Clinical Implications of the Association between Respiratory and Gastrointestinal Disorders in Migraine and Non-Migraine Headache Patients
Abstract
:1. Introduction
2. Materials and Methods
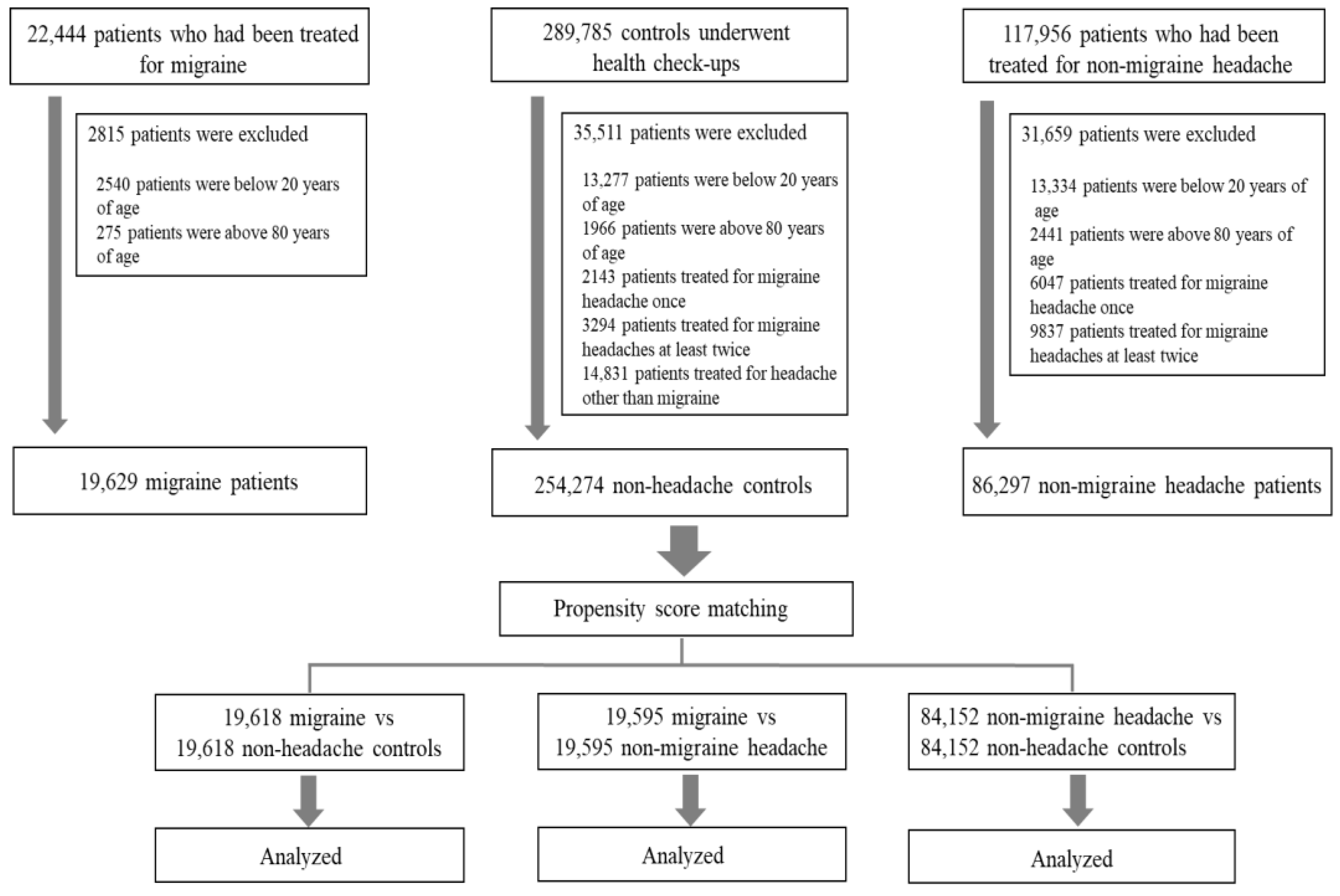
2.1. Subjects
2.2. Migraine or nMH, Respiratory Disorders, and Covariates
2.3. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. ORs for Respiratory and GI Disorders in Patients with Migraine and Patients with nMH
3.3. Differences in Respiratory and GI Disorders between Patients with Migraine and Patients with nMH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Before Matching | After Matching | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Migraine | Controls | nMH | Migraine | Controls | nMH | Controls | Migraine | nMH | |
| n = 19,629 | n = 254,274 | n = 86,297 | n = 19,618 | n = 19,618 | n = 84,152 | n = 84,152 | n = 19,595 | n = 19,595 | |
| Asthma | 585 (3.0%) | 4051 (1.6%) | 2313 (2.7%) | 584 (3.0%) | 505 (2.6%) | 2233 (2.7%) | 1972 (2.3%) | 582 (3.0%) | 523 (2.7%) |
| Bronchitis | 431 (2.2%) | 3050 (1.2%) | 1844 (2.1%) | 430 (2.2%) | 389 (2.0%) | 1778 (2.1%) | 1360 (1.6%) | 430 (2.2%) | 446 (2.3%) |
| COPD | 129 (0.7%) | 2179 (0.9%) | 984 (1.1%) | 129 (0.7%) | 200 (1.0%) | 941 (1.1%) | 1052 (1.3%) | 128 (0.7%) | 143 (0.7%) |
| GERD | 2676 (13.6%) | 19,015 (7.5%) | 9518 (11.0%) | 2671 (13.6%) | 2050 (10.4%) | 9177 (10.9%) | 8184 (9.7%) | 2665 (13.6%) | 2217 (11.3%) |
| Gastritis | 3600 (18.3%) | 21,424 (8.4%) | 12,038 (13.9%) | 3596 (18.3%) | 2279 (11.6%) | 11,602 (13.8%) | 8976 (10.7%) | 3591 (18.3%) | 2845 (14.5%) |
| FGID | 619 (3.2%) | 4302 (1.7%) | 2140 (2.5%) | 616 (3.1%) | 522 (2.7%) | 2059 (2.4%) | 1998 (2.4%) | 615 (3.1%) | 525 (2.7%) |
| IBS | 584 (3.0%) | 3227 (1.3%) | 1534 (1.8%) | 584 (3.0%) | 370 (1.9%) | 1471 (1.7%) | 1394 (1.7%) | 579 (3.0%) | 357 (1.8%) |
References
- Aurora, S.K.; Shrewsbury, S.B.; Ray, S.; Hindiyeh, N.; Nguyen, L. A link between gastrointestinal disorders and migraine: Insights into the gut–brain connection. Headache 2021, 61, 576–589. [Google Scholar] [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Siacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. JHP 2020, 21, 15. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Rhew, K. Association between Gastrointestinal Diseases and Migraine. Int. J. Environ. Res. Public Health 2022, 19, 4018. [Google Scholar] [CrossRef]
- Noghani, M.T.; Rezaeizadeh, H.; Fazljoo, S.M.B.; Keshavarz, M. Gastrointestinal Headache; a Narrative Review. Emerg. 2016, 4, 171–183. [Google Scholar]
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef]
