1. Introduction
The treatment of femoral non-unions is a huge challenge for every surgeon. Non-unions after reamed intramedullary nailing are rare. Nevertheless, their existence causes severe pain, a prolonged disability, poor quality of life, and poor mental health, as well as high costs due to an increased re-hospitalization rate [
1,
2,
3]. The rate of delayed unions or non-unions in femoral shaft fractures varies from study to study. In the literature, figures from 1.6 to 12% after treatment with intramedullary nailing are reported [
4,
5]. In a recently published study, Mills et al. evaluated the overall risk of non-union at 1.9% [
6].
Reasons for non-unions are, most commonly, biomechanical instability and infections, as well as patient-based criteria such as comorbidities or nicotine abuse. Especially instabilities caused by a too small nail diameter, fracture dehiscence, and misalignment are relevant [
7,
8]. According to the fracture diamond model of Giannoudis et al., bone healing is mainly influenced or compromised by biological, vascular, mechanical, and patient-specific factors [
9] (
Figure 1).
Despite this knowledge, the correct prognosis of non-union is difficult and mainly depends on the surgeon’s experience. Additionally, the exact timing of the revision is hard to identify.
Multiple clinical-radiological scores have been established to identify possible non-unions, especially tibial (NURD, LEG-NUI, etc.) [
10] and subtrochanteric non-unions [
11]. No score exists for diaphyseal femur fractures. The NUSS score, created by Calori et al., can be used as a multifactorial approach to the estimation of non-union risk, taking bone stock, surrounding soft tissue, and patient health into consideration. Fifteen factors influencing non-union are evaluated, and the scores are added and multiplied by two. A maximum of 100 points can be reached, indicating maximum risk for impaired healing. Using the ladder strategy, a certain treatment is recommended [
12]. The score has been validated by several authors [
13,
14]. The main point of criticism is the underestimation of necessary treatment due to biological factors [
15]. All scores are based on patient comorbidities and surgical factors, as well as on the evaluation of postoperative and follow-up X-rays. A further development might be to use computational methods to take biomechanical aspects as well as vascularity into consideration.
In addition to these purely radiological rating systems, it has been proposed to use measures characterizing mechanical stability as a predictor for healing success instead. For instance, Perren defined the interfragmentary strain (IFS) for characterizing the biomechanical environment at the fracture site [
16]. Using simple global measures, such as IFS, as a predictor for healing success in real-world cases, however, is challenged not only by the difficulty of even defining them for complex fractures with varying fracture gap sizes and complex physiological motion, but also by the fact that a single value can hardly sufficiently describe the complex nature of the actual strain field.
Over the course of the last three decades, multiple mechano-regulation hypotheses of tissue differentiation have been proposed and implemented in numerical simulation models of bone remodeling and fracture healing that consider the full strain field in order to predict local tissue differentiation [
17,
18]. One of these models, the Ulm tissue-level bone healing model [
19,
20], based on the biomechanical thesis by Pauwels [
21] with further refinements by Claes and Heigele [
22], unifies both remodeling and fracture healing in a single model and is able to integrate patient-specific information and all current complex information on bone healing. Local distortional and dilatational strains have been identified as the main mechanical stimuli for tissue differentiation and remodeling (woven and lamellar bone, cartilage, and connective tissue) [
20]. Aside from mechanical stimuli, the fuzzy logic rules take local tissue composition as well as vascularization into account. Through iterative numerical procedures, the tissue differentiation processes of fracture healing are simulated [
23]. After having been corroborated by in vivo animal experiments, the next step will be using the model on a human collective under clinical conditions [
24].
The aim of this study was to establish a simulation-based method that allows for the identification of patients at risk for developing non-unions due to biomechanical problems by predicting the individual healing process based on postoperative data. If successful, this method would enable improved individualized care by providing surgeons with additional data to base their clinical decisions on. Due to the limited nature of the available retrospective data, we focus on the model’s capability of predicting the outcome as “union”/“non-union”. Consequently, predictions of the exact tissue differentiation process or the callus’ size, etc., could not be validated within the context of this study and should therefore be considered purely hypothetical.
