Morpho-Functional Assessment of Retinal Ganglion Cells and Visual Pathways in Patients with Optic Disc Drusen: Superficial Drusen Visible Height as a Marker of Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
- (1)
- Age ranging from 18 to 80 years;
- (2)
- HFA 30-2 VF with defects that preserved the ability to maintain stable fixation comparable to that of normal subjects (fixation loss rate higher than 4%);
- (3)
- Capacity to clearly distinguish a target of fixation placed in the center of the screen, at a viewing distance of 114 cm, in which the visual stimuli of PERG and VEP were presented (see below);
- (4)
- BCVA between 0.00 and 0.40 logarithm of the minimum angle of resolution (logMAR);
- (5)
- Refractive error (when present) between −3.00 and +3.00 spherical equivalent;
- (6)
- IOP less than 18 mmHg;
- (7)
- Absence of cornea, lens, and retina/macula diseases or detectable spontaneous eye movements (i.e., nystagmus).
2.2. Procedures
2.2.1. Visual Acuity Assessment
2.2.2. Visual Field Examination
2.2.3. Electrophysiological Examinations
PERG Recordings
VEP Recordings
2.2.4. Spectral-Domain Optical Coherence Tomography
2.2.5. Statistics
3. Results
3.1. Demographic and Clinical Features
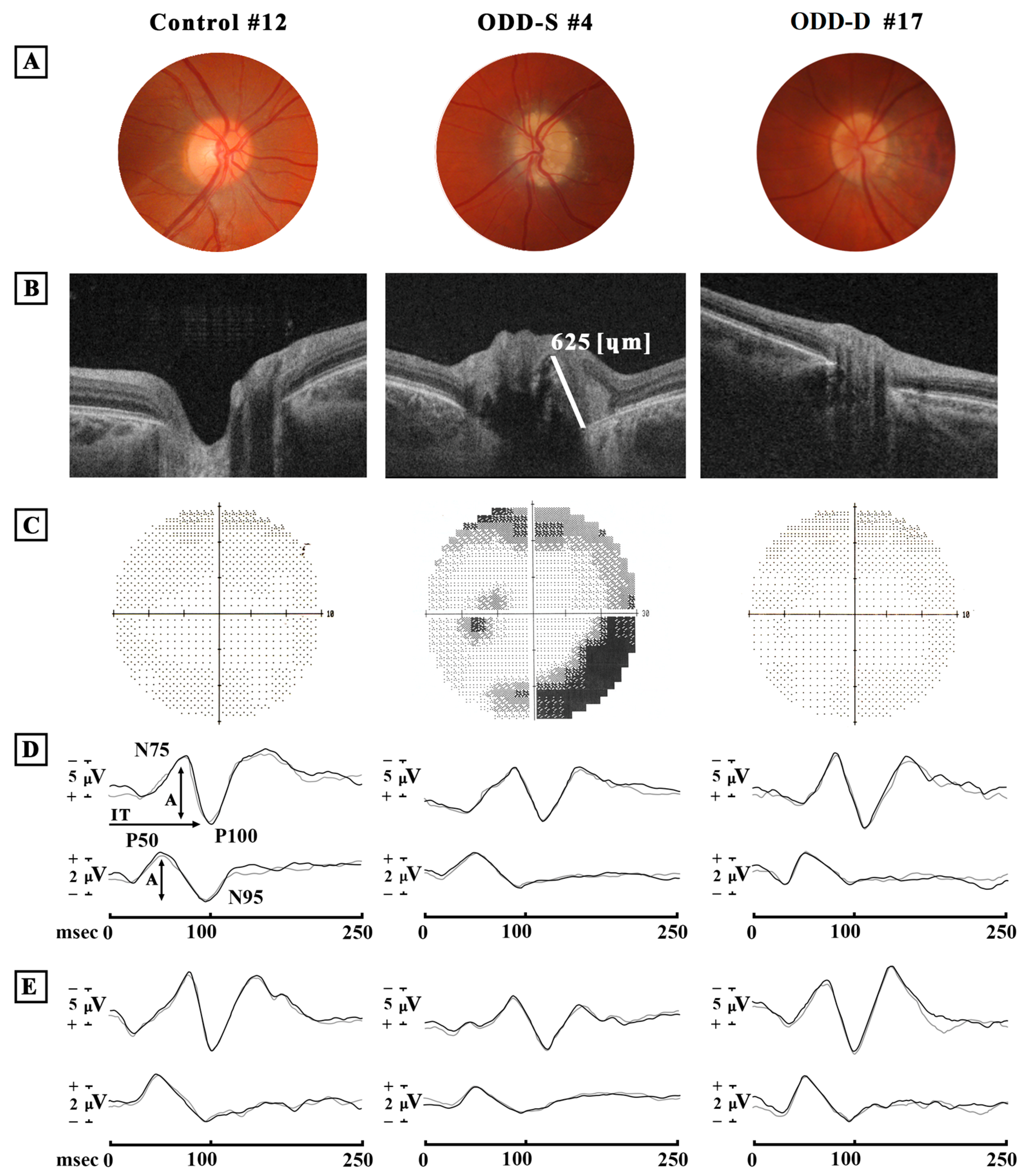
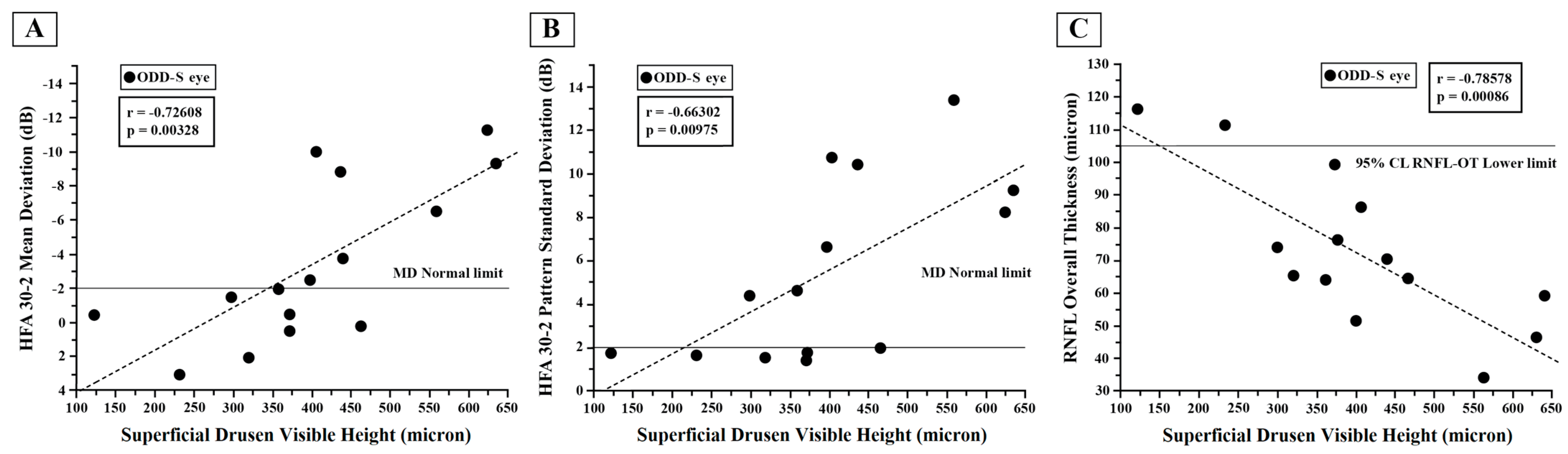
3.2. Optic Disc Druse Characteristics
3.3. Best-Corrected Visual Acuity Data
3.4. Visual Field Changes: HFA Data
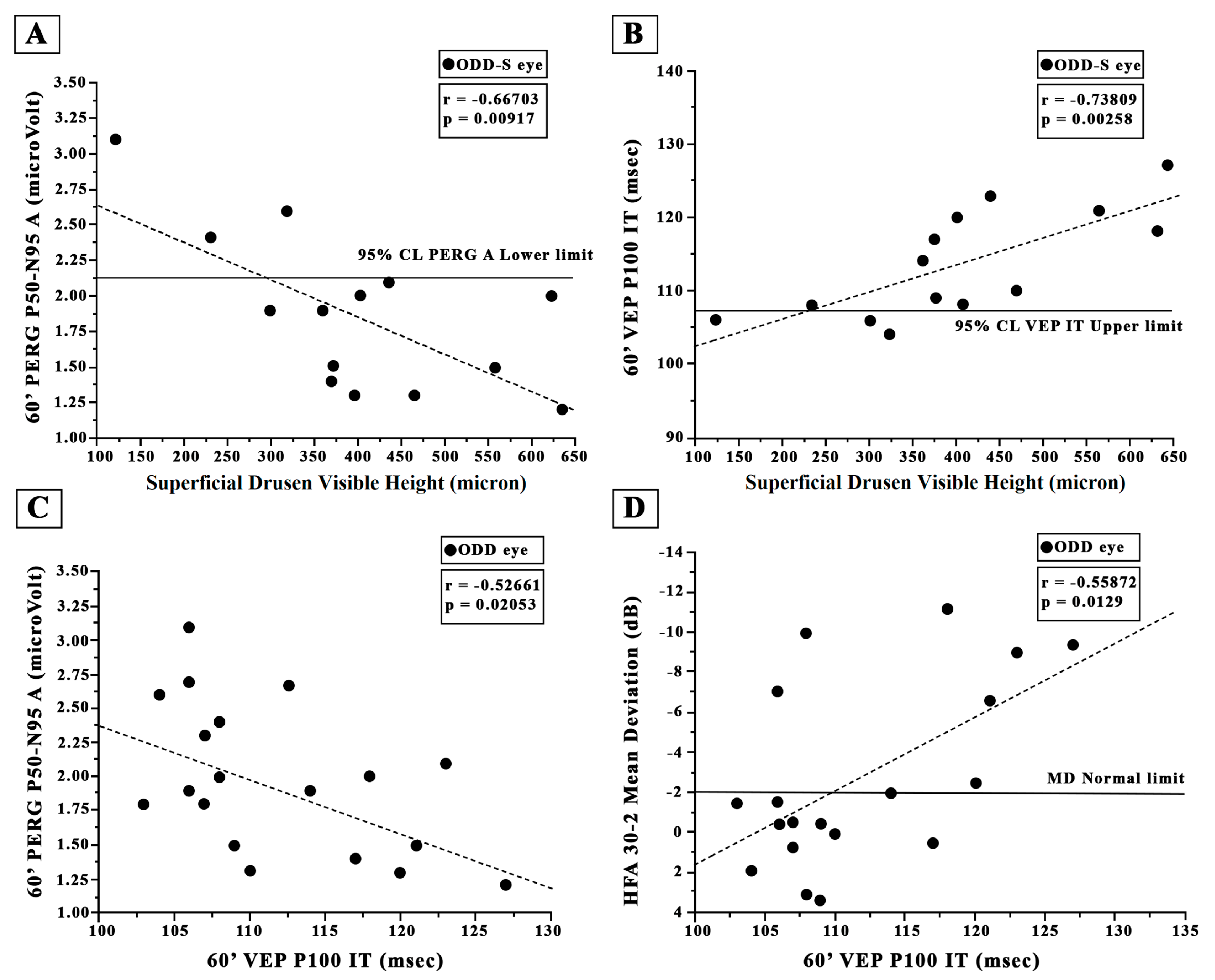
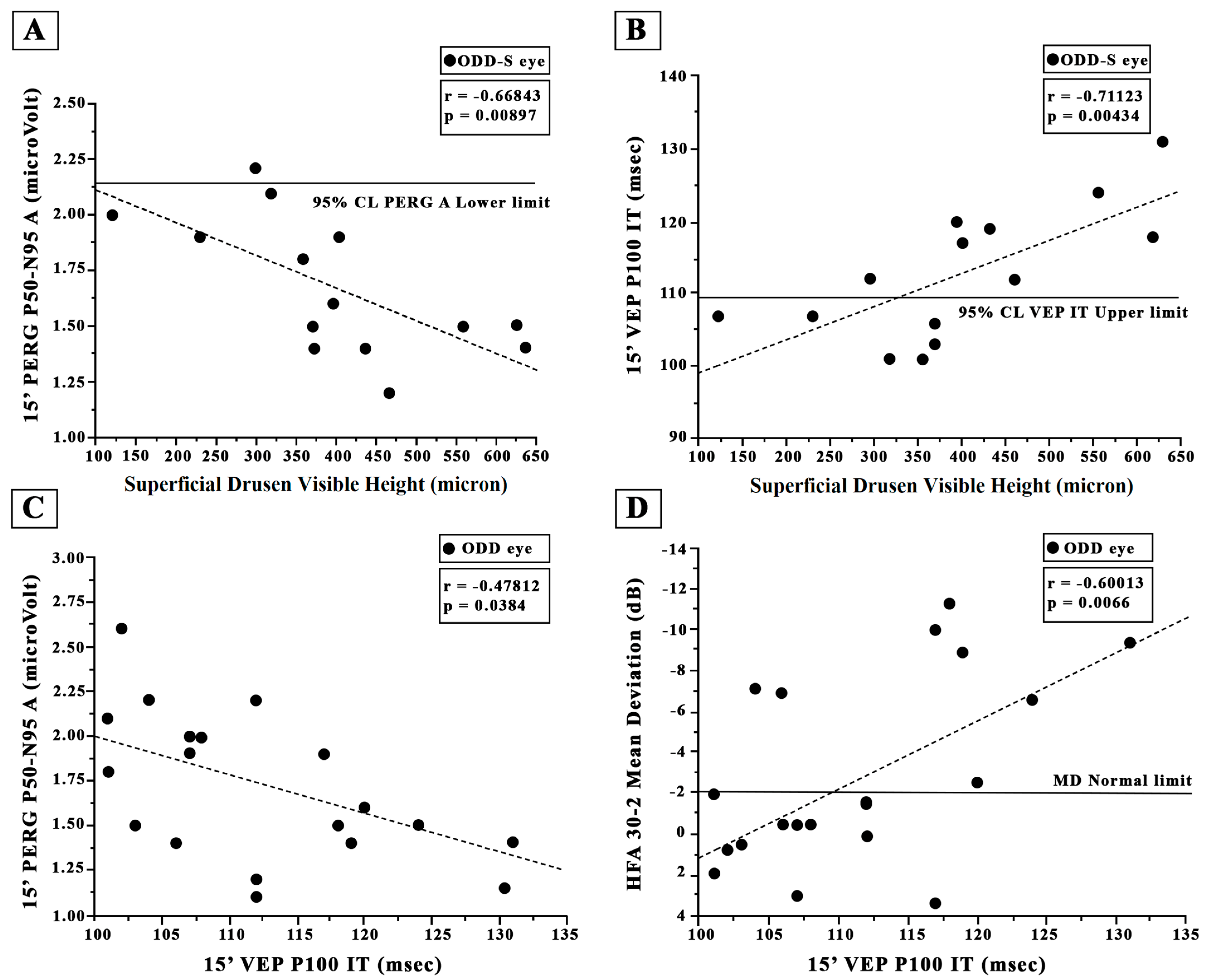
3.5. Retinal Ganglion Cells’ Functional Changes: PERG Data
3.6. Neural Conduction along the Visual Pathways’ Changes: VEP Data
3.7. Retinal Nerve Fiber Layer and Ganglion Cell Thicknesses: OCT Data
4. Discussion
- (1)
- A normal BCVA (0.0 LogMAR) but a significantly reduced HFA MD and increased HFA PSD values, correlated with increased 15′ VEP ITs;
- (2)
- Significantly reduced 60′ and 15′ PERG and VEP A values and significantly increased 60′ and 15′ VEP IT values;
- (3)
- A significantly reduced RNFL-T in all sectors apart from the nasal one and a reduced GC-T.
