Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy?
Abstract
:1. Introduction
2. Materials and Methods
- Definitions
- Inclusion and exclusion criteria for the analysis of the ERCP-based stent program
- Endpoints
- Statistical methods
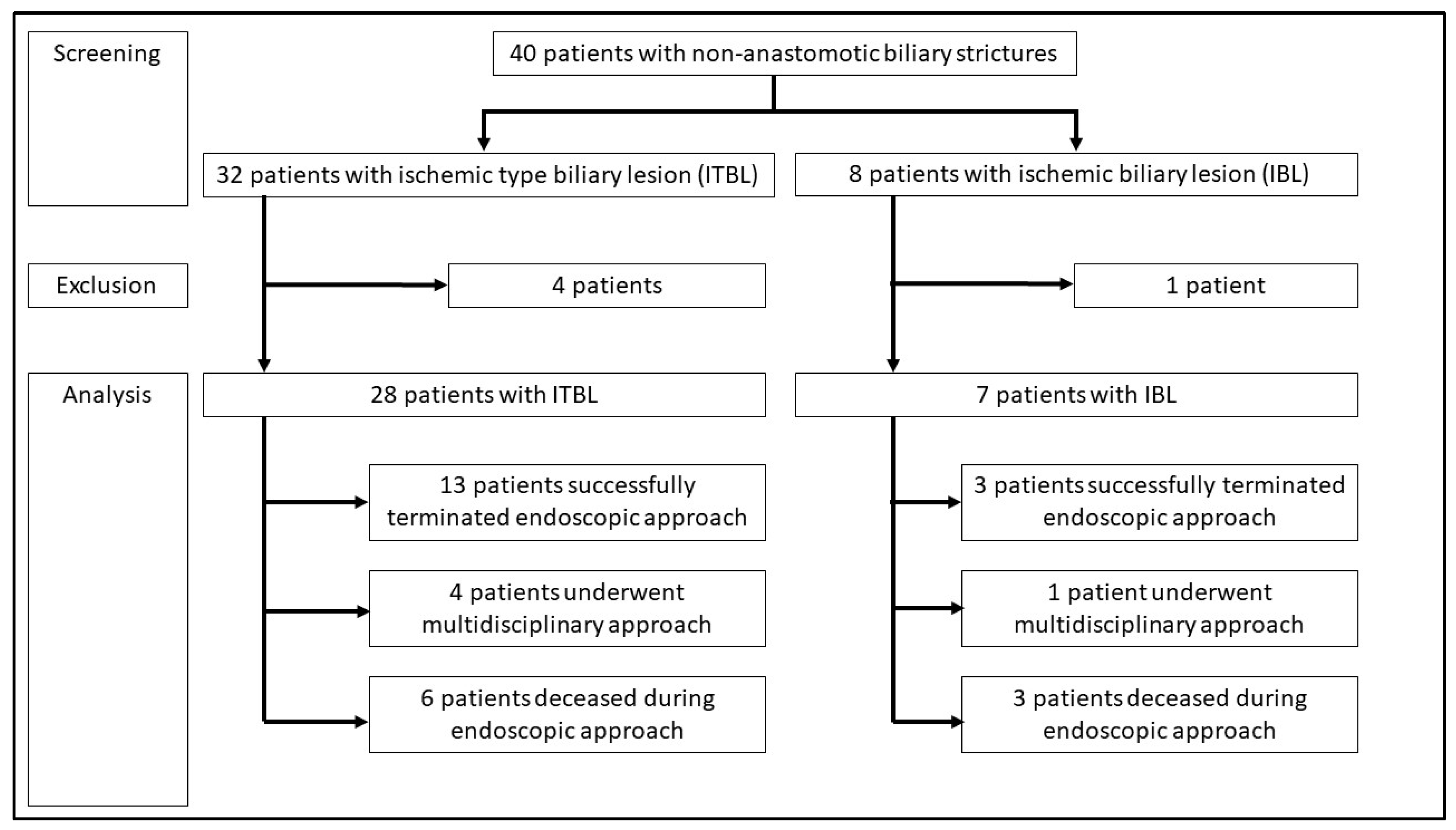
3. Results
- Descriptive analysis of all patients with NAS
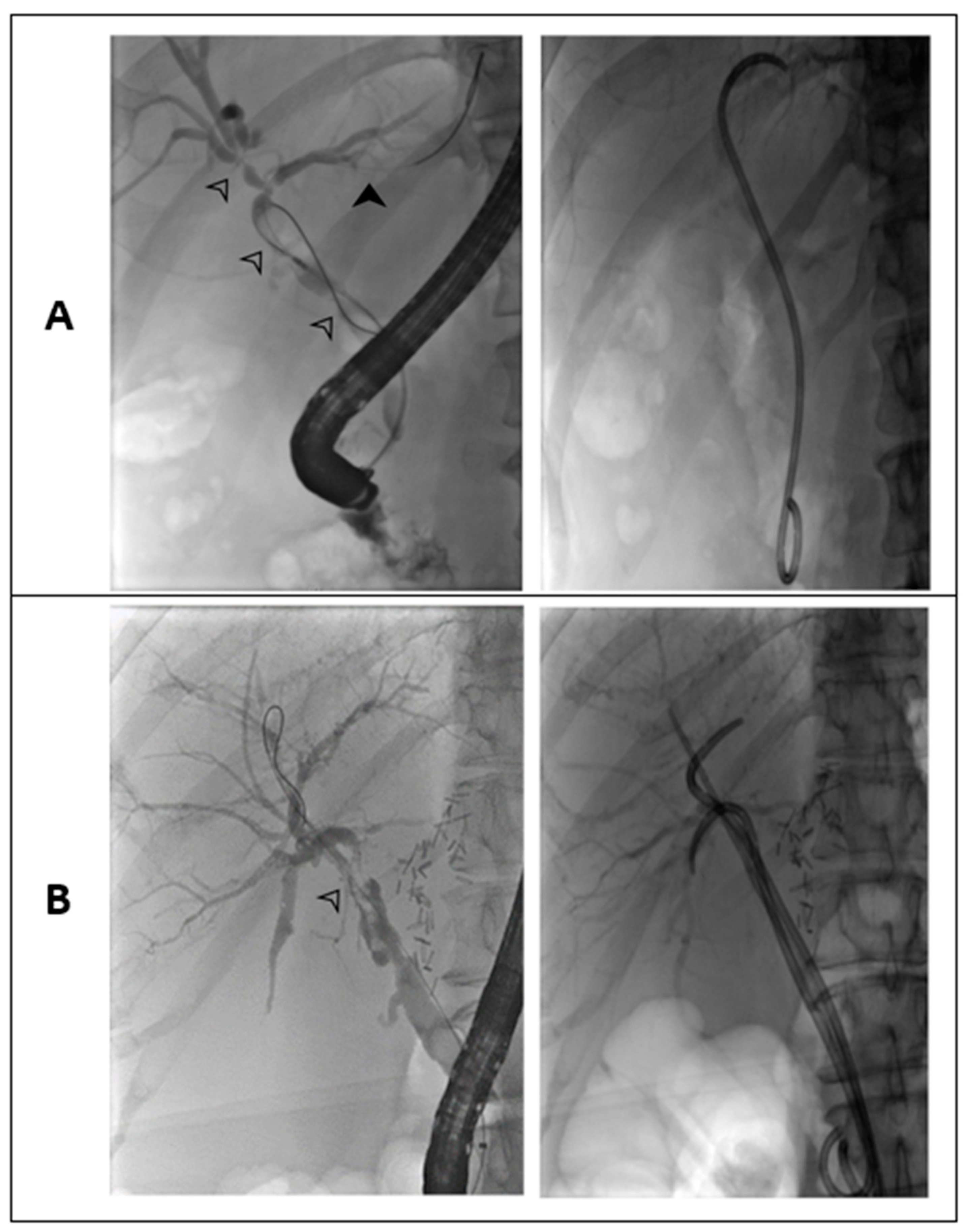
- Analysis of the ERCP-based stent program
- Variables for termination of ERCP-based stent program and overall mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochimica et biophysica acta. Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Villa, N.A.; Harrison, M.E. Management of Biliary Strictures After Liver Transplantation. Gastroenterol. Hepatol. 2015, 11, 316–328. [Google Scholar]
- Verdonk, R.C.; Buis, C.I.; Porte, R.J.; Haagsma, E.B. Biliary complications after liver transplantation: A review. Scand. J. Gastroenterol. 2006, 41, 89–101. [Google Scholar] [CrossRef]
- Akamatsu, N.; Sugawara, Y.; Hashimoto, D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: A systematic review of the incidence, risk factors and outcome. Transpl. Int. Off. J. Eur. Soc. Organ Transplant. 2011, 24, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Mekeel, K.L.; Crowell, M.D.; Nguyen, C.C.; Das, A.; Aqel, B.A.; Carey, E.J.; Byrne, T.J.; Vargas, H.E.; Douglas, D.D.; et al. Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: Outcomes after maximal stent therapy. Gastrointest. Endosc. 2013, 77, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, H.; Itamoto, T.; Sasaki, T.; Ohdan, H.; Fudaba, Y.; Amano, H.; Fukuda, S.; Nakahara, H.; Ishiyama, K.; Ohshita, A.; et al. Biliary complications after duct-to-duct biliary reconstruction in living-donor liver transplantation: Causes and treatment. World J. Surg. 2007, 31, 2222–2229. [Google Scholar] [CrossRef]
- Morelli, J.; Mulcahy, H.E.; Willner, I.R.; Cunningham, J.T.; Draganov, P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest. Endosc. 2003, 58, 374–379. [Google Scholar] [CrossRef]
- Dechêne, A.; Kodde, C.; Kathemann, S.; