amado, I.; Mouchet-Mages, S.; Olie, J.-P.; Krebs, M.-O. The role of the cerebellum in schizophrenia: An update of clinical, cognitive, and functional evidences. Schizophr. Bull. 2008, 34, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.V.; Abreu, R.; Castelo-Branco, M. A two-stage framework for neural processing of biological motion. NeuroImage 2022, 259, 119403. [Google Scholar] [CrossRef]
- Duarte, J.V.; Costa, G.N.; Martins, R.; Castelo-Branco, M. Pivotal role of hMT+ in long-range disambiguation of interhemispheric bistable surface motion. Hum. Brain Mapp. 2017, 38, 4882–4897. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.V.; Faustino, R.; Lobo, M.; Cunha, G.; Nunes, C.; Ferreira, C.; Januário, C.; Castelo-Branco, M. Parametric fMRI of paced motor responses uncovers novel whole-brain imaging biomarkers in spinocerebellar ataxia type 3. Hum. Brain Mapp. 2016, 37, 3656–3668. [Google Scholar] [CrossRef]
- Chrobak, A.A.; Siuda-Krzywicka, K.; Sołtys, Z.; Siwek, G.P.; Bohaterewicz, B.; Sobczak, A.M.; Ceglarek, A.; Tereszko, A.; Starowicz-Filip, A.; Fąfrowicz, M.; et al. Relationship between neurological and cerebellar soft signs, and implicit motor learning in schizophrenia and bipolar disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 111, 110137. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, G.; Jayakumar, P.N.; Gangadhar, B.N.; Keshavan, M.S. Neuroanatomical correlates of neurological soft signs in antipsychotic-naive schizophrenia. Psychiatry Res. Neuroimaging 2008, 164, 215–222. [Google Scholar] [CrossRef]
- Lei, W.; Kirkpatrick, B.; Wang, Q.; Deng, W.; Li, M.; Guo, W.; Liang, S.; Li, Y.; Zhang, C.; Li, X.; et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res. Neuroimaging 2019, 288, 12–20. [Google Scholar] [CrossRef]
- Stein, J. Sensorimotor control. In The Curated Reference Collection in Neuroscience and Biobehavioral Psychology; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hirjak, D.; Meyer-Lindenberg, A.; Fritze, S.; Sambataro, F.; Kubera, K.M.; Wolf, R.C. Motor dysfunction as research domain across bipolar, obsessive-compulsive and neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2018, 95, 315–335. [Google Scholar] [CrossRef]