4.1. ODD and Visual Field Changes: HFA Data
4.2. ODD and Retinal Ganglion Cell Function: PERG Data
4.3. ODD and Neural Conduction along the Visual Pathways: VEP Data
4.4. ODD and Retinal Nerve Fiber Layer and Ganglion Cell Thicknesses: OCT Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ODD | Optic disc drusen |
| ODD-S | Superficial optic disc drusen |
| ODD-D | Deep optic disc drusen |
| EDI-OCT | Enhanced depth imaging OCT |
| RGCs | Retinal ganglion cells |
| PERG | Pattern Electroretinogram |
| VEP | Visual Evoked Potential |
| CL | Confidence limit |
| ANOVA | One-way analysis of variance |
| RNFL | Retinal nerve fiber layer |
| BCVA | Best-corrected visual acuity measurement |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| LogMAR | Logarithm of the minimum angle of resolution |
| IOP | Intraocular pressure |
| HFA | Humphrey Field Analyzer |
| MD | Mean deviation |
| PSD | Pattern standard deviation |
| ISCEV | International Society for Clinical Electrophysiology of Vision |
| PERG A | Pattern Electroretinogram amplitude |
| VEP IT | Visual Evoked Potential implicit time |
| VEP A | Visual Evoked Potential amplitude |
| SNR | Signal-to-noise ratio |
| BMO | Bruch’s membrane opening |
| SD | Standard deviation |
| GC | Ganglion cell |
| OHT | Ocular hypertension |
| VF | Visual field |
| SS-OCT | Swept-source optical coherence tomography |
| TT | Temporal Thickness |
| ST | Superior Thickness |
| IT | Inferior Thickness |
| NT | Nasal Thickness |
References
- Friedman, A.H.; Beckerman, B.; Gold, D.H.; Walsh, J.B.; Gartner, S. Drusen of the Optic Disc. Surv. Ophthalmol. 1977, 21, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Tso, M.O.M. Pathology and Pathogenesis of Drusen of the Optic Nervehead. Ophthalmology 1981, 88, 1066–1080. [Google Scholar] [CrossRef] [PubMed]
- Auw-Haedrich, C.; Staubach, F.; Witschel, H. Optic Disk Drusen. Surv. Ophthalmol. 2002, 47, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Allegrini, D.; Pagano, L.; Ferrara, M.; Borgia, A.; Sorrentino, T.; Montesano, G.; Angi, M.; Romano, M.R. Optic Disc Drusen: A Systematic Review: Up-to-Date and Future Perspective. Int. Ophthalmol. 2020, 40, 2119–2127. [Google Scholar] [CrossRef]
- Roh, S.; Noecker, R.J.; Schuman, J.S.; Hedges, T.R.; Weiter, J.J.; Mattox, C. Effect of Optic Nerve Head Drusen on Nerve Fiber Layer Thickness. Ophthalmology 1998, 105, 878–885. [Google Scholar] [CrossRef]
- Malmqvist, L.; Bursztyn, L.; Costello, F.; Digre, K.; Fraser, J.A.; Fraser, C.; Katz, B.; Lawlor, M.; Petzold, A.; Sibony, P.; et al. The Optic Disc Drusen Studies Consortium Recommendations for Diagnosis of Optic Disc Drusen Using Optical Coherence Tomography. J. Neuroophthalmol. 2018, 38, 299–307. [Google Scholar] [CrossRef]
- Pojda-Wilczek, D.; Wycisło-Gawron, P. The Effect of a Decrease in Intraocular Pressure on Optic Nerve Function in Patients with Optic Nerve Drusen. Ophthalmic. Res. 2019, 61, 153–158. [Google Scholar] [CrossRef]
- Silverman, A.L.; Tatham, A.J.; Medeiros, F.A.; Weinreb, R.N. Assessment of Optic Nerve Head Drusen Using Enhanced Depth Imaging and Swept Source Optical Coherence Tomography. J. Neuroophthalmol. 2014, 34, 198–205. [Google Scholar] [CrossRef]
- Morris, R.W.; Ellerbrock, J.M.; Hamp, A.M.; Joy, J.T.; Roels, P.; Davis, C.N. Advanced Visual Field Loss Secondary to Optic Nerve Head Drusen: Case Report and Literature Review. Optometry 2009, 80, 83–100. [Google Scholar] [CrossRef]
- Hamann, S.; Malmqvist, L.; Costello, F. Optic Disc Drusen: Understanding an Old Problem from a New Perspective. Acta Ophthalmol. 2018, 96, 673–684. [Google Scholar] [CrossRef]
