Association between Triglyceride-Glucose Index and Early Neurological Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke
Abstract
:1. Introduction
2. Methods
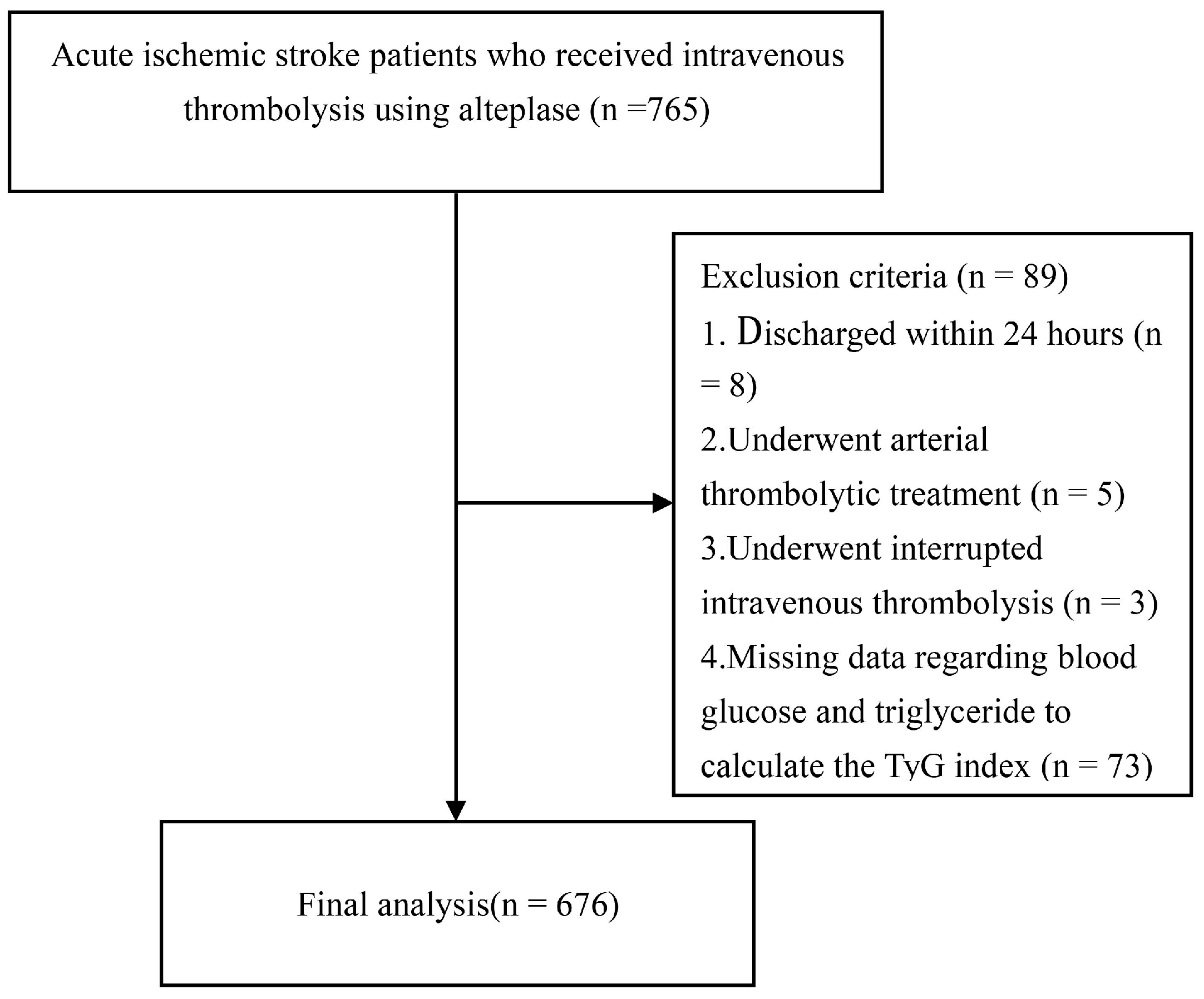
2.1. Study Design and Participants
2.2. Data Acquisition
2.3. Triglyceride Glucose (TyG) Index Evaluation
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. TyG Index and END
3.3. TyG Index and ENI
3.4. TyG Index and NIHSS Score Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Michael, B.; Demaerschalk, B.M.; Hoh, B.; et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Neurology, C.; Society, C.S. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin. J. Neurol. 2018, 51, 666–682. [Google Scholar]
- Emberson, J.; Lees, K.R.; Lyden, P.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke A meta-analysis of individual patient data from randomised trials. Lancet 2014, 384, 1929–1935. [Google Scholar] [PubMed]
- Whiteley, W.N.; Emberson, J.; Lees, K.R.; Blackwell, L.; Albers, G.; Bluhmki, E.; Brott, T.; Cohen, G.; Davis, S.; Donnan, G.; et al. Stroke Thrombolysis Trialists’ Collaboration. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke A secondary analysis of an individual patient data meta-analysis. Lancet Neurol. 2016, 15, 925–933. [Google Scholar] [CrossRef]
- Seners, P.; Turc, G.; Oppenheim, C.; Baron, J.C. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke A systematic review with pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 2015, 86, 87–94. [Google Scholar] [CrossRef]
- Weyland, C.S.; Mokli, Y.; Vey, J.A.; Kieser, M.; Herweh, C.; Schönenberger, S.; Bendszus, M.; Möhlenbruch, M.A.; Ringleb, P.A.; Nagel, S. Predictors for Failure of Early Neurological Improvement After Successful Thrombectomy in the Anterior Circulation. Stroke 2021, 52, 1291–1298. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. NESS-China Investigators. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480,687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Yeo, L.L.; Paliwal, P.; Teoh, H.L.; Seet, R.C.; Chan, B.P.; Wakerley, B.; Liang, S.; Rathakrishnan, R.; Chong, V.F.; Ting, E.Y.S.; et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, e590–e596. [Google Scholar] [CrossRef]
- Nagpal, M.; De, D.; Handa, S.; Pal, A.; Sachdeva, N. Insulin Resistance and Metabolic Syndrome in Young Men with Acne. JAMA Dermatol. 2016, 152, 399–404. [Google Scholar]
- Holden, S.E.; Currie, C.J. Endogenous hyperinsulinaemia and exogenous insulin A common theme between atherosclerosis, increased cancer risk and other morbidities. Atherosclerosis 2012, 222, 26–28. [Google Scholar] [CrossRef]