- Intaitė, M.; Duarte, J.V.; Castelo-Branco, M. Working memory load influences perceptual ambiguity by competing for fronto-parietal attentional resources. Brain Res. 2016, 1650, 142–151. [Google Scholar] [CrossRef]
- Molina, V.; Galindo, G.; Cortés, B.; Hernández, J.A. Voxel-based morphometry comparison between chronic schizophrenia and bipolar patients and healthy controls. Schizophr. Res. 2010, 117, 341. [Google Scholar] [CrossRef]
- Caligiuri, M.P.; Brown, G.G.; Meloy, M.; Eyler, L.T.; Kindermann, S.S.; Eberson, S.; Frank, L.R.; Lohr, J.B. A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord. 2004, 6, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Exner, C.; Weniger, G.; Schmidt-Samoa, C.; Irle, E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophr. Res. 2006, 84, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Cotovio, G.; da Silva, D.R.; Lage, E.R.; Seybert, C.; Oliveira-Maia, A.J. Hemispheric asymmetry of motor cortex excitability in mood disorders–Evidence from a systematic review and meta-analysis. Clin. Neurophysiol. 2022, 137, 25–37. [Google Scholar] [CrossRef]
- Yang, H.; Qin, Q.; Jiang, J.; Xiang, H.; Yang, Z.; Zhou, Y.; Wu, Y.; Zhang, M. Explore functional brain changes in bipolar disorder: A whole brain ale meta-analysis. Rev. Psiquiatr. Clín. 2021, 48, 208–215. [Google Scholar]
- Jung, S.; Lee, A.; Bang, M.; Lee, S.-H. Gray matter abnormalities in language processing areas and their associations with verbal ability and positive symptoms in first-episode patients with schizophrenia spectrum psychosis. NeuroImage Clin. 2019, 24, 102022. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, E.; Jiang, W.; Calhoun, V.D.; van Erp, T.G.; Agartz, I.; Ford, J.M.; Mueller, B.A.; Liu, J.; Turner, J.A. Positive and general psychopathology associated with specific gray matter reductions in inferior temporal regions in patients with schizophrenia. Schizophr. Res. 2019, 208, 242–249. [Google Scholar] [CrossRef]