- Robson, A.G.; Frishman, L.J.; Grigg, J.; Hamilton, R.; Jeffrey, B.G.; Kondo, M.; Li, S.; McCulloch, D.L. ISCEV Standard for Full-Field Clinical Electroretinography (2022 Update). Doc. Ophthalmol. 2022, 144, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.V.; Bach, M.; Brigell, M.; Holder, G.E.; McCulloch, D.L.; Tormene, A.P.; Vaegan. ISCEV Standard for Clinical Visual Evoked Potentials (2009 Update). Doc. Ophthalmol. 2010, 120, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Pineles, S.L. Optic Disc Drusen in Children. Surv. Ophthalmol. 2016, 61, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Scholl, G.B.; Song, H.S.; Winkler, D.E.; Wray, S.H. The Pattern Visual Evoked Potential and Pattern Electroretinogram in Drusen-Associated Optic Neuropathy. Arch. Ophthalmol. 1992, 110, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Frishman, L.J. Retinal Pathway Origins of the Pattern Electroretinogram (PERG). Investig. Ophthalmol. Vis. Sci. 2011, 52, 8571–8584. [Google Scholar] [CrossRef]
- Katz, B.J.; Pomeranz, H.D. Visual Field Defects and Retinal Nerve Fiber Layer Defects in Eyes with Buried Optic Nerve Drusen. Am. J. Ophthalmol. 2006, 141, 248–253. [Google Scholar] [CrossRef]
- Gili, P.; Flores-Rodríguez, P.; Martin-Ríos, M.D.; Carrasco Font, C. Anatomical and Functional Impairment of the Nerve Fiber Layer in Patients with Optic Nerve Head Drusen. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2421–2428. [Google Scholar] [CrossRef]
- Lee, K.M.; Woo, S.J.; Hwang, J.-M. Morphologic Characteristics of Optic Nerve Head Drusen on Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2013, 155, 1139–1147.e1. [Google Scholar] [CrossRef]
- Engelke, H.; Shajari, M.; Riedel, J.; Mohr, N.; Priglinger, S.G.; Mackert, M.J. OCT Angiography in Optic Disc Drusen: Comparison with Structural and Functional Parameters. Br. J. Ophthalmol. 2020, 104, 1109–1113. [Google Scholar] [CrossRef]
- Tsikata, E.; Verticchio Vercellin, A.C.; Falkenstein, I.; Poon, L.Y.-C.; Brauner, S.; Khoueir, Z.; Miller, J.B.; Chen, T.C. Volumetric Measurement of Optic Nerve Head Drusen Using Swept Source Optical Coherence Tomography. J. Glaucoma 2017, 26, 798–804. [Google Scholar] [CrossRef]
- Lee, K.M.; Woo, S.J.; Hwang, J.-M. Factors Associated with Visual Field Defects of Optic Disc Drusen. PLoS ONE 2018, 13, e0196001. [Google Scholar] [CrossRef]
- Malmqvist, L.; Lindberg, A.-S.W.; Dahl, V.A.; Jørgensen, T.M.; Hamann, S. Quantitatively Measured Anatomic Location and Volume of Optic Disc Drusen: An Enhanced Depth Imaging Optical Coherence Tomography Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2491–2497. [Google Scholar] [CrossRef]
- Sato, T.; Mrejen, S.; Spaide, R.F. Multimodal Imaging of Optic Disc Drusen. Am. J. Ophthalmol. 2013, 156, 275–282.e1. [Google Scholar] [CrossRef]
- Traber, G.L.; Weber, K.P.; Sabah, M.; Keane, P.A.; Plant, G.T. Enhanced Depth Imaging Optical Coherence Tomography of Optic Nerve Head Drusen: A Comparison of Cases with and without Visual Field Loss. Ophthalmology 2017, 124, 66–73. [Google Scholar] [CrossRef]
- Scuderi, G.L.; Cesareo, M.; Perdicchi, A.; Recupero, S.M. Standard Automated Perimetry and Algorithms for Monitoring Glaucoma Progression. Prog. Brain Res. 2008, 173, 77–99. [Google Scholar] [CrossRef]
- Parisi, V.; Scarale, M.E.; Balducci, N.; Fresina, M.; Campos, E.C. Electrophysiological Detection of Delayed Postretinal Neural Conduction in Human Amblyopia. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5041–5048. [Google Scholar] [CrossRef]
- Parisi, V.; Ziccardi, L.; Sadun, F.; De Negri, A.M.; La Morgia, C.; Barbano, L.; Carelli, V.; Barboni, P. Functional Changes of Retinal Ganglion Cells and Visual Pathways in Patients with Chronic Leber’s Hereditary Optic Neuropathy during One Year of Follow-Up. Ophthalmology 2019, 126, 1033–1044. [Google Scholar] [CrossRef]
- Parisi, V.; Manni, G.; Spadaro, M.; Colacino, G.; Restuccia, R.; Marchi, S.; Bucci, M.G.; Pierelli, F. Correlation between Morphological and Functional Retinal Impairment in Multiple Sclerosis Patients. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2520–2527. [Google Scholar]