- Lee, M.; Saver, J.L.; Hong, K.S.; Song, S.; Chang, K.H.; Ovbiagele, B. Effect of pre-diabetes on future risk of stroke Meta-analysis. BMJ 2012, 344, e3564. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Alizargar, J.; Bai, C.H.; Hsieh, N.C.; Wu, S.V. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc. Diabetol. 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ahn, C.W.; Lee, B.K.; Kang, S.; Nam, J.S.; You, J.H.; Kim, M.J.; Kim, M.K.; Park, J.S. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc. Diabetol. 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, L.; Shao, Q.; Cheng, Y.; Lv, S.; Sun, Y.; Shen, H.; Wang, Z.; Zhou, Y.; Liu, X.; et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 31. [Google Scholar] [CrossRef]
- Kim, J.; Shin, S.J.; Kang, H.T. The association between triglyceride-glucose index, cardio-cerebrovascular diseases, and death in Korean adults: A retrospective study based on the NHIS-HEALS cohort. PLoS ONE 2021, 16, e0259212. [Google Scholar] [CrossRef]
- Kwon, H.M.; Lim, J.S.; Park, H.K.; Lee, Y.S. Hypertriglyceridemia as a possible predictor of early neurological deterioration in acute lacunar stroke. J. Neurol. Sci. 2011, 309, 128–130. [Google Scholar] [CrossRef]
- Nair, S.B.; Somarajan, D.; Pillai, R.K.; Balachandran, K.; Sathian, S. Predictors of Early Neurological Deterioration Following Intravenous Thrombolysis: Difference between Risk Factors for Ischemic and Hemorrhagic Worsening. Ann. Indian Acad Neurol. 2022, 25, 627–633. [Google Scholar]
- Si, S.; Li, J.; Li, Y.; Li, W.; Chen, X.; Yuan, T.; Liu, C.; Li, H.; Hou, L.; Wang, B.; et al. Causal Effect of the Triglyceride-Glucose Index and the Joint Exposure of Higher Glucose and Triglyceride With Extensive Cardio-Cerebrovascular Metabolic Outcomes in the UK Biobank: A Mendelian Randomization Study. Front. Cardiovasc. Med. 2021, 7, 583473. [Google Scholar] [CrossRef]
- Gökçal, E.; Niftaliyev, E.; Asil, T. Etiological classification of ischemic stroke in young patients A comparative study of TOAST, CCS, and ASCO. Acta Neurol. Belg. 2017, 117, 643–648. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab. Syndr. Relat. Disord. 2008, 6, 299–304. [Google Scholar] [CrossRef]
- Vynckier, J.; Maamari, B.; Grunder, L.; Goeldlin, M.B.; Meinel, T.R.; Kaesmacher, J.; Hakim, A.; Arnold, M.; Gralla, J.; Seiffge, D.J.; et al. Early Neurologic Deterioration in Lacunar Stroke: Clinical and Imaging Predictors and Association with Long-term Outcome. Neurology 2021, 16, 1212. [Google Scholar] [CrossRef]
- Seners, P.; Hassen, W.B.; Lapergue, B.; Arquizan, C.; Heldner, M.R.; Henon, H.; Perrin, C.; Strambo, D.; Cottier, J.P.; Sablot, D.; et al. MINOR-STROKE Collaborators. Prediction of Early Neurological Deterioration in Individuals With Minor Stroke and Large Vessel Occlusion Intended for Intravenous Thrombolysis Alone. JAMA Neurol. 2021, 78, 321–328. [Google Scholar] [CrossRef]