- Takahashi, T.; Zhou, S.-Y.; Nakamura, K.; Tanino, R.; Furuichi, A.; Kido, M.; Kawasaki, Y.; Noguchi, K.; Seto, H.; Kurachi, M.; et al. A follow-up MRI study of the fusiform gyrus and middle and inferior temporal gyri in schizophrenia spectrum. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1957–1964. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, M.; Zhou, S.-Y.; Tanino, R.; Hagino, H.; Niu, L.; Kawasaki, Y.; Seto, H.; Kurachi, M. Temporal lobe gray matter in schizophrenia spectrum: A volumetric MRI study of the fusiform gyrus, parahippocampal gyrus, and middle and inferior temporal gyri. Schizophr. Res. 2006, 87, 116–126. [Google Scholar] [CrossRef]
- Goldman, D.A.; Sankar, A.; Rich, A.; Kim, J.A.; Pittman, B.; Constable, R.T.; Scheinost, D.; Blumberg, H.P. A graph theory neuroimaging approach to distinguish the depression of bipolar disorder from major depressive disorder in adolescents and young adults. J. Affect. Disord. 2022, 319, 15–26. [Google Scholar] [CrossRef]
- Jung, S.; Kim, J.-H.; Kang, N.-O.; Sung, G.; Ko, Y.-G.; Bang, M.; Park, C.I.; Lee, S.-H. Fusiform gyrus volume reduction associated with impaired facial expressed emotion recognition and emotional intensity recognition in patients with schizophrenia spectrum psychosis. Psychiatry Res. Neuroimaging 2021, 307, 111226. [Google Scholar] [CrossRef]
- Chen, M.-H.; Kao, Z.-K.; Chang, W.-C.; Tu, P.-C.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Tsai, S.-J.; et al. Increased Proinflammatory Cytokines, Executive Dysfunction, and Reduced Gray Matter Volumes In First-Episode Bipolar Disorder and Major Depressive Disorder. J. Affect. Disord. 2020, 274, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Z.; Zuo, J.; Xi, C.; Long, Y.; Li, M.D.; Ouyang, X.; Yang, J. Decreased Cortical Folding of the Fusiform Gyrus and Its Hypoconnectivity with Sensorimotor Areas in Major Depressive Disorder. J. Affect. Disord. 2021, 295, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Cui, Y.; Liu, K.; Qu, H.; Lu, Y.; Li, W.; Zhang, L.; Zhang, Y.; Song, J.; et al. Reduced Gray Matter Volume in Orbitofrontal Cortex Across Schizophrenia, Major Depressive Disorder, and Bipolar Disorder: A Comparative Imaging Study. Front. Neurosci. 