- Ziccardi, L.; Sadun, F.; De Negri, A.M.; Barboni, P.; Savini, G.; Borrelli, E.; La Morgia, C.; Carelli, V.; Parisi, V. Retinal Function and Neural Conduction along the Visual Pathways in Affected and Unaffected Carriers with Leber’s Hereditary Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6893–6901. [Google Scholar] [CrossRef]
- Parisi, V.; Miglior, S.; Manni, G.; Centofanti, M.; Bucci, M.G. Clinical Ability of Pattern Electroretinograms and Visual Evoked Potentials in Detecting Visual Dysfunction in Ocular Hypertension and Glaucoma. Ophthalmology 2006, 113, 216–228. [Google Scholar] [CrossRef]
- Parisi, V. Impaired Visual Function in Glaucoma. Clin. Neurophysiol. 2001, 112, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Harter, M.R.; White, C.T. Evoked Cortical Responses to Checkerboard Patterns: Effect of Check-Size as a Function of Visual Acuity. Electroencephalogr. Clin. Neurophysiol. 1970, 28, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, A.; Maffei, L.; Pirchio, M.; Spinelli, D.; Porciatti, V. The ERG in Response to Alternating Gratings in Patients with Diseases of the Peripheral Visual Pathway. Investig. Ophthalmol. Vis. Sci. 1981, 21, 490–493. [Google Scholar]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The Ten-Twenty Electrode System of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar] [PubMed]
- Cruz-Herranz, A.; Balk, L.J.; Oberwahrenbrock, T.; Saidha, S.; Martinez-Lapiscina, E.H.; Lagreze, W.A.; Schuman, J.S.; Villoslada, P.; Calabresi, P.; Balcer, L.; et al. The APOSTEL Recommendations for Reporting Quantitative Optical Coherence Tomography Studies. Neurology 2016, 86, 2303–2309. [Google Scholar] [CrossRef]
- Savino, P.J.; Glaser, J.S.; Rosenberg, M.A. A Clinical Analysis of Pseudopapilledema. II. Visual Field Defects. Arch. Ophthalmol. 1979, 97, 71–75. [Google Scholar] [CrossRef]
- Lorentzen, S.E. Drusen of the Optic Disk. A Clinical and Genetic Study. Acta Ophthalmol. Suppl. 1966, 90, 1–180. [Google Scholar]
- Mistlberger, A.; Sitte, S.; Hommer, A.; Emesz, M.; Dengg, S.; Hitzl, W.; Grabner, G. Scanning Laser Polarimetry (SLP) for Optic Nerve Head Drusen. Int. Ophthalmol. 2001, 23, 233–237. [Google Scholar] [CrossRef]
- Malmqvist, L.; Wegener, M.; Sander, B.A.; Hamann, S. Peripapillary Retinal Nerve Fiber Layer Thickness Corresponds to Drusen Location and Extent of Visual Field Defects in Superficial and Buried Optic Disc Drusen. J. Neuroophthalmol. 2016, 36, 41–45. [Google Scholar] [CrossRef]
- Wilkins, J.M.; Pomeranz, H.D. Visual Manifestations of Visible and Buried Optic Disc Drusen. J. Neuroophthalmol. 2004, 24, 125–129. [Google Scholar] [CrossRef]
- Mustonen, E. Pseudopapilloedema with and without Verified Optic Disc Drusen. A Clinical Analysis II: Visual Fields. Acta Ophthalmol. 1983, 61, 1057–1066. [Google Scholar] [CrossRef]
- Malmqvist, L.; Lund-Andersen, H.; Hamann, S. Long-Term Evolution of Superficial Optic Disc Drusen. Acta Ophthalmol. 2017, 95, 352–356. [Google Scholar] [CrossRef]
- Frisén, L. Evolution of Drusen of the Optic Nerve Head over 23 Years. Acta Ophthalmol. 2008, 86, 111–112. [Google Scholar] [CrossRef]
- Spencer, W.H. XXXIV Edward Jackson Memorial Lecture: Drusen of the Optic Disc and Aberrant Axoplasmic Transport. Ophthalmology 1978, 85, 21–38. [Google Scholar] [CrossRef]
- Purvin, V.; King, R.; Kawasaki, A.; Yee, R. Anterior Ischemic Optic Neuropathy in Eyes with Optic Disc Drusen. Arch. Ophthalmol. 2004, 122, 48–53. [Google Scholar] [CrossRef]
- Farah, S.G.; Mansour, A.M. Central Retinal Artery Occlusion and Optic Disc Drusen. Eye 1998, 12 Pt 3a, 480–482. [Google Scholar] [CrossRef]