- Hosang, L.; Canals, R.C.; van der Flier, F.J.; Hollensteiner, J.; Daniel, R.; Flügel, A.; Odoardi, F. The lung microbiome regulates brain autoimmunity. Nature 2022, 603, 138–144. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain-Lung Axis. Cell. Mol. Neurobiol. 2022, 43, 991–1003. [Google Scholar] [CrossRef]
- Portegies, M.L.; Lahousse, L.; Joos, G.F.; Hofman, A.; Koudstaal, P.J.; Stricker, B.H.; Brusselle, G.G.; Ikram, M.A. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 2016, 193, 251–258. [Google Scholar] [CrossRef]
- Morgan, A.D.; Sharma, C.; Rothnie, K.J.; Potts, J.; Smeeth, L.; Quint, J.K. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. Ann. Am. Thorac. Soc. 2017, 14, 754–765. [Google Scholar] [CrossRef]
- Lahousse, L.; Tiemeier, H.; Ikram, M.A.; Brusselle, G.G. Chronic obstructive pulmonary disease and cerebrovascular disease: A comprehensive review. Respir. Med. 2015, 109, 1371–1380. [Google Scholar] [CrossRef]
- Kerr, N.A.; de Rivero Vaccari, J.P.; Umland, O.; Bullock, M.R.; Conner, G.E.; Dietrich, W.D.; Keane, R.W. Human Lung Cell Pyroptosis Following Traumatic Brain Injury. Cells 2019, 8, 69. [Google Scholar] [CrossRef]
- Kerr, N.A.; de Rivero Vaccari, J.P.; Abbassi, S.; Kaur, H.; Zambrano, R.; Wu, S.; Dietrich, W.D.; Keane, R.W. Traumatic Brain Injury-Induced Acute Lung Injury: Evidence for Activation and Inhibition of a Neural-Respiratory-Inflammasome Axis. J. Neurotrauma. 2018, 35, 2067–2076. [Google Scholar] [CrossRef]
- Kerr, N.A.; de Rivero Vaccari, J.P.; Weaver, C.; Dietrich, W.D.; Ahmed, T.; Keane, R.W. Enoxaparin Attenuates Acute Lung Injury and Inflammasome Activation after Traumatic Brain Injury. J. Neurotrauma. 2021, 38, 646–654. [Google Scholar] [CrossRef]
- Schonhoff, A.M.; Mazmanian, S.K. Lung microbes mediate spinal-cord autoimmunity. Nature 2022, 603, 38–40. [Google Scholar] [CrossRef]
- Bajinka, O.; Simbilyabo, L.; Tan, Y.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2022, 48, 257–269. [Google Scholar] [CrossRef]
- Buse, D.C.; Manack, A.; Serrano, D.; Turkel, C.; Lipton, R.B. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J. Neurol. Neurosurg. Psychiatry 2010, 81, 428–432. [Google Scholar] [CrossRef]
- Sayyah, M.; Saki-Malehi, A.; Javanmardi, F.; Forouzan, A.; Shirbandi, K.; Rahim, F. Which came first, the risk of migraine or the risk of asthma? A systematic review. Neurol. Neurochir. Pol. 2018, 52, 562–569. [Google Scholar] [CrossRef]
- Wilmshurst, P.; Nightingale, S. The role of cardiac and pulmonary pathology in migraine: A hypothesis. Headache 2006, 46, 429–434. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Peng, Y.H.; Chen, K.F.; Liao, W.C.; Hsia, T.C.; Chen, H.J.; Yin, M.C.; Ho, W.C. Association of migraine with asthma risk: A retrospective population-based cohort study. Clin. Respir. J. 2018, 12, 1030–1037. [Google Scholar] [CrossRef]
