Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. OCTA Findings before and after Intravitreal Injection of Triamcinolone Acetonide
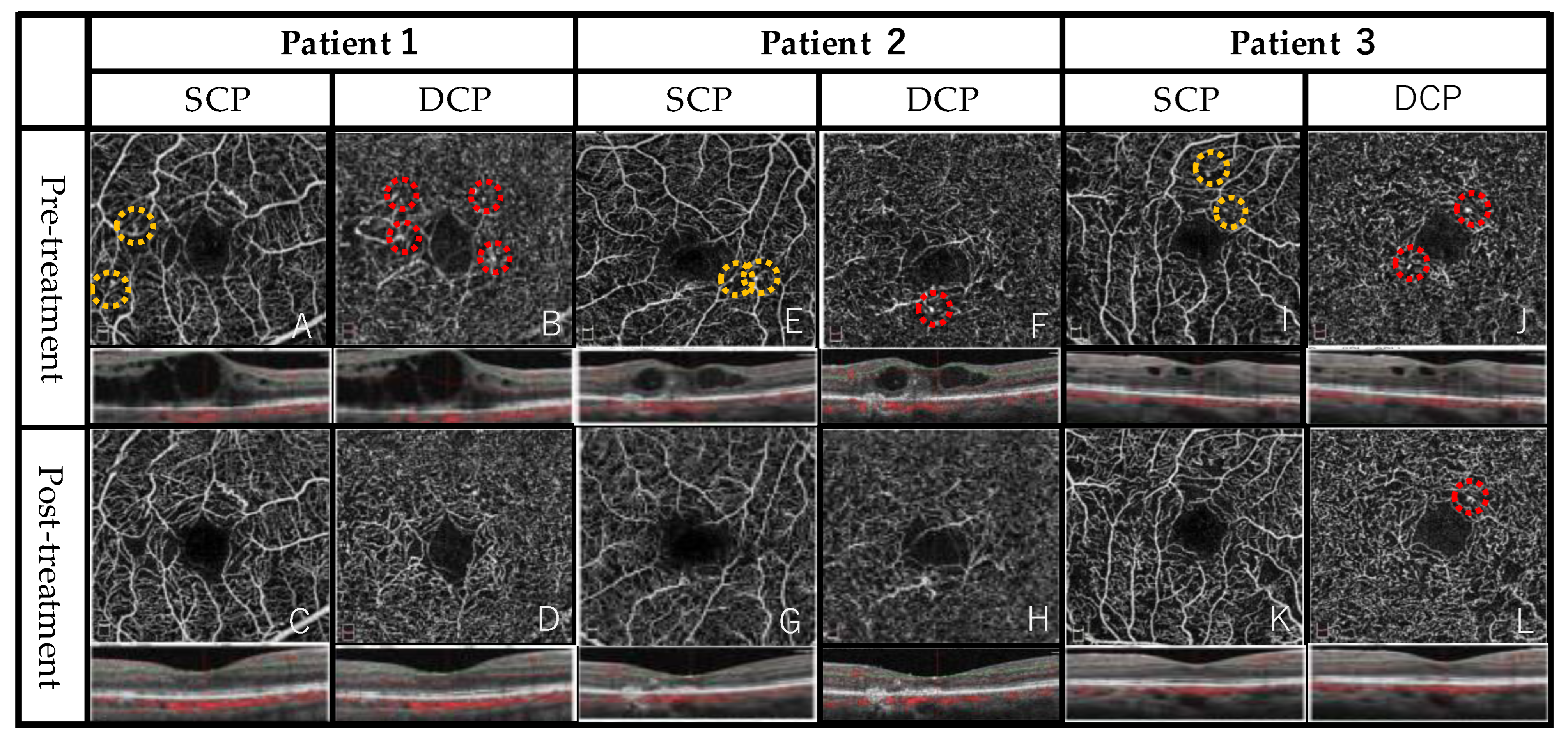
3.2. Representative Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, R. The epidemiology of diabetic retinopathy: Findings from the Wisconsin epidemiologic study of diabetic retinopathy. Int. Ophthalmol. Clin. 1987, 27, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Stefánsson, E.; Bek, T.; Porta, M.; Larsen, N.; Kristinsson, J.K.; Agardh, E. Screening and prevention of diabetic blindness. Acta Ophthalmol. Scand. 2000, 78, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Murakami, T.; Nozaki, M.; Suzuma, K.; Baba, T.; Hirano, T.; Sawada, O.; Sugimoto, M.; Takamura, Y.; Tsuiki, E. Review of clinical studies and recommendation for a therapeutic flow chart for diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McGuire, P.G. Diabetic macular edema: Pathophysiology and novel therapeutic targets. Ophthalmology 2015, 122, 1375–1394. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Rogers, S.L. Meta-analysis for eye disease (META-EYE) study group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Funatsu, H.; Noma, H. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009, 116, 73–79. [Google Scholar] [CrossRef]
- Miyamoto, K.; Khosrof, S.; Bursell, S.-E.; Rohan, R.; Murata, T.; Clermont, A.C.; Aiello, L.P.; Ogura, Y.; Adamis, A.P. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc. Natl. Acad. Sci. USA 1999, 96, 10836–10841. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ogura, Y. Pathogenetic potential of leukocytes in diabetic retinopathy. Semin. Ophthalmol. 1999, 14, 233–239. [Google Scholar] [CrossRef]
- Ogura, S.; Kurata, K.; Hattori, Y.; Takase, H.; Ishiguro-Oonuma, T.; Hwang, Y.; Ahn, S.; Park, I.; Ikeda, W.; Kusuhara, S.; et al. Sustained inflammation after pericyte depletion induces irreversible blood-retina barrier breakdown. JCI Insight 2017, 2, e90905. [Google Scholar] [CrossRef]
- Hong, I.H.; Choi, W.; Han, J.R. The effects of intravitreal triamcinolone acetonide in diabetic macular edema refractory to anti-VEGF treatment. Jpn. J. Ophthalmol. 2020, 64, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T.; Oshitari, T.; Baba, T.; Takatsuna, Y.; Yamamoto, S. Effects of switching from anti-VEGF treatment to triamcinolone acetonide in eyes with refractory macular edema associated with diabetic retinopathy or retinal vein occlusion. Biomed. Res. Int. 2020, 20, 4529850. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Falavarjani, K.G.; Habibi, A.; Anvari, P.; Ghasemizadeh, S.; Khorasani, M.A.; Shenazandi, H.; Sarraf, D. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br. J. Ophthalmol. 2019, 104, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Krawitz, B.D.; Mo, S.; Geyman, L.S.; Agemy, S.A.; Scripsema, N.K.; Garcia, P.M.; Chui, T.Y.; Rosen, R.B. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vis. Res. 2017, 139, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Bagley, S.; Ireland, G.; McLeod, D.; Boulton, M.E. Three dimensional analysis of microaneurysms in the human diabetic retina. J. Anat. 1999, 194, 89–100. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Falavarjani, G.K.; Iafe, N.A.; Hubschman, J.P.; Tsui, I.; Sadda, S.R.; Sarraf, D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 2017, 58, 30–34. [Google Scholar] [CrossRef]
- Sorour, O.A.; Sabrosa, A.S.; Alibhai, A.Y.; Arya, M.; Ishibazawa, A.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Waheed, N.K. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int. Ophthalmol. 2019, 39, 2361–2371. [Google Scholar] [CrossRef]
- Bonini-Filho, M.; Costa, R.A.; Calucci, D.; Jorge, R.; Melo, L.A.; Scott, I.U. Intravitreal Bevacizumab for Diabetic Macular Edema Associated with Severe Capillary Loss: One-Year Results of a Pilot Study. Am. J. Ophthalmol. 2009, 147, 1022–1030.e5. [Google Scholar] [CrossRef]
- Karst, S.G.; Deak, G.G.; Gerendas, B.S.; Waldstein, S.M.; Lammer, J.; Simader, C.; Guerin, T.; Schmidt-Erfurth, U.M. Association of Changes in macular perfusion with Ranibizumab treatment for diabetic macular edema: A subanalysis of the RESTORE (extension) study. JAMA Ophthalmol. 2018, 136, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Toto, L.; D’Aloisio, R.; Di Nicola, M.; Di Martino, G.; Di Staso, S.; Ciancaglini, M.; Tognetto, D.; Mastropasqua, L. Qualitative and quantitative assessment of vascular changes in diabetic macular edema after dexamethasone implant using optical coherence tomography angiography. Int. J. Mol. Sci. 2017, 18, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, A.; Dogan, M.E.; Demircan, A.; Akar, Y. Evaluation of macular vascular density and foveal avascular zone changes by optical coherence tomography angiography (OCT-A) after intravitreal dexamethasone implant in diabetic macular edema resistant to Anti-VEGF treatment. J. Int. Ophthalmol. 