- Green, W.R.; Chan, C.C.; Hutchins, G.M.; Terry, J.M. Central Retinal Vein Occlusion: A Prospective Histopathologic Study of 29 Eyes in 28 Cases. Retina 1981, 1, 27–55. [Google Scholar] [CrossRef]
- Duncan, J.E.; Freedman, S.F.; El-Dairi, M.A. The Incidence of Neovascular Membranes and Visual Field Defects from Optic Nerve Head Drusen in Children. J. AAPOS 2016, 20, 44–48. [Google Scholar] [CrossRef]
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Retinal Ganglion Cell Atrophy Correlated with Automated Perimetry in Human Eyes with Glaucoma. Am. J. Ophthalmol. 1989, 107, 453–464. [Google Scholar] [CrossRef]
- Maffei, L.; Fiorentini, A. Electroretinographic Responses to Alternating Gratings in the Cat. Exp. Brain Res. 1982, 48, 327–334. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G. The Uniform Field and Pattern ERG in Macaques with Experimental Glaucoma: Removal of Spiking Activity. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2797–2810. [Google Scholar]
- Parisi, V. Correlation between Morphological and Functional Retinal Impairment in Patients Affected by Ocular Hypertension, Glaucoma, Demyelinating Optic Neuritis and Alzheimer’s Disease. Semin. Ophthalmol. 2003, 18, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-H.; Park, K.K.; Luo, X.; Porciatti, V. Retrograde Signaling in the Optic Nerve Is Necessary for Electrical Responsiveness of Retinal Ganglion Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Brudet-Wickel, C.L.; Van Lith, G.H.; Graniewski-Wijnands, H.S. Drusen of the Optic Disc and Occipital Transient Pattern Reversal Responses. Doc. Ophthalmol. 1981, 50, 243–248. [Google Scholar] [CrossRef]
- Mustonen, E.; Sulg, I.; Kallanranta, T. Electroretinogram (ERG) and Visual Evoked Response (VER) Studies in Patients with Optic Disc Drusen. Acta Ophthalmol. 1980, 58, 539–549. [Google Scholar] [CrossRef]
- Bishara, S.; Feinsod, M. Visual Evoked Response as an Aid in Diagnosing Optic Nerve Head Drusen: Case Report. J. Pediatr. Ophthalmol. Strabismus 1980, 17, 396–398. [Google Scholar] [CrossRef]
- Casado, A.; Rebolleda, G.; Guerrero, L.; Leal, M.; Contreras, I.; Oblanca, N.; Muñoz-Negrete, F.J. Measurement of Retinal Nerve Fiber Layer and Macular Ganglion Cell-Inner Plexiform Layer with Spectral-Domain Optical Coherence Tomography in Patients with Optic Nerve Head Drusen. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1653–1660. [Google Scholar] [CrossRef]
- Rothenbuehler, S.P.; Malmqvist, L.; Belmouhand, M.; Bjerager, J.; Maloca, P.M.; Larsen, M.; Hamann, S. Comparison of Spectral-Domain OCT versus Swept-Source OCT for the Detection of Deep Optic Disc Drusen. Diagnostics 2022, 12, 2515. [Google Scholar] [CrossRef]
| ODD a | HFA b 30-2 | 60′ PERG c | 60′ VEP d | 15′ PERG c | 15′ VEP d | RNFL e | GC-T f | |||||||||
| Type | Visible Height (µ g) | MD h (dB i) | PSD l (dB i) | A m (µV n) | IT o (msec p) | A m (µV n) | A m (µV n) | IT o (msec p) | A m (µV n) | TT q (µ g) | ST r (µ g) | IT s (µ g) | NT t (µ g) | OT u (µ g) | (µ g) | |
| ODD#1 | Deep | −7.06 | 7.5 | 2.7 | 106 | 7.1 | 2.2 | 104 | 2.4 | 65 | 68 | 51 | 87 | 67.75 | 50 | |
| ODD#2 | Deep | 3.46 | 1.81 | 1.5 | 109 | 6.2 | 1.9 | 117 | 3.7 | 79 | 138 | 73 | 152 | 110.50 | 67 | |
| ODD#3 | Superficial | 559 | −6.57 | 13.41 | 1.5 | 121 | 4.2 | 1.5 | 124 | 2.7 | 32 | 44 | 20 | 40 | 34.00 | 54 |
| ODD#4 | Superficial | 625 | −11.22 | 8.24 | 2.0 | 118 | 8.1 | 1.5 | 118 | 8.1 | 33 | 37 | 50 | 65 | 46.25 | 56 |
| ODD#5 | Superficial | 371 | 0.53 | 1.45 | 1.4 | 117 | 7.5 | 1.5 | 103 | 4.4 | 94 | 85 | 68 | 150 | 99.25 | 50 |
| ODD#6 | Superficial | 121 | −0.39 | 1.72 | 3.1 | 106 | 11.5 | 2.0 | 107 | 11.6 | 82 | 147 | 77 | 155 | 116.24 | 66 |
| ODD#7 | Superficial | 358 | −1.91 | 4.62 | 1.9 | 114 | 5.2 | 1.8 | 101 | 6.3 | 59 | 61 | 51 | 84 | 63.75 | 58 |
| ODD#8 | Superficial | 397 | −2.43 | 6.62 | 1.3 | 120 | 14.8 | 1.6 | 120 | 15.5 | 36 | 56 | 45 | 69 | 51.53 | 50 |