- Knüppel, S.; Stang, A. DAG Program: Identifying Minimal Sufficient Adjustment Sets. Epidemiology 2010, 21, 159. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, X.; Gong, Y.; Liu, Y.; Wang, S.; Li, Z.; Chen, W.; Zhou, F.; Zhou, J.; Jiang, T.; et al. A novel nomogram to predict early neurological deterioration in patients with acute ischaemic stroke. Eur. J. Neurol. 2020, 27, 1996–2005. [Google Scholar] [CrossRef]
- Seners, P.; Turc, G.; Tisserand, M.; Legrand, L.; Labeyrie, M.A.; Calvet, D.; Meder, J.F.; Mas, J.L.; Oppenheim, C.; Baron, J.C.; et al. Unexplained early neurological deterioration after intravenous thrombolysis Incidence, predictors, and associated factors. Stroke 2014, 45, 2004–2009. [Google Scholar] [CrossRef]
- Simonsen, C.Z.; Schmitz, M.L.; Madsen, M.H.; Mikkelsen, I.K.; Chandra, R.V.; Leslie-Mazwi, T.; Anderse, G. Early neurological deterioration after thrombolysis: Clinical and imaging predictors. Int. J. Stroke 2016, 11, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Hu, H.H.; Chao, H.L.; Ho, B.L.; Chen, C.H.; Chan, L.; Lin, H.J.; Sun, Y.; Lin, Y.Y.; Chen, P.L.; et al. Triglyceride-Glucose Index and Intravenous Thrombolysis Outcomes for Acute Ischemic Stroke: A Multicenter Prospective-Cohort Study. Front. Neurol. 2022, 13, 737441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, L.; Ruan, H.; Zhu, Q.; Yu, D.; Yang, Y.; Men, X.; Lu, Z. Triglyceride-Glucose Index Linked to Hospital Mortality in Critically Ill Stroke: An Observational Multicentre Study on eICU Database. Front. Med. 2020, 7, 591036. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Lee, Y.S. High triglyceride-glucose index is associated with early recurrent ischemic lesion in acute ischemic stroke. Sci. Rep. 2021, 11, 15335. [Google Scholar] [CrossRef]
- Toh, E.M.S.; Lim, A.Y.L.; Ming, C.; Yeo, L.L.L.; Sia, C.H.; Tan, B.W.Q.; Leow, A.S.T.; Ho, J.S.Y.; Chan, B.P.L.; Sharma, V.K.; et al. Association of triglyceride-glucose index with clinical outcomes in patients with acute ischemic stroke receiving intravenous thrombolysis. Sci. Rep. 2022, 12, 1596. [Google Scholar] [CrossRef] [PubMed]
- Palomo, I.; Alarcón, M.; Moore-Carrasco, R.; Argilés, J.M. Hemostasis alterations in metabolic syndrome (Review). Int. J. Mol. Med. 2006, 18, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Juhan-Vague, I. Metabolic syndrome, haemostasis and thrombosis. Thromb. Haemost. 2008, 99, 995–1000. [Google Scholar] [PubMed]