2022, 42, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Ozcalıskan, S.; Pehlıvanoglu, S.; Huseyınhan, Z.; Alagoz, C.; Erdogan, G.; Artunay, O. Macular and peripapillary microvasculature after dexamethasone injection in diabetic macular edema. Eur. J. Ophthalmol. 2021, 32, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Carnota-Méndez, P.; Méndez-Vázquez, C.; Pérez-Gavela, C. OCT-angiography changes in patients with diabetic macular edema treated with intravitreal dexamethasone implant. Clin. Ophthalmol. 2022, 2, 247–263. [Google Scholar] [CrossRef]
- Semeraro, F.; Russo, A.; Rizzoni, D.; Danzi, P.; Morescalchi, F.; Costagliola, C. Diameters and wall-to-lumen ratio of retinal arterioles in patients with retinal vein occlusion before and after treatment with dexamethasone intravitreal implants. J. Ocul. Pharmacol. Ther. 2014, 30, 573–579. [Google Scholar] [CrossRef]
- Wickremasinghe, S.S.; Rogers, S.L.; Gillies, M.C.; Zhu, M.D.; Wong, T.Y. Retinal vascular caliber changes after intravitreal triamcinolone treatment for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4707–4711. [Google Scholar] [CrossRef]
- Dong, A.; Bryan, T.; Dao-Yi, Y.; Balaratnasingam, C. Differentiating microaneurysm pathophysiology in diabetic retinopathy through objective analysis of capillary nonperfusion, inflammation, and pericytes. Diabetes 2022, 71, 733–746. [Google Scholar]
- Tamura, H.; Miyamoto, K.; Kiryu, J.; Miyahara, S.; Katsuta, H.; Hirose, F.; Musashi, K.; Yoshimura, N. Intravitreal Injection of Corticosteroid Attenuates Leukostasis and Vascular Leakage in Experimental Diabetic Retina. Investig. Opthalmology Vis. Sci. 2005, 46, 1440–1444. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, M.; Gao, M.; Liu, H.; Sun, X. Factors affecting the foveal avascular zone area in healthy eyes among young chinese adults. Biomed. Res. Int. 2020, 2020, 7361492. [Google Scholar] [CrossRef]
- Kwon, J.; Choi, J.; Shin, J.W.; Lee, J.; Kook, M.S. An optical coherence tomography angiography study of the relationship between foveal avascular zone size and retinal vessel density. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.S.; Kulikov, A.N.; Kazak, A.A.; Freund, K.B. Suspended scattering particles in motion may influence optical coherence tomography angiography vessel density metrics in eyes with diabetic macular edema. Retina 2021, 41, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Green, K.M.; Kwon, J.; Chu, Z.; Zhang, Q.; Wang, R.K.; Garrity, S.; Sarraf, D.; Rebhun, C.B.; Waheed, N.K.; et al. Suspended Scattering Particles in Motion: A Novel Feature of OCT Angiography in Exudative Maculopathies. Ophthalmol. Retin. 2018, 2, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Moon, B.G.; Cho, A.R.; Yoon, Y.H. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology 2016, 123, 2368–2375. [Google Scholar] [CrossRef]