2022, 16, 919272. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Takahashi, T.; Orikabe, L.; Masuda, N.; Mozue, Y.; Nakamura, K.; Kawasaki, Y.; Itokawa, M.; Sato, Y.; Yamasue, H.; et al. Volume reduction and altered sulco-gyral pattern of the orbitofrontal cortex in first-episode schizophrenia. Schizophr. Res. 2010, 121, 55–65. [Google Scholar] [CrossRef]
- Carroll, A.L.; Damme, K.S.; Alloy, L.B.; Bart, C.P.; Ng, T.H.; Titone, M.K.; Chein, J.; Cichocki, A.C.; Armstrong, C.C.; Nusslock, R. Risk for bipolar spectrum disorders associated with positive urgency and orbitofrontal cortical grey matter volume. NeuroImage Clin. 2022, 36, 103225. [Google Scholar] [CrossRef]
- Nelson, B.D.; Bjorkquist, O.A.; Olsen, E.K.; Herbener, E.S. Schizophrenia symptom and functional correlates of anterior cingulate cortex activation to emotion stimuli: An fMRI investigation. Psychiatry Res. Neuroimaging 2015, 234, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Frascarelli, M.; Tognin, S.; Mirigliani, A.; Parente, F.; Buzzanca, A.; Torti, M.C.; Tinelli, E.; Caramia, F.; Di Fabio, F.; Biondi, M.; et al. Medial frontal gyrus alterations in schizophrenia: Relationship with duration of illness and executive dysfunction. Psychiatry Res. Neuroimaging 2015, 231, 103–110. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Q.; Tian, F.; Cheng, B.; Qiu, L.; Wang, S.; He, M.; Wang, H.; Duan, M.; Jia, Z. Brain grey-matter volume alteration in adult patients with bipolar disorder under different conditions: A voxel-based meta-analysis. J. Psychiatry Neurosci. 2019, 44, 89–101. [Google Scholar] [CrossRef]
- Madeira, N.; Martins, R.; Duarte, J.V.; Costa, G.; Macedo, A.; Castelo-Branco, M. A Fundamental Distinction in Early Neural Processing of Implicit Social Interpretation in Schizophrenia and Bipolar Disorder. NeuroImage Clin. 2021, 32, 102836. [Google Scholar] [CrossRef]
| Schizophrenia n = 20 | Bipolar Disorder n = 20 | Healthy Controls n = 20 | Test Statistics | p-Value | |
|---|---|---|---|---|---|