- Calleja, A.I.; García-Bermejo, P.; Cortijo, E.; Bustamante, R.; Martínez, E.R.; Sarmiento, E.G.; Fernández-Herranz, R.; Arenillas, J.F. Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care 2011, 34, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Cymbalista, C.M.; Spector, T.D.; Grant, P.J. EuroCLOT Investigators. Heritability of clot formation, morphology, and lysis the EuroCLOT study. Arterioscler Thromb. Vasc. Biol. 2007, 27, 2783–2789. [Google Scholar] [CrossRef]
- Juutilainen, A.; Kortelainen, S.; Lehto, S.; Rönnemaa, T.; Pyörälä, K.; Laakso, M. Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 2004, 27, 2898–2904. [Google Scholar] [CrossRef]
- Tiano, J.P.; Mauvais-Jarvis, F. Importance of oestrogen receptors to preserve functional β–cell mass in diabetes. Nat. Rev. Endocrinol. 2012, 8, 342–351. [Google Scholar] [CrossRef]
- Duncan, A.C.; Lyall, H.; Roberts, R.N.; Petrie, J.R.; Perera, M.J.; Monaghan, S.; Hart, D.M.; Connell, J.M.C.; Lumsden, M.A. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy pos-menopausal women. J. Clin. Endocrinol. Metab. 1999, 84, 2402–2407. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Rodríguez-Gutiérrez, R.; Mancillas-Adame, L.; González-Nava, V.; González-Colmenero, A.D.; Solis, R.C.; Álvarez-Villalobos, N.A.; González-González, J.G. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef]
| Variable | Total (n = 676) | Tertile I (Lowest) (n = 226) | Tertile II (Medium) (n = 227) | Tertile III (Highest) (n = 223) | p for Trend |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, y, median (IQR) | 68 (60–76) | 70 (62–77) | 68 (59–75) | 67 (59–74) | 0.064 |
| Sex, n (%) | 0.118 | ||||
| Male, n (%) | 432 (63.9) | 89 (39.4) | 83 (36.6) | 72 (32.3) | |
| Female, n (%) | 244 (36.1) | 137 (60.6) | 144 (63.4) | 151 (67.7) | |
| Vascular risk factors | |||||
| Current smoker, n (%) | 198 (29.3) | 61 (27.0) | 64 (28.2) | 73 (32.7) | 0.153 |
| Regular alcohol user, n (%) | 62 (9.2) | 12 (5.3) | 24 (10.6) | 26 (11.7) | 0.034 |
| Previous stroke, n (%) | 102 (15.1) | 41 (18.1) | 33 (14.5) | 28 (12.6) | 0.098 |
| Hypertension, n (%) | 448 (66.3) | 143 (63.3) | 156 (68.7) | 149 (66.8) | 0.426 |
| Diabetes, n (%) | 142 (21.0) | 31 (13.7) | 48 (21.1) | 63 (28.3) | <0.001 |
| Dyslipidemia, n (%) | 207 (30.6) | 42 (18.6) | 55 (24.2) | 110 (49.3) | <0.001 |
| Atrial fibrillation, n (%) | 210 (31.1) | 78 (34.5) | 68 (30.0) | 64 (28.7) | 0.183 |
| Coronary artery disease, n (%) | 81 (12.0) | 20 (8.8) | 30 (13.2) | 31 (13.9) | 0.099 |
| Medication use history | |||||
| Previous antiplatelet, n (%) | 75 (11.1) | 29 (12.8) | 27 (11.9) | 19 (8.5) | 0.147 |
| Previous anticoagulants, n (%) | 14 (2.1) | 1 (0.4) | 10 (4.4) | 3 (1.3) | 0.495 |
| Previous statin, n (%) | 50 (7.4) | 24 (10.6) | 15 (6.6) | 11 (4.9) | 0.021 |
| Previous antihypertension, n (%) | 264 (39.1) | 85(37.6) | 99 (43.6) | 80 (35.9) | 0.711 |