Our hypothesis proposes that our computer-based simulation correctly predicts healing, or non-union, of femoral shaft fractures after surgical treatment with intramedullary nailing. The bridging dates predicted by the simulation are then correlated retrospectively with the clinical and radiological healing processes of the patients, and the accuracy of the prognosis is thus evaluated.
4. Results
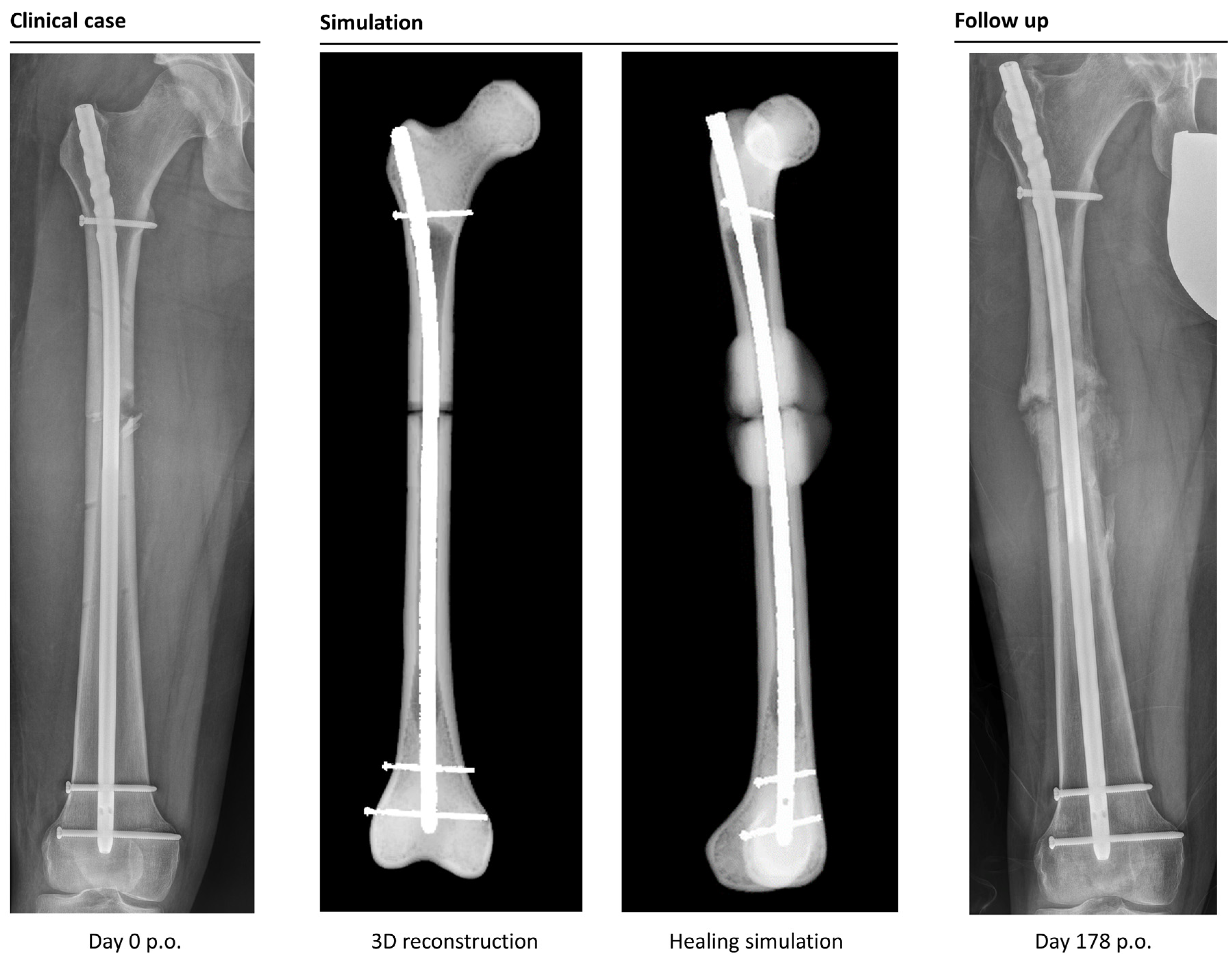
All patients were treated between 2010 and 2020 in a level 1 trauma center in Germany. Applying the above-mentioned inclusion and exclusion criteria, we identified 32 suitable cases. We decided to focus on osteosynthesis with LFN, PFNA long, and FRN (DePuy, Synthes, Johnson & Johnson Services, New Brunswick, NJ, USA) and excluded 5 patients who were treated with nails from other manufacturers (AFN, Trigen). One patient could not be simulated due to meshing problems, and one patient had to be excluded because of cerclages and one due to insufficient X-ray data. Simulating cerclages is per se possible, but the positioning of the cerclages is dependent on the fracture configuration, so there is no standardization for implantation. Therefore, including cerclages would have increased the amount of time needed for simulation significantly.