| ODD#9 | Superficial | 635 | −9.36 | 9.24 | 1.2 | 127 | 8.2 | 1.4 | 131 | 10.7 | 72 | 68 | 45 | 52 | 59.25 | 50 |
| ODD#10 | Superficial | 464 | 0.12 | 1.97 | 1.3 | 110 | 5.1 | 1.2 | 112 | 5.5 | 82 | 67 | 43 | 66 | 64.52 | 55 |
| ODD#11 | Superficial | 436 | −8.9 | 10.43 | 2.1 | 123 | 4.2 | 1.4 | 119 | 6.1 | 62 | 84 | 55 | 82 | 70.75 | 50 |
| ODD#12 | Superficial | 404 | −9.97 | 10.74 | 2.0 | 108 | 15.3 | 1.9 | 117 | 7.2 | 45 | 133 | 72 | 86 | 86.28 | 28 |
| ODD#13 | Superficial | 372 | −0.45 | 1.72 | 1.5 | 109 | 9.1 | 1.4 | 106 | 3.5 | 81 | 63 | 65 | 95 | 76.05 | 53 |
| ODD#14 | Superficial | 298 | −1.54 | 4.38 | 1.9 | 106 | 3.2 | 2.2 | 112 | 4.5 | 104 | 63 | 30 | 100 | 74.25 | 62 |
| ODD#15 | Deep | −0.48 | 2.68 | 2.3 | 107 | 4.5 | 2 | 108 | 5.1 | 100 | 79 | 47 | 110 | 84.00 | 63 | |
| ODD#16 | Superficial | 319 | 1.96 | 1.51 | 2.6 | 104 | 16.6 | 2.1 | 101 | 12.2 | 62 | 56 | 57 | 87 | 65.50 | 52 |
| ODD#17 | Deep | 0.78 | 2.37 | 1.8 | 107 | 11.5 | 2.6 | 102 | 10.5 | 64 | 87 | 63 | 82 | 74.00 | 55 | |
| ODD#18 | Superficial | 230 | 3.08 | 1.62 | 2.4 | 108 | 18.7 | 1.9 | 107 | 10.6 | 68 | 149 | 75 | 153 | 111.25 | 69 |
| ODD#19 | Deep | −1.45 | 1.38 | 1.8 | 103 | 10 | 1.1 | 112 | 13 | 60 | 133 | 58 | 139 | 97.50 | 55 | |
| 95%CL v | −2.0 | 2.0 | 2.14 | 107.19 | 8.38 | 2.14 | 109.59 | 7.48 | 76.65 | 109.74 | 119.13 | 78.68 | 104.73 | 56.00 | ||
| Controls (N a = 20) | ODD b (N a = 19) | ODD b Ab c (N a) | Ab c % | ANOVA d: ODD b vs. Controls | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | 1SD e | Mean | 1SD e | f (1,38) = | p= | |||
| Age (years) | 58.62 | 8.77 | 59.10 | 12.68 | 0.02 | 0.894 | ||
| HFA f MD g (dB i) | 0.82 | 0.38 | −2.69 | 4.50 | 7 | 36.84 | 12.09 | <0.001 |
| HFA f PSD h (dB i) | 0.68 | 1.36 | 4.77 | 3.85 | 11 | 57.89 | 19.97 | <0.0001 |
| 60′ PERG l A m (µV n) | 2.68 | 0.27 | 1.92 | 0.52 | 14 | 73.68 | 33.30 | <0.0001 |
| 60′ VEP o IT p (msec q) | 101.29 | 2.95 | 111.51 | 7.00 | 12 | 63.15 | 35.95 | <0.0001 |
| 60′ VEP o A m (µV n) | 13.4 | 2.51 | 8.97 | 4.49 | 11 | 57.89 | 14.66 | <0.0001 |
| 15′ PERG i A l (µV n) | 2.66 | 0.26 | 1.77 | 0.39 | 16 | 84.21 | 71.00 | <0.0001 |
| 15′ VEP o IT p (msec q) | 104.21 | 2.69 | 111.53 | 8.25 | 10 | 52.63 | 14.18 | <0.0001 |
| 15′ VEP o A l (µV n) | 12.52 | 2.52 | 7.75 | 3.80 | 11 | 57.89 | 21.55 | <0.0001 |
| RNFL r-TT s (µ) | 86.39 | 4.87 | 67.83 | 20.63 | 12 | 63.15 | 15.31 | <0.0001 |
| RNFL r-ST t (µ) | 136.22 | 13.24 | 86.39 | 35.60 | 14 | 73.68 | 34.24 | <0.0001 |
| RNFL r-IT u (µ) | 142.37 | 11.62 | 58.21 | 20.60 | 19 | 100.00 | 250.24 | <0.0001 |
| RNFL r-NT v (µ) | 97.42 | 9.37 | 96.63 | 35.24 | 5 | 26.31 | 0.01 | 0.923 |
| RNFL r-OT w (µ) | 116.21 | 5.74 | 77.87 | 22.95 | 16 | 84.21 | 52.43 | <0.0001 |
| GC-T x (µ) | 62.4 | 3.2 | 54.95 | 8.74 | 13 | 68.42 | 12.75 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, G.; Ziccardi, L.; Barbano, L.; Di Renzo, A.; Parisi, V. Morpho-Functional Assessment of Retinal Ganglion Cells and Visual Pathways in Patients with Optic Disc Drusen: Superficial Drusen Visible Height as a Marker of Impairment. J. Clin. Med. 2023, 12, 3432. https://doi.org/10.3390/jcm12103432
Antonelli G, Ziccardi L, Barbano L, Di Renzo A, Parisi V. Morpho-Functional Assessment of Retinal Ganglion Cells and Visual Pathways in Patients with Optic Disc Drusen: Superficial Drusen Visible Height as a Marker of Impairment. Journal of Clinical Medicine. 2023; 12(10):3432. https://doi.org/10.3390/jcm12103432
Chicago/Turabian StyleAntonelli, Giulio, Lucia Ziccardi, Lucilla Barbano, Antonio Di Renzo, and Vincenzo Parisi. 2023. "Morpho-Functional Assessment of Retinal Ganglion Cells and Visual Pathways in Patients with Optic Disc Drusen: Superficial Drusen Visible Height as a Marker of Impairment" Journal of Clinical Medicine 12, no. 10: 3432. https://doi.org/10.3390/jcm12103432