- Davey, G.; Sedgwick, P.; Maier, W.; Visick, G.; Strachan, D.P.; Anderson, H.R. Association between migraine and asthma: Matched case-control study. Br. J. Gen. Prac. 2002, 52, 723–727. [Google Scholar]
- Graif, Y.; Shohat, T.; Machluf, Y.; Farkash, R.; Chaiter, Y. Association between asthma and migraine: A cross-sectional study of over 110 000 adolescents. Clin. Respir. J. 2018, 12, 2491–2496. [Google Scholar] [CrossRef]
- Turan, M.O.; Susuz, Ç.; Turan, P.A. Presence of Headache and Migraine in Asthma Patients. Turk. Thorac. J. 2017, 18, 47–51. [Google Scholar] [CrossRef]
- Peng, Y.H.; Chen, K.F.; Kao, C.H.; Chen, H.J.; Hsia, T.C.; Chen, C.H.; Liao, W.C. Risk of Migraine in Patients with Asthma: A Nationwide Cohort Study. Medicine 2016, 95, e2911. [Google Scholar] [CrossRef]
- Kang, L.L.; Chen, P.E.; Tung, T.H.; Chien, C.W. Association Between Asthma and Migraine: A Systematic Review and Meta-Analysis of Observational Studies. Front. Allergy 2021, 2, 741135. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Lim, J.S.; Choi, H.G. Bidirectional association between asthma and migraines in adults: Two longitudinal follow-up studies. Sci. Rep. 2019, 9, 18343. [Google Scholar] [CrossRef]
- Martin, V.T.; Fanning, K.M.; Serrano, D.; Buse, D.C.; Reed, M.L.; Lipton, R.B. Asthma is a risk factor for new onset chronic migraine: Results from the American migraine prevalence and prevention study. Headache 2016, 56, 118–131. [Google Scholar] [CrossRef]
- Dirican, N.; Demirci, S.; Cakir, M. The relationship between migraine headache and asthma features. Acta. Neurol. Belg. 2017, 117, 531–536. [Google Scholar] [CrossRef]
- Steinemann, A. National Prevalence and Effects of Multiple Chemical Sensitivities. J. Occup. Environ. Med. 2018, 60, e152–e156. [Google Scholar] [CrossRef]
- Özge, A.; Uluduz, D.; Bolay, H. Co-occurrence of migraine and atopy in children and adolescents: Myth or a casual relationship? Curr. Opin. Neurol. 2017, 30, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K. Recurrent syncope, hypotension, asthma, and migraine with aura: Role of metoclopramide. Headache 2005, 45, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, A.H.; Stovner, L.J.; Langhammer, A.; Hagen, K.; Zwart, J.A. Is headache related to asthma, hay fever, and chronic bronchitis? The Head-HUNT Study. Headache 2007, 47, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, L.; De Vanna, G.; Cresta, E.; Bellotti, A.; Corbelli, I.; Letizia Cupini, M.; Calabresi, P.; Sarchielli, P. Immunological findings in patients with migraine and other primary headaches: A narrative review. Clin. Exp. Immunol. 2022, 207, 11–26. [Google Scholar] [CrossRef]