| Gender (Female | Male) | 7 | 13 | 7 | 13 | 7 | 13 | 0.000 | 1.000 |
| Age—years (SD) | 31.5 (10.3) | 31.65 (10.00) | 31.5 (10.3) | F 0.001 | 0.992 |
| Education—years (SD) | 13.6 (3.7) | 13.85 (2.64) | 14.9 (4.52) | F 0.756 | 0.474 |
| Age of disease onset—years (SD) | 25.6 (6.9) | 26.5 (8.8) | - | t −0.276 | 0.784 |
| Duration of disease—years (SD) | 6.0 (7.9) | 5.2 (4.3) | - | t 0.297 | 0.769 |
| Number of lifetime admissions (min–max) | 1.25 (0–7) | 1.25 (0–4) | - | t 0.000 | 1.000 |
| Antipsychotic exposure (CPZE)—mg (SD) | 380.0 (337.3) | 160.8 (272.3) | - | t 2.226 | 0.032 |
| History of psychotic symptoms | 20 | 16 | - | 0.035 | 0.106 |
| History of substance abuse | 5 | 7 | - | 0.557 | 0.731 |
| History of suicidal behaviors | 4 | 4 | - | 0.000 | 1.000 |
| Psychopathology—BPRS (SD) | 35.65 (6.41) | 29.11 (2.61) | - | t 3.991 | 0.000 |
| Functioning—PSP (SD) | 80.22 (12.36) | 92.00 (4.00) | - | t −3.845 | 0.001 |
| Insight—ITAQ (SD) | 17.12 (3.16) | 19.13 (2.22) | - | t −2.100 | 0.044 |
| Schizo-Bipolar Scale (min–max) | 8.00 (7–9) | 0.94 (0–2) | - | t 28.356 | 0.000 |
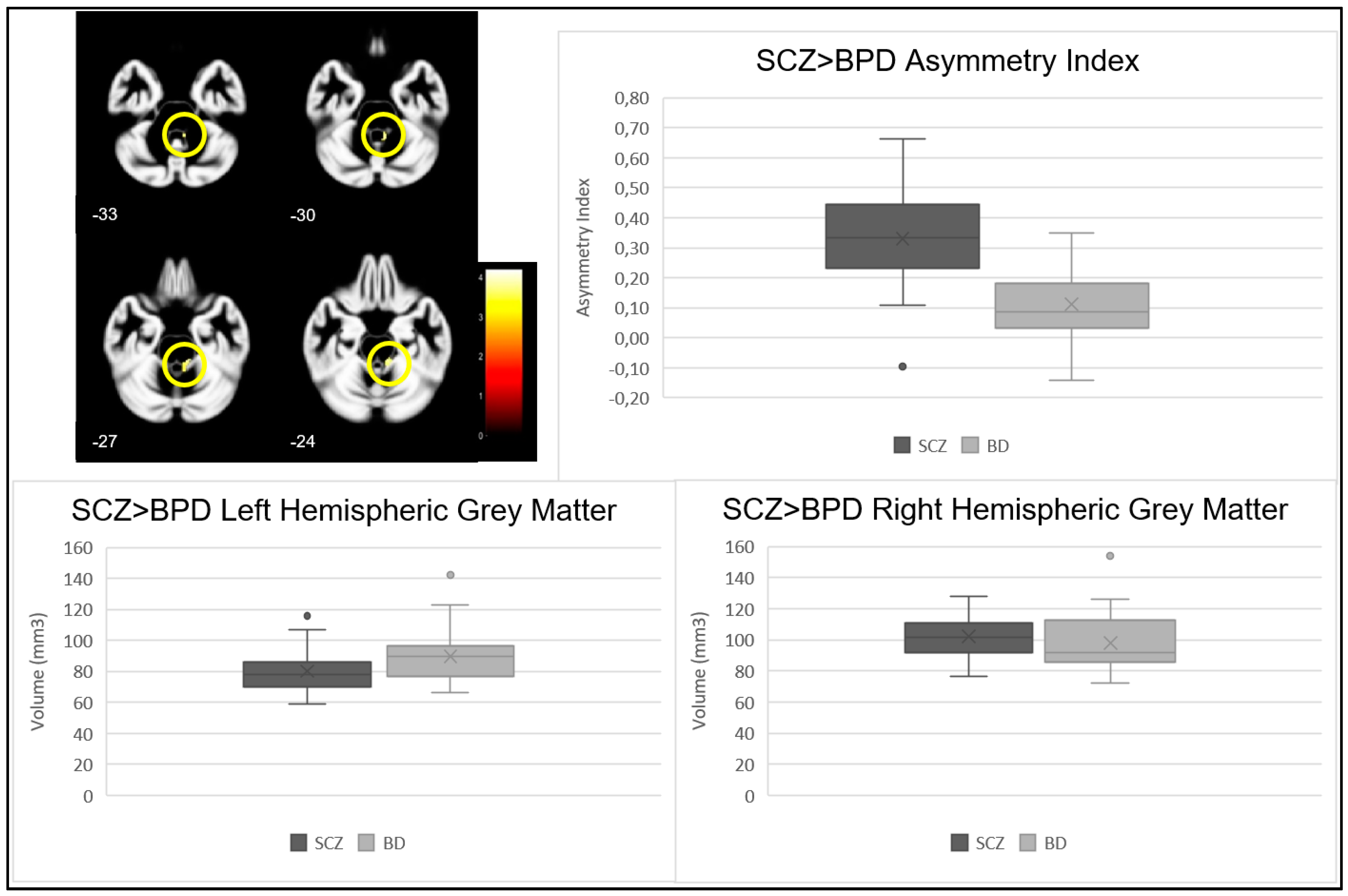
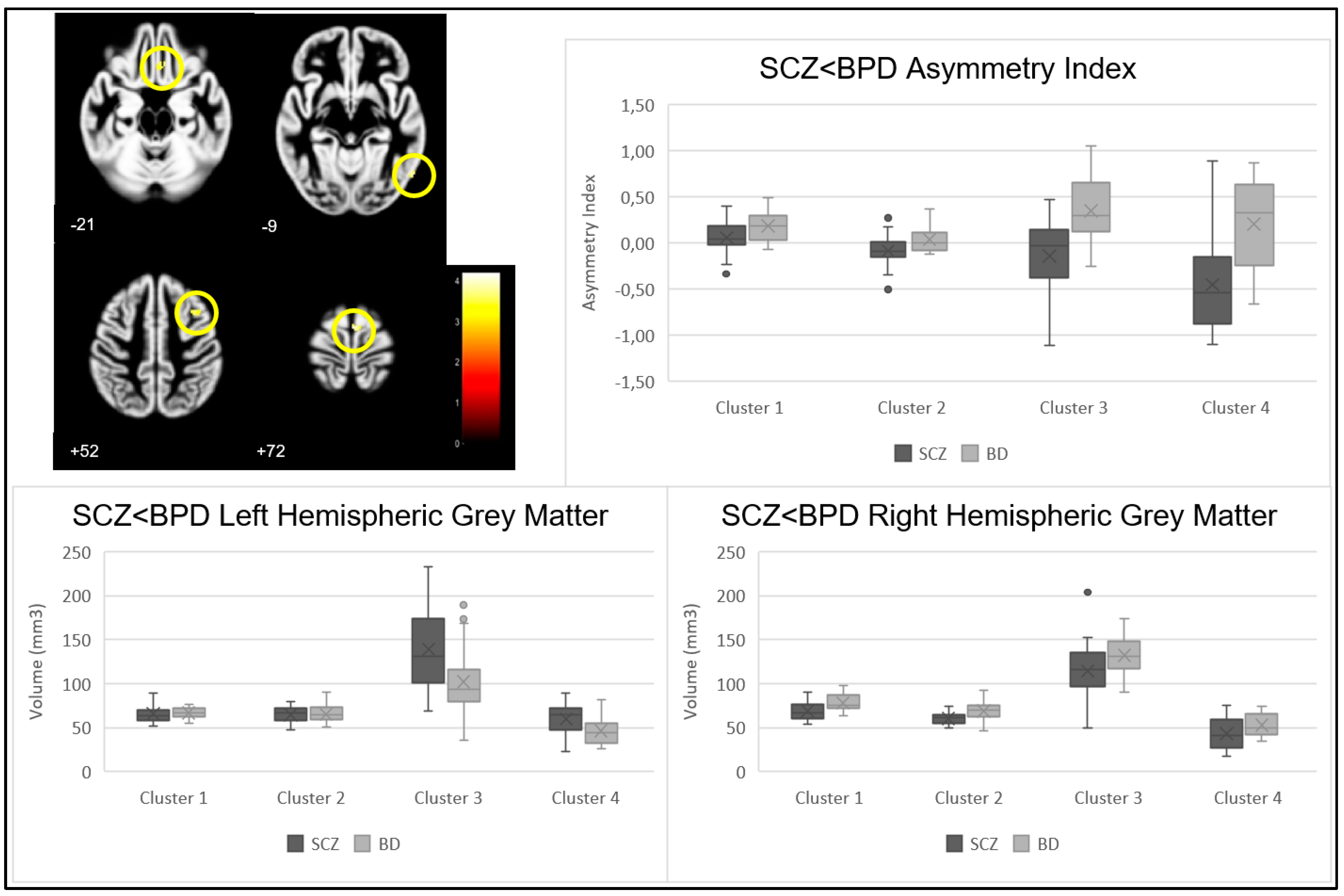
| Cluster | p-Value | Z Statistic | Cluster Size | MNI Coordinates (X, Y, Z) | Brain Region | |
|---|---|---|---|---|---|---|
| SCZ > BPD | 1 | <0.001 | 3.78 | 82 | (11, −34, −26) | Right Cerebellum hemisphere, Anterior Lobe, Gyrus Culmen |
| <0.001 | 3.52 | (6, −45, −30) | ||||
| SCZ < BPD | 1 | <0.001 | 3.92 | 38 | (3, −6,72) | Right Cerebrum, Frontal Lobe, Medial Frontal Gyrus, Brodmann area 6 |
| 2 | <0.001 | 3.78 | 35 | (57, −66, −9) | Right Cerebrum, Temporal Lobe, Fusiform, Inferior Temporal, and Middle Temporal Gyrus, Brodmann area 37 | |
| 3 | <0.001 | 3.47 | 60 | (33, 14, 52) | Right Cerebrum, Frontal Lobe, Medial Frontal Gyrus, Brodmann area 6 | |
| 4 | <0.001 | 3.44 | 33 | (5, 29, −21) | Right Cerebrum, Frontal and Limbic Lobe, Medial Frontal Gyrus and Anterior Cingulate Cortex, Brodmann area 11, 25 and 32 | |
| SCZ > HC | 1 | <0.001 | 4.37 | 207 | (44, 21, −9) | Right Cerebrum, Frontal Lobe, Inferior Frontal Gyrus, Brodmann area 47 |