In summary, 27 patients (7 females, 20 males), aged 16–60 with a mean age of 30.4 ± 14.5 years could be evaluated, including 10 right and 17 left femora. The mean patient height was 173.4 ± 12.8 cm, and the mean patient weight was 80.4 ± 18.3 kg, with a mean BMI of 26.5 ± 5.4 kg/m2. A total of 17 patients fractured their femur during high-velocity traffic accidents; 1 trauma mechanism was unknown; the other fractures occurred due to falls.
In total, 32 simulations were performed. The 29 LFN, 1 FRN, and 2 PFNA were used for osteosynthesis. Clinically, in 25 cases, healing took place without problems; in 7 cases, non-union occurred, including the 5 non-union patients who were simulated again, according to the new osteosynthesis, after revision was performed. After revision, 4 patients healed without further complications; 1 patient developed non-union again (
Figure 3).
The simulation classified 26 cases as “healing”, while it categorized 6 cases as non-unions. In retrospective comparison with the clinical outcomes, the simulation correctly predicted 23 uncomplicated healing fractures. Three patients would have been expected to heal according to the simulation, but clinically turned out as non-unions (false negatives). Four out of six predicted non-unions were correctly recognized by the simulation, but two cases were wrongfully diagnosed as developing non-unions (false positives). This means the simulation correctly predicted the right outcome in 85% of the cases (
Figure 4 and
Figure 5).
To compare virtual and clinical outcomes, the mRUST score was evaluated for all cases. “Healing” was defined as the first time an mRUST score over 9 was reached, which means a bridging of at least 3 cortices. The mean mRUST score was 9.5, whereas the time of follow-up X-rays varied in such a range that no direct comparison is possible (
Figure 6).
For the 23 cases where the simulation correctly predicted consolidation, according to the simulation, average consolidation took place 189.5 ± 61.4 days after surgery. Due to the retrospective comparison, no definitive clinical healing time can be calculated, and therefore a direct comparison is not possible. Clinical consolidation took place from days 120 to 626 after surgery, so all healed patients developed an mRUST score of at least 9 during this time (see
Figure 7).
A further aim of the study was the comparison of the bridging times predicted by the simulation with the clinically observed ones. For 16 of these 23 cases (P01–P16), the predicted healing time point was compatible with the clinical consolidation time range that could be deduced from the retrospective radiographic data. In five cases (P17–P23), clinical healing was faster than the simulated healing, and in two cases, it was slower.
5. Discussion
In this study, to the best of our knowledge, the Ulm fracture-healing model was used for the first time in a clinical context to predict non-unions. After further adjustment of the calculation on human bones, this system could be used as an individualized approach to early detection of delayed or non-union. Clinical intervention could be derived, or alternatively delayed, in cases of retarded but late healing.
Clinical consolidation was correctly predicted in 23 cases; 3 cases were wrongfully diagnosed as healing in the simulation but resulted in non-union clinically. In the retrospective analysis of the cases, no general, documented clinical deviation could be detected. In these cases, solely relying on the simulation would have led to an overly optimistic estimation of the healing chances. Aside from shortcomings of the simulation model, possible extrinsic reasons for non-detection could be undocumented patient-specific factors impairing fracture healing, e.g., unreported nicotine consumption or incompliance concerning weight-bearing. Patient compliance concerning weight-bearing as well as physiotherapeutic exercise contributes to healing. This compliance has not been observed, and actual loading can only be estimated by the simulation.
Furthermore, a need for further adaptation and improvement of the model is under discussion. For instance, the simulation assumes the same muscle insertion points for all individuals as well as the same muscle force distribution. This may also contribute to inexact results. For example, musculoskeletal forces are calculated indirectly, and direct measurement in vivo is not possible. Additionally, the initial soft tissue distribution and vascularization can only be estimated and approximately calculated.