| Previous hypoglycemic agents, n (%) | 81 (12.0) | 20 (8.8) | 25 (11.0) | 36 (16.1) | 0.018 |
| Clinical assessment | |||||
| Initial NIHSS score, median (IQR) | 6 (3–12) | 7 (3–12) | 6 (4–12) | 6 (3–12) | 0.527 |
| Discharge NIHSS score, median (IQR) | 3 (1–7) | 3 (1–6) | 3 (1–7) | 2 (0–7) | 0.221 |
| SBP, mmHg, mean ± SD | 149 ± 23 | 150 ± 25 | 148 ± 22 | 149 ± 22 | 0.726 |
| DBP, mmHg, median (IQR) | 89 (80–98) | 90 (80–98) | 87 (80–98) | 90 (80–99) | 0.285 |
| OTT, minute, median (IQR) | 180 (120–210) | 180 (120–210) | 180 (120–211) | 180 (120–210) | 0.928 |
| Thrombectomy treatment, n (%) | 93 (13.8) | 30 (13.3) | 33 (14.5) | 30 (13.5) | 1.000 |
| 24 h sICH, n (%) | 28 (4.1) | 9 (4.0) | 4 (1.8) | 15 (6.7) | 0.147 |
| Any ICH, n (%) | 127(18.9) | 45 (19.9) | 39 (17.2) | 43 (19.3) | 0.862 |
| ASCO Stroke subtype | 0.454 | ||||
| Atherosclerosis, n (%) | 250 (37.0) | 72(31.9) | 92 (40.5) | 86 (38.6) | |
| Cardioembolic, n (%) | 211 (31.2) | 78 (34.5) | 70 (30.8) | 63 (28.3) | |
| Small vessel disease, n (%) | 70 (10.4) | 25 (11.1) | 20 (8.8) | 25 (11.2) | |
| Other causes, n (%) | 145 (21.4) | 51 (22.6) | 45 (19.8) | 49 (22.0) | |
| Laboratory data | |||||
| FBG, mg/dL, median (IQR) | 102.9 (88.6–124.3) | 93.2 (84.2–107.6) | 101.7 (88.7–115.5) | 119.3 (100.1–164.8) | <0.001 |
| TG, mg/dL, median (IQR) | 99.2 (69.1–144.4) | 60.3 (46.0–73.5) | 105.4 (88.6–121.3) | 174.4 (133.2–231.2) | <0.001 |
| TC, mg/dL, median (IQR) | 173.3 (144.4–200.7) | 165.4 (132.7–191.0) | 168.4 (142.9–195.1) | 186.5 (159.4–214.8) | <0.001 |
| LDL, mg/dL, median (IQR) | 114.1 (87.1–139.2) | 106.2 (78.1–125.7) | 114.1 (89.4–136.7) | 130.3 (98.1–150.2) | <0.001 |
| Early Neurological Outcome | |||||
| END2 | 89 (13.2) | 21 (9.3) | 21 (9.3) | 47 (21.1) | <0.001 |
| END4 | 61 (9.0) | 13 (5.8) | 14 (6.2) | 34 (15.2) | <0.001 |
| ENI | 492 (72.7) | 171 (75.7) | 171 (75.3) | 150 (67.3) | 0.046 |
| Variable | Without END2 (n = 587) | With END2 (n = 89) | p Value | Without END4 (n = 615) | With END4 (n = 61) | p Value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age, y, median (IQR) | 68 (59–75) | 70 (62–76) | 0.048 | 68 (59–76) | 70 (67–76) | 0.019 |
| Sex, n (%) | 0.790 | 0.784 | ||||
| Male, n (%) | 374 (63.7) | 58 (65.2) | 394 (64.1) | 38 (62.3) | ||
| Female, n (%) | 213 (36.3) | 31 (34.8) | 221 (35.9) | 23 (37.7) | ||
| Vascular risk factors | ||||||
| Current smoker, n (%) | 170 (29.0) | 28 (31.5) | 0.629 | 181 (29.4) | 17 (27.9) | 0.798 |
| Regular alcohol user, n (%) | 54 (9.2) | 8 (9.0) | 0.949 | 59 (9.6) | 3 (4.9) | 0.228 |
| Previous stroke, n (%) | 94 (16.0) | 8 (9.0) | 0.084 | 95 (15.4) | 7 (11.5) | 0.408 |
| Hypertension, n (%) | 383 (65.2) | 65 (73.0) | 0.148 | 406 (66.0) | 42 (68.9) | 0.655 |