- Busch, C.; Wakabayashi, T.; Sato, T.; Fukushima, Y.; Hara, C.; Shiraki, N.; Winegarner, A.; Nishida, K.; Sakaguchi, H.; Nishida, K. Retinal microvasculature and visual acuity after Intravitreal aflibercept in diabetic macular edema: An optical coherence tomography angiography study. Sci. Rep. 2019, 9, 1561. [Google Scholar] [CrossRef]
- Pongsachareonnont, P.; Charoenphol, P.; Hurst, C.; Somkijrungroj, T. The effect of anti-vascular endothelial growth factor on retinal microvascular changes in diabetic macular edema using swept-source optical coherence tomography angiography. Clin. Ophthalmol. 2020, 14, 3871–3880. [Google Scholar] [CrossRef]
- Mori, K.; Yoshida, S.; Kobayashi, Y.; Ishikawa, K.; Nakao, S.; Hisatomi, T.; Haruta, M.; Isihibashi, T.; Sonoda, K. Decrease in the number of microaneurysms in diabetic macular edema after antivascular endothelial growth factor therapy: Implications for indocyanine green angiography-guided detection of refractory microaneurysms. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 735–741. [Google Scholar] [CrossRef]
- Usui-Ouchi, A.; Tamaki, A.; Sakanishi, Y.; Tamaki, K.; Mashimo, K.; Sakuma, T.; Ebihara, N. Factors affecting a short-term response to anti-VEGF therapy in diabetic macular edema. Life 2021, 11, 83. [Google Scholar] [CrossRef]
- Querques, G.; Borrelli, E.; Battista, M.; Sacconi, R.; Bandello, F. Optical coherence tomography angiography in diabetes: Focus on microaneurysms. Eye 2021, 35, 142–148. [Google Scholar] [CrossRef]
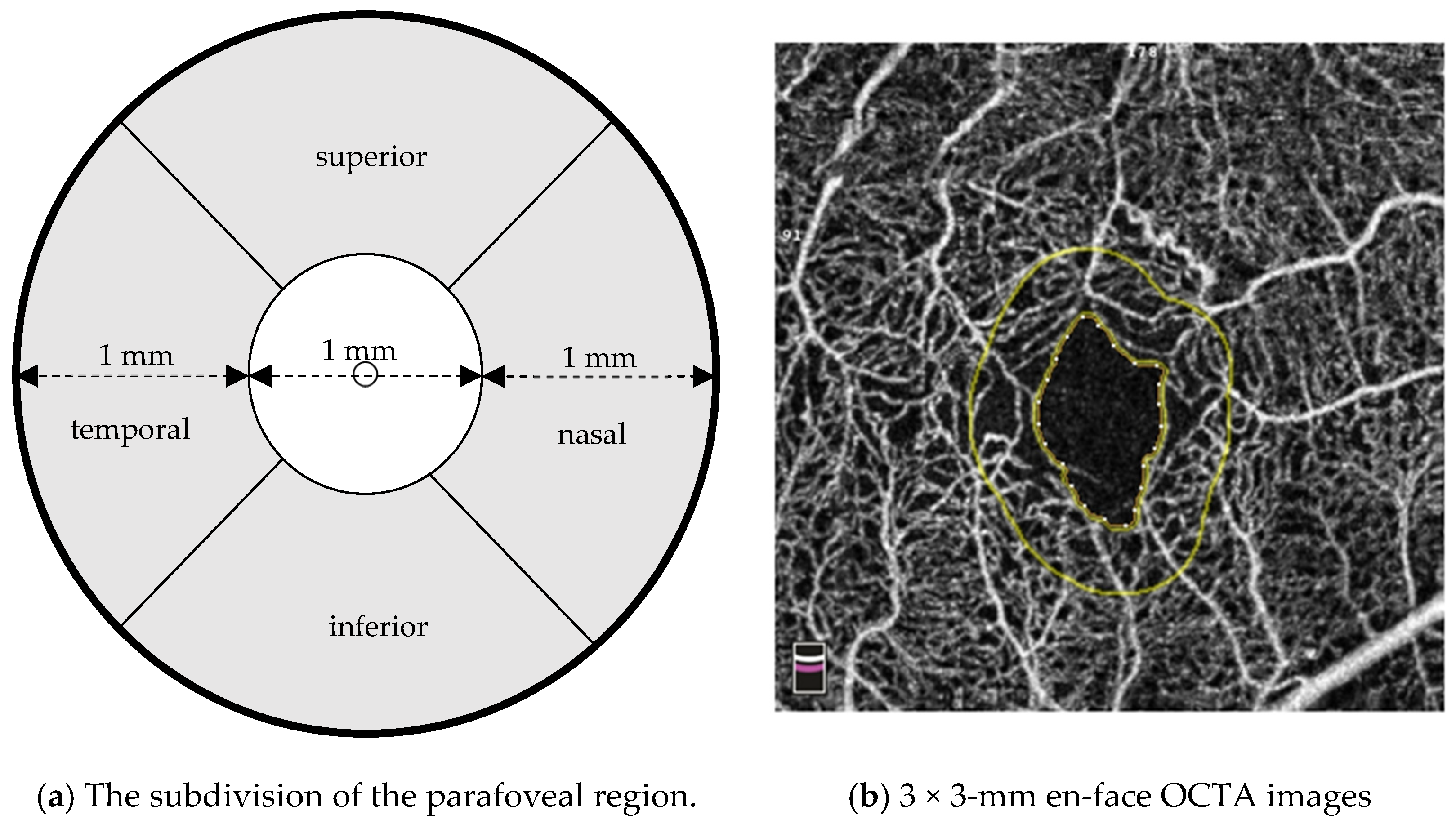
| Number of eyes/patients | 12/11 |
| Age (years) (mean ± SD; range) | 65.9 ± 11.4 (44–81) |
| Sex (number of men/women) | 4/7 |
| Lens status | |
| phakia/pseudophakia | 0/12 |
| Eyes with diabetic retinopathy (%) | |
| Mild NPDR | 1 (8.3) |
| Moderate NPDR | 2 (16.6) |
| Severe NPDR | 6 (50) |
| PDR | 3 (25) |
| Types of DME morphology in eyes (%) | |
| cystoid macular edema | 8 (66.7) |
| sponge-like diffuse retinal thickening | 3 (25) |
| serous retinal detachment | 1 (8.3) |
| Previous treatments in eyes (%) | |
| Anti-VEGF + TA + LP | 3 (25) |
| Anti-VEGF + TA | 3 (25) |
| Anti-VEGF + LP | 1 (8.3) |
| TA + LP | 1 (8.3) |
| Anti-VEGF | 1 (8.3) |
| TA | 1 (8.3) |
| LP | 1 (8.3) |