| <0.001 | 3.82 | (39, 23, −17) | ||||
| 2 | <0.001 | 3.56 | 57 | (30, −21, 74) | Right Cerebrum, Frontal Lobe, Precentral Gyrus, Brodmann area 4 and 3 | |
| SCZ < HC | 1 | <0.001 | 3.71 | 83 | (35, −70, 54) | Right Cerebrum, Parietal Lobe, Superior Parietal Lobule, Brodmann area 7 |
| <0.001 | 3.59 | (29, −64.52) | ||||
| 2 | <0.001 | 3.68 | 33 | (17, 11, 10) | Right Cerebrum, Caudate Body | |
| BPD > HC | 1 | <0.001 | 4.50 | 112 | (47, 23, 9) | Right Cerebrum, Frontal Lobe, Sub-gyral, White Matter |
| 2 | <0.001 | 4.43 | 81 | (38, 38, 6) | Right Cerebrum, Frontal Lobe, Sub-gyral, White Matter | |
| 3 | <0.001 | 3.61 | 33 | (15, 11, −3) | Right Cerebrum, Caudate Head | |
| BPD < HC | 1 | <0.001 | 4.29 | 45 | (16, −60, 60) | Right Cerebrum, Parietal Lobe, Precuneus, Brodmann area 7 |
| p-Value | |||
|---|---|---|---|
| Cluster | Left Hemisphere | Right Hemisphere | |
| SCZ > BPD | 1 | 0.080 | 0.406 |
| SCZ < BPD | 1 | 0.731 | 0.007 |
| 2 | 0.900 | 0.002 | |
| 3 | 0.012 | 0.078 | |
| 4 | 0.018 | 0.044 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, D.; Martins, R.; Macedo, A.; Castelo Branco, M.; Valente Duarte, J.; Madeira, N. Brain Hemispheric Asymmetry in Schizophrenia and Bipolar Disorder. J. Clin. Med. 2023, 12, 3421. https://doi.org/10.3390/jcm12103421
Pinto D, Martins R, Macedo A, Castelo Branco M, Valente Duarte J, Madeira N. Brain Hemispheric Asymmetry in Schizophrenia and Bipolar Disorder. Journal of Clinical Medicine. 2023; 12(10):3421. https://doi.org/10.3390/jcm12103421
Chicago/Turabian StylePinto, Diogo, Ricardo Martins, António Macedo, Miguel Castelo Branco, João Valente Duarte, and Nuno Madeira. 2023. "Brain Hemispheric Asymmetry in Schizophrenia and Bipolar Disorder" Journal of Clinical Medicine 12, no. 10: 3421. https://doi.org/10.3390/jcm12103421
APA StylePinto, D., Martins, R., Macedo, A., Castelo Branco, M., Valente Duarte, J., & Madeira, N. (2023). Brain Hemispheric Asymmetry in Schizophrenia and Bipolar Disorder. Journal of Clinical Medicine, 12(10), 3421. https://doi.org/10.3390/jcm12103421