A further modification of the model for compromised bones (e.g., poor bone quality caused by osteoporosis) might be necessary in order to predict healing in severe cases of osteoporosis. After speeding up the process of simulation, large numbers of patients with different conditions impairing fracture consolidation could be analyzed to take co-morbidities into consideration. At this time, further approaches are being made by other scientific groups to adjust fracture healing algorithms, e.g., in humans with diabetes [
18]. Integration of these findings into the simulation is possible in principle.
Concerning the six cases where the simulation predicted a non-union, four matched the clinical outcome. Taking a closer look at the two falsely detected non-union cases, a possible reason is the fracture configuration. Patients 24 and 25 both showed fractures with high distortional strain and, therefore, prolonged vascularization of the formed callus in the simulation. In both cases, the clinical course showed a rather large callus formation but a normal consolidation time. A possible explanation may be, on the one hand, an overestimated loading and, on the other hand, underestimated osteosynthesis stability. A second possible explanation might be that the model allows a too small window of distortional strain. As published by Dailey et al., the tissue destruction cutoff for distortional strain seems to have to be increased from the previously published cutoff of 0.17 to an upper limit of 1.0 to accurately depict the actual influence of distortional strains [
18]. This issue might be an artifact of deriving the differentiation rules from animal models. Further adjustment may be necessary to adapt the model to human bone healing.
The average time for the bridging of three cortices according to the simulation was 184 days, which is longer than the clinically expected 6 to 12 weeks. Yet, comparing the radiologically determined healing intervals to the healing time points predicted by the simulation model, in 16 out of 23 cases, the predicted consolidation speed was compatible with the clinical data. Due to the retrospective character of the study, X-ray intervals varied widely between cases, and further narrowing down the time of actual consolidation was not possible. In six cases, consolidation took place earlier than predicted by the simulation. We concluded that this may also indicate the necessity of adapting the calculation to human healing potential. Narrowing down the time of radiologic healing may help to further evaluate the exactness of the simulation. Due to the retrospective character of this study, this has proven difficult due to the lack of follow-up examinations or the large amounts of time between X-rays. This could be further evaluated by a prospective study and an extension of the patient collective.
There is a lack of certain tools to assess fracture healing. Currently, the evaluation of the progress of fracture healing is mainly based on clinical and radiological findings as well as on surgeons’ experience. Especially in the so called “forgotten phase” (the time 6 to 9 months after surgery), in which delayed union cannot be detected clinically or radiologically, surgical intervention is stalled. This leads to decelerated treatment [
32].
Using radiology as the main tool to detect non-union is only possible during healing, and a reliable prediction of consolidation is not possible. X-rays to assess fracture healing are a cheap and quick method, but nevertheless, various studies show inaccurate and inconclusive findings concerning the diagnosis of non-union, as well as a strong dependence on the viewer’s experience. Scoring systems such as RUSH and RUST scores have been established to create a comparable and reliable score for radiological assessment of fracture healing, but their accuracy is still under evaluation [
12]. CT offers high sensitivity to detect non-unions but low specificity. Due to cost, radiation dose, and metal artifacts, diminishing the accuracy, CT diagnostics is not a widespread way for early detection of non-union [
13]. Ultrasound, as used by Moed et al. in tibial fractures, is not tested for femur fractures [
14]. DCE-MRI perfusion analysis after revision surgery was shown to successfully predict the postoperative outcome but is not common and expensive [
33].
Currently, scientific approaches to measuring fracture healing include, e.g., implanted magnetoelastic wireless electronic devices, analysis of vibrations through the bone, and fluidic X-ray-sensitive sensors attached to osteosynthesis material. None of these methods is practical or fit for widespread clinical use, and they have not yet been adequately tested [
15].
As serological markers for fracture healing, bone-specific alkaline phosphatase (BALP), procollagen type-III N-terminal propeptide (PIIINP), procollagen type-I C-terminal propeptide (PICP), and osteocalcin (OC) are often discussed. Further evaluation and interpretation of these findings in the context of non-union development is under current examination [
16,
17].