- Biscetti, L.; De Vanna, G.; Cresta, E.; Corbelli, I.; Gaetani, L.; Cupini, L.; Calabresi, P.; Sarchielli, P. Headache and immunological/autoimmune disorders: A comprehensive review of available epidemiological evidence with insights on potential underlying mechanisms. J. Neuroinflammation 2021, 18, 259. [Google Scholar] [CrossRef]
- Talafi Noghani, M.; Namdar, H. Migraine associated with gastrointestinal disorders: A pathophysiological explanation. Med. Hypotheses 2019, 125, 90–93. [Google Scholar] [CrossRef]
- Martami, F.; Ghorbani, Z.; Abolhasani, M.; Togha, M.; Meysamie, A.; Sharifi, A.; Razeghi Jahromi, S. Comorbidity of gastrointestinal disorders, migraine, and tension-type headache: A cross-sectional study in Iran. Neurol. Sci. 2018, 39, 63–70. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.J.; Kwon, Y.; Kim, J.H.; Sohn, J.H. Clinical Implications of Associations between Headache and Gastrointestinal Disorders: A Study Using the Hallym Smart Clinical Data Warehouse. Frontie. Neurol. 2017, 8, 526. [Google Scholar] [CrossRef]
- Aamodt, A.H.; Stovner, L.J.; Hagen, K.; Zwart, J.A. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia 2008, 28, 144–151. [Google Scholar] [CrossRef]
- Faraji, F.; Zarinfar, N.; Zanjani, A.T.; Morteza, A. The effect of Helicobacter pylori eradication on migraine: A randomized, double blind, controlled trial. Pain Physician 2012, 15, 495–498. [Google Scholar]
- Spierings, E.L. Reflux-triggered migraine headache originating from the upper gum/teeth. Cephalalgia 2002, 22, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Francavilla, R.; Maiuri, L.; Ruggieri, M.; Spina, M.; Pavone, P.; Francavilla, T.; Magistà, A.M.; Pavone, L. Headache in pediatric patients with celiac disease and its prevalence as a diagnostic clue. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhao, Y.; Han, Y.; Guo, W.; Wang, J.; Li, X.; Han, Y.; Fan, D. Reversal of migraine symptoms by Helicobacter pylori eradication therapy in patients with hepatitis-B-related liver cirrhosis. Helicobacter 2007, 12, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Cremonini, F.; Fiore, G.; Addolorato, G.; Padalino, C.; Candelli, M.; De Leo, M.E.; Santarelli, L.; Giacovazzo, M.; Gasbarrini, A.; et al. Association between migraine and Celiac disease: Results from a preliminary case-control and therapeutic study. Am. J. Gastroenterol. 2003, 98, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiol. Pain 2022, 11, 100090. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef]



| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Migraine | Control | ASD | Migraine | Control | ASD | |
| n = 19,629 | n = 254,274 | n = 19,618 | n = 19,618 | |||
| Female sex | 14,357 (73.1%) | 124,936 (49.1%) | 0.542 | 14,346 (73.1%) | 14,231 (72.5%) | 0.013 |
| Age (y) | 44.5 ± 14.5 | 45.7 ± 15.0 | 0.079 | 44.5 ± 14.5 | 46.0 ± 15.3 | 0.106 |
| DM | 976 (5.0%) | 13,150 (5.2%) | 0.009 | 976 (5.0%) | 1030 (5.3%) | 0.013 |
| HTN | 2274 (11.6%) | 20,391 (8.0%) | 0.111 | 2272 (11.6%) | 2437 (12.4%) | 0.026 |
| Dyslipidemia | 1847 (9.4%) | 21,278 (8.4%) | 0.036 | 1846 (9.4%) | 1997 (10.2%) | 0.026 |
| Angina | 1110 (5.7%) | 8161 (3.2%) | 0.106 | 1109 (5.7%) | 1164 (5.9%) | 0.012 |
| AF | 150 (0.8%) | 2231 (0.9%) | 0.013 | 150 (0.8%) | 177 (0.9%) | 0.016 |
| Heart disease | 882 (4.5%) | 6762 (2.7%) | 0.089 | 881 (4.5%) | 954 (4.9%) | 0.018 |
| CVD | 2779 (14.2%) | 9601 (3.8%) | 0.298 | 2768 (14.1%) | 2989 (15.2%) | 0.032 |
| Renal failure | 263 (1.3%) | 3145 (1.2%) | 0.009 | 263 (1.3%) | 312 (1.6%) | 0.022 |
| Chronic hepatitis | 476 (2.4%) | 10,099 (4.0%) | 0.101 | 475 (2.4%) | 475 (2.4%) | <0.001 |
| Anxiety | 818 (4.2%) | 2217 (0.9%) | 0.165 | 813 (4.1%) | 788 (4.0%) | 0.006 |
| Depression | 2315 (11.8%) | 4896 (1.9%) | 0.306 | 2304 (11.7%) | 2302 (11.7%) | <0.001 |
| Sleep disorder | 1553 (7.9%) | 4227 (1.7%) | 0.232 | 1542 (7.9%) | 1495 (7.6%) | 0.009 |
| Menopause | 708 (3.6%) | 6386 (2.5%) | 0.059 | 707 (3.6%) | 760 (3.9%) | 0.014 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| nMH | Control | ASD | nMH | Control | ASD | |
| n = 86,297 | n = 254,274 | n = 84,152 | n = 84,152 | |||
| Female sex | 50,760 (58.8%) | 124,936 (49.1%) | 0.197 | 49,071 (58.3%) | 47,979 (57.0%) | 0.026 |
| Age (y) | 49.2 ± 14.6 | 45.7 ± 15.0 | 0.242 | 49.0 ± 14.7 | 49.0 ± 15.2 | <0.001 |
| DM | 5248 (6.1%) | 13,150 (5.2%) | 0.038 | 5089 (6.0%) | 5397 (6.4%) | 0.015 |
| HTN | 10,978 (12.7%) | 20,391 (8.0%) | 0.141 | 10,468 (12.4%) | 10,648 (12.7%) | 0.006 |
| Dyslipidemia | 8217 (9.5%) | 21,278 (8.4%) | 0.039 | 7970 (9.5%) | 8388 (10.0%) | 0.017 |
| Angina | 5445 (6.3%) | 8161 (3.2%) | 0.128 | 5209 (6.2%) | 5219 (6.2%) | <0.001 |
| AF | 1093 (1.3%) | 2231 (0.9%) | 0.035 | 1054 (1.3%) | 1092 (1.3%) | 0.004 |
| Heart disease | 4029 (4.7%) | 6762 (2.7%) | 0.095 | 3876 (4.6%) | 3826 (4.5%) | 0.003 |
| CVD | 11,834 (13.7%) | 9601 (3.8%) | 0.289 | 9939 (11.8%) | 9467 (11.2%) | 0.016 |
| Renal failure | 1407 (1.6%) | 3145 (1.2%) | 0.031 | 1382 (1.6%) | 1467 (1.7%) | 0.008 |
| Chronic hepatitis | 2767 (3.2%) | 10,099 (4.0%) | 0.043 | 2744 (3.3%) | 2922 (3.5%) | 0.012 |