| Diabetes, n (%) | 115 (19.6) | 27 (30.3) | 0.020 | 120 (19.5) | 22 (36.1) | 0.002 |
| Dyslipidemia, n (%) | 179 (30.5) | 28 (31.5) | 0.854 | 193 (31.4) | 14 (23.0) | 0.173 |
| Atrial fibrillation, n (%) | 161 (27.4) | 49 (55.1) | <0.001 | 168 (27.3) | 42 (68.9) | <0.001 |
| Coronary artery disease, n (%) | 64 (10.9) | 17 (19.1) | 0.026 | 67 (10.9) | 14 (23.0) | 0.006 |
| Medication use history | ||||||
| Previous antiplatelet, n (%) | 63 (10.7) | 12 (13.5) | 0.441 | 66 (10.7) | 9 (14.8) | 0.340 |
| Previous anticoagulants, n (%) | 13 (2.2) | 1 (1.1) | 0.784 | 13 (2.1) | 1 (1.6) | 0.804 |
| Previous statin, n (%) | 45 (7.7) | 5 (5.6) | 0.491 | 46 (7.5) | 4 (6.6) | 0.793 |
| Previous antihypertension, n (%) | 228 (38.8) | 36 (40.4) | 0.772 | 239 (38.9) | 25 (41.0) | 0.746 |
| Previous hypoglycemic agents, n (%) | 63 (10.7) | 18 (20.2) | 0.010 | 66 (10.7) | 15 (24.6) | <0.001 |
| Clinical assessment | ||||||
| Initial NIHSS score, median (IQR) | 6 (3–12) | 8 (4–12) | 0.102 | 6 (3–12) | 12 (7–15) | <0.001 |
| Discharge NIHSS score, median (IQR) | 4 (2–9) | 16 (8–24) | <0.001 | 4 (2–9) | 18 (15–29) | <0.001 |
| SBP, mmHg, mean ± SD | 149 ± 22 | 151 ± 26 | 0.621 | 149 ± 22 | 151 ± 28 | 0.476 |
| DBP, mmHg, median (IQR) | 89 (80–98) | 90 (80–90) | 0.533 | 89 (80–98) | 87 (78–99) | 0.922 |
| OTT, minute, median (IQR) | 180 (120–210) | 169 (120–228) | 0.513 | 180 (120–210) | 160 (120–220) | 0.206 |
| Thrombectomy treatment, n (%) | 65 (11.1) | 28 (31.5) | <0.001 | 68 (11.1) | 25 (41.0) | <0.001 |
| 24 h sICH, n (%) | 0 (0.0) | 28 (31.5) | <0.001 | 0 (0.0) | 28 (45.9) | <0.001 |
| Any ICH, n (%) | 87 (14.8) | 40 (44.9) | <0.001 | 92 (15.0) | 45 (57.4) | <0.001 |
| ASCO Stroke subtype | <0.001 | <0.001 | ||||
| Atherosclerosis, n (%) | 222 (37.9) | 28 (32.5) | 232 (37.7) | 18 (29.5) | ||
| Cardioembolic, n (%) | 167 (27.3) | 44 (50.6) | 172 (28.0) | 39 (63.9) | ||
| Small vessel disease, n (%) | 60 (8.5) | 10 (9.6) | 67 (10.9) | 3 (4.9) | ||
| Other causes, n (%) | 138 (26.3) | 7 (7.2) | 144 (23.4) | 1 (1.6) | ||
| Laboratory data | ||||||
| FBG, mg/dL, median (IQR) | 101.3 (87.9–119.2) | 121.9 (95.2–177.7) | <0.001 | 100.8 (87.8–119.2) | 136.5 (110.3–201.6) | <0.001 |
| TG, mg/dL, median (IQR) | 97.5 (69.1–144.4) | 103.6 (77.9–143.4) | 0.305 | 99.2 (69.1–147.0) | 98.3 (77.0–129.3) | 0.712 |
| TC, mg/dL, median (IQR) | 173.2 (144.2–200.0) | 175.3 (145.4–207.3) | 0.643 | 173.4 (144.7–200.7) | 168.6 (138.1–199.9) | 0.649 |
| LDL, mg/dL, median (IQR) | 113.3 (86.4–138.7) | 120.3 (95.1–141.9) | 0.178 | 113.7 (87.0–139.4) | 118.3 (92.8–139.2) | 0.757 |
| TyG index, mean ± SD | 8.58 ± 0.69 | 8.87 ± 0.68 | <0.001 | 8.59 ± 0.69 | 8.90 ± 0.68 | <0.001 |
| TyG tertiles | <0.001 | <0.001 | ||||