| None (naïve case) | 1 (8.3) |
| Pre-Treatment | Post-Treatment | p Value | ||
|---|---|---|---|---|
| LogMAR BCVA (mean ± SD) | 0.27 ± 0.25 | 0.23 ± 0.21 | 0.144 | |
| CMT, μm (mean ± SD) | 452 ± 105 | 273 ± 47 | 0.002 | |
| Number of microaneurysms | ||||
| SCP (mean ± SD) | 2.1 ± 1.1 | 1.0 ± 1.0 | 0.018 | |
| DCP (mean ± SD) | 2.0 ± 1.1 | 0.8 ± 0.8 | 0.008 | |
| Vessel density, % | ||||
| SCP (mean ± SD, range) | whole | 40.4 ± 3.5 | 41.8 ± 3.1 | 0.100 |
| temporal | 41.3 ± 4.8 | 42.1 ± 3.5 | 0.136 | |
| superior | 43.0 ± 5.4 | 45.8 ± 4.9 | 0.028 | |
| nasal | 41.7 ± 3.6 | 42.5 ± 4.5 | 0.722 | |
| inferior | 42.3 ± 4.8 | 44.7 ± 3.8 | 0.023 | |
| DCP (mean ± SD, range) | whole | 40.7 ± 5.8 | 41.2 ± 2.3 | 0.424 |
| temporal | 40.7 ± 5.9 | 41.8 ± 3.4 | 0.480 | |
| superior | 40.3 ± 6.1 | 42.4 ± 2.6 | 0.182 | |
| nasal | 41.3 ± 6.1 | 42.0 ± 3.6 | 0.209 | |
| inferior | 42.7 ± 7.2 | 42.6 ± 3.4 | 0.638 | |
| FAZ area, mm2 (mean ± SD, range) | 0.29 ± 0.10 (0.31–0.51) | 0.32 ± 0.13 (0.096–0.59) | 0.041 | |
| FD-300, % | 45.0 ± 3.6 | 44.8 ± 3.4 | 0.657 | |
| FAZ acircularity index | 1.20 ± 0.07 | 1.19 ± 0.06 | 0.789 | |
| Pre-Treatment | Post-Treatment | |||
|---|---|---|---|---|
| Practice | P1 | P2 | P1 | P2 |
| Number of microaneurysms in the SCP (mean ± SD, range) | 1.8 ± 1.2 (0–4) | 2.5 ± 1.4 (0–4) | 0.7 ± 1.0 (0–3) | 1.2 ± 1.1 (0–3) |
| Mean | 2.1 ± 1.1 | 1.0 ± 1.0 | ||
| ICC in the SCP | 0.70 | 0.78 | ||
| Number of microaneurysms in the DCP (mean ± SD, range) | 1.9 ± 1.3 (0–4) | 2.0 ± 1.2 (0–4) | 0.6 ± 0.7 (0–2) | 1.0 ± 1.0 (0–3) |
| Mean | 2.0 ± 1.1 | 0.8 ± 0.8 | ||
| ICC in the DCP | 0.71 | 0.78 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, F.; Nozaki, M.; Kato, A.; Yasukawa, T. Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema. J. Clin. Med. 2023, 12, 3475. https://doi.org/10.3390/jcm12103475
Kato F, Nozaki M, Kato A, Yasukawa T. Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema. Journal of Clinical Medicine. 2023; 12(10):3475. https://doi.org/10.3390/jcm12103475
Chicago/Turabian StyleKato, Fusae, Miho Nozaki, Aki Kato, and Tsutomu Yasukawa. 2023. "Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema" Journal of Clinical Medicine 12, no. 10: 3475. https://doi.org/10.3390/jcm12103475
APA StyleKato, F., Nozaki, M., Kato, A., & Yasukawa, T. (2023). Retinal Microvascular Changes after Intravitreal Triamcinolone Acetonide in Diabetic Macular Edema. Journal of Clinical Medicine, 12(10), 3475. https://doi.org/10.3390/jcm12103475