At this point, none of the above-mentioned conventional and experimental tools can reliably predict non-unions early enough. Using big data may be a solution to the problem of early detection.
This study was performed as a feasibility approach to use the Ulm fracture healing model to simulate consolidation. The model has been used for different scientific questions but has never been used on clinical human data [
28,
34,
35]. Ideally, we would like to obtain detailed data about the temporal evolution of the different tissue types at each point of the fracture site in order to enable a quantitative comparison of the primary simulation output with reality. Unfortunately, obtaining such data, retrospectively, is currently not feasible. We therefore focused the discussion on derived predictions, such as classifying union/non-union cases, that can readily be compared to clinical data. One obvious limitation of this approach is that the predicted evolution of tissue distribution, callus growth, and calcification remains hypothetical. Perhaps more serious, however, is the fact that it does not allow us to infer why the model might have failed in a specific case, making it virtually impossible to use the outcome of this study to improve the model.
While it might be tempting to replace complex tissue differentiation models with their many unknown parameters with a simpler model that is easier to validate, it is not a straightforward task to define such an indicator. For instance, when applying Perren’s idea of IFS to the data set at hand, we did not find any significant difference between the groups of healing/non-union cases with respect to initial IFS. Again, given enough data, it is conceivable to identify more complex patterns of the initial biomechanical condition (e.g., via machine learning) that are indeed useful predictors of the healing outcome, but that comes at the expense of replacing a simulation model that is built on first principles and could—at least in theory—offer some explanation for its predictions with an opaque black box model.
Tibial non-unions are more often reported than femoral non-unions, but they are mostly accompanied by or caused by extensive soft tissue damage. To focus on the biomechanical aspect of the model, we decided to focus on femur fractures before including tibial fractures. Additionally, the medical relevance of femoral non-unions is given by the relevant impact on patients’ health caused by misdiagnosis or delayed diagnosis of non-unions concerning femur fractures. Geometric variation and study data were sufficient to use femora as a starting point.
A limitation of this study is the use of biplanar X-rays to assess the initial fracture geometry. Complicated or multi-fragment fractures can only be reconstructed approximatively. To recreate the real geometry, CT data would be necessary. Yet, CT imaging is not needed for definitive treatment and therefore only exists in rare cases or to detect delayed union.
Furthermore, given the limited amount of information we had available about each case and given that we explicitly excluded patients with comorbidities that are known to influence fracture healing specifically, such as patients with osteoporosis or smokers, we assumed the same “biology” (i.e., tissue remodeling rates, sensitivity to stimuli) for all patients.
Regarding patient-specific loading, we assumed full weight-bearing for all cases throughout the healing process. It is known that patients are typically not able to follow conventional loading recommendations [
36]. By treating all cases equally, we avoid introducing artificial bias in individual cases. Assuming that biomechanically induced non-unions in the case of fractures treated with an intramedullary nail tend to be a consequence of insufficient stability, we implicitly increase the sensitivity of the model for detecting non-union cases, with the trade-off of an increased false-positive rate.
After further re-evaluation and testing of the model on a larger collective, we are positive that it is possible to assess the risk for non-unions using our simulation. Further work must be conducted to accelerate the speed of calculation to make the model usable for clinical work as a diagnostic tool for personalized treatment. One possibility of use might be to evaluate the risk of non-union directly after surgery to prevent long-term disability and pain for the patient and evaluate the need for immediate revision. To achieve this goal, further studies with larger sample sizes need to be conducted in order to tune the model further to human differentiation rates, as it was originally established and calibrated using in vivo data from sheep and mouse models.
Within the hospital workflow, another benefit, apart from detecting non-unions early on, might be in planning further examinations and giving recommendations for weight bearing. For patients with a positive prognosis for successful healing, it might be possible to extend the intervals of routine control, reducing radiation exposure. Finally, an intraoperative risk assessment would enable surgeons to adjust the treatment plan during the surgery based on real-time information, thereby increasing the precision and effectiveness of the procedure. Furthermore, visualizing the healing process may also help increase patient compliance.
As a further step, it might be possible to develop a tool for preoperative estimation of healing potential depending on different osteosynthesis options, e.g., nail diameter, dynamic vs. static locking, or decision nail vs. plate, even before the surgical intervention takes place.