| Lowest, n (%) | 205 (34.9) | 21 (23.6) | 213 (34.6) | 13 (21.3) | ||
| Medium, n (%) | 206 (35.1) | 21 (23.6) | 213 (34.6) | 14 (23.0) | ||
| Highest, n (%) | 176 (30.0) | 47 (52.8) | 189 (30.7) | 34 (55.7) |
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| TyG index | 1.77 (1.30–2.42) | <0.001 | 1.91 (1.38–2.66) | <0.001 | 1.88 (1.37–2.58) | <0.001 |
| TyG tertiles | <0.001 | <0.001 | <0.001 | |||
| Lowest | Ref | Ref | Ref | |||
| Medium | 1.00 (0.53–1.88) | 1.05 (0.54–2.02) | 1.06 (0.56–2.02) | |||
| Highest | 2.61 (1.50–4.53) | 2.94 (1.64–5.27) | 2.82 (1.61–4.95) | |||
| TyG binary classification | ||||||
| Lowest to medium | Ref | Ref | Ref | |||
| Highest | 2.61 (1.66–4.11) | <0.001 | 2.87 (1.78–4.62) | <0.001 | 2.74 (1.73–4.33) | <0.001 |
| Unadjusted | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| TyG index | 1.80 (1.26–2.57) | 0.001 | 2.07 (1.40–3.07) | <0.001 | 1.99 (1.37–2.87) | <0.001 |
| TyG tertiles | <0.001 | <0.001 | <0.001 | |||
| Lowest | Ref | Ref | Ref | |||
| Medium | 1.08 (0.49–2.35) | 1.21 (0.54–2.74) | 1.20 (0.55–2.62) | |||
| Highest | 2.95 (1.51–5.75) | 3.80 (1.85–7.79) | 3.37 (1.71–6.65) | |||
| TyG binary classification | ||||||
| Lowest to medium | Ref | Ref | Ref | |||
| Highest | 2.84 (1.67–4.84) | <0.001 | 3.45 (1.94–6.12) | <0.001 | 3.08 (1.79–5.30) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Lei, H.; Ambler, G.; Werring, D.J.; Fang, S.; Li, H.; Chen, R.; Wei, J.; Chen, G.; Liu, N.; et al. Association between Triglyceride-Glucose Index and Early Neurological Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke. J. Clin. Med. 2023, 12, 3471. https://doi.org/10.3390/jcm12103471
Zhang B, Lei H, Ambler G, Werring DJ, Fang S, Li H, Chen R, Wei J, Chen G, Liu N, et al. Association between Triglyceride-Glucose Index and Early Neurological Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke. Journal of Clinical Medicine. 2023; 12(10):3471. https://doi.org/10.3390/jcm12103471
Chicago/Turabian StyleZhang, Baixiang, Hanhan Lei, Gareth Ambler, David J. Werring, Shuangfang Fang, Hangfeng Li, Ronghua Chen, Jin Wei, Guangliang Chen, Nan Liu, and et al. 2023. "Association between Triglyceride-Glucose Index and Early Neurological Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke" Journal of Clinical Medicine 12, no. 10: 3471. https://doi.org/10.3390/jcm12103471
APA StyleZhang, B., Lei, H., Ambler, G., Werring, D. J., Fang, S., Li, H., Chen, R., Wei, J., Chen, G., Liu, N., & Du, H. (2023). Association between Triglyceride-Glucose Index and Early Neurological Outcomes after Thrombolysis in Patients with Acute Ischemic Stroke. Journal of Clinical Medicine, 12(10), 3471. https://doi.org/10.3390/jcm12103471