| Anxiety | 2973 (3.4%) | 2217 (0.9%) | 0.141 | 2631 (3.1%) | 2138 (2.5%) | 0.032 |
| Depression | 5145 (6.0%) | 4896 (1.9%) | 0.170 | 4775 (5.7%) | 4356 (5.2%) | 0.021 |
| Sleep disorder | 3546 (4.1%) | 4227 (1.7%) | 0.123 | 3334 (4.0%) | 3159 (3.8%) | 0.010 |
| Menopause | 2864 (3.3%) | 6386 (2.5%) | 0.045 | 2794 (3.3%) | 2724 (3.2%) | 0.005 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Migraine | nMH | ASD | Migraine | nMH | ASD | |
| n = 19,629 | n = 86,297 | n = 19,595 | n = 19,595 | |||
| Female sex | 14,357 (73.1%) | 50,760 (58.8%) | 0.323 | 14,323 (73.1%) | 14,208 (72.5%) | 0.013 |
| Age | 44.5 ± 14.5 | 49.2 ± 14.6 | 0.323 | 44.5 ± 14.5 | 44.8 ± 14.5 | 0.018 |
| DM | 976 (5.0%) | 5248 (6.1%) | 0.051 | 974 (5.0%) | 1039 (5.3%) | 0.015 |
| HTN | 2274 (11.6%) | 10,978 (12.7%) | 0.036 | 2269 (11.6%) | 2319 (11.8%) | 0.008 |
| Dyslipidemia | 1847 (9.4%) | 8217 (9.5%) | 0.004 | 1841 (9.4%) | 1870 (9.5%) | 0.005 |
| Angina | 1110 (5.7%) | 5445 (6.3%) | 0.028 | 1108 (5.7%) | 1157 (5.9%) | 0.011 |
| AF | 150 (0.8%) | 1093 (1.3%) | 0.058 | 150 (0.8%) | 165 (0.8%) | 0.009 |
| Heart disease | 882 (4.5%) | 4029 (4.7%) | 0.008 | 878 (4.5%) | 855 (4.4%) | 0.006 |
| CVD | 2779 (14.2%) | 11,834 (13.7%) | 0.013 | 2772 (14.1%) | 2803 (14.3%) | 0.005 |
| Renal failure | 263 (1.3%) | 1407 (1.6%) | 0.025 | 262 (1.3%) | 307 (1.6%) | 0.020 |
| Chronic hepatitis | 476 (2.4%) | 2767 (3.2%) | 0.051 | 476 (2.4%) | 480 (2.4%) | 0.001 |
| Anxiety | 818 (4.2%) | 2973 (3.4%) | 0.036 | 811 (4.1%) | 846 (4.3%) | 0.009 |
| Depression | 2315 (11.8%) | 5145 (6.0%) | 0.181 | 2281 (11.6%) | 2304 (11.8%) | 0.004 |
| Sleep disorder | 1553 (7.9%) | 3546 (4.1%) | 0.141 | 1519 (7.8%) | 1533 (7.8%) | 0.003 |
| Menopause | 708 (3.6%) | 2864 (3.3%) | 0.015 | 708 (3.6%) | 690 (3.5%) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Lee, Y.; Kwon, Y.-S.; Sohn, J.-H. Clinical Implications of the Association between Respiratory and Gastrointestinal Disorders in Migraine and Non-Migraine Headache Patients. J. Clin. Med. 2023, 12, 3434. https://doi.org/10.3390/jcm12103434
Kim J-H, Lee Y, Kwon Y-S, Sohn J-H. Clinical Implications of the Association between Respiratory and Gastrointestinal Disorders in Migraine and Non-Migraine Headache Patients. Journal of Clinical Medicine. 2023; 12(10):3434. https://doi.org/10.3390/jcm12103434
Chicago/Turabian StyleKim, Jong-Ho, Yeonkyeong Lee, Young-Suk Kwon, and Jong-Hee Sohn. 2023. "Clinical Implications of the Association between Respiratory and Gastrointestinal Disorders in Migraine and Non-Migraine Headache Patients" Journal of Clinical Medicine 12, no. 10: 3434. https://doi.org/10.3390/jcm12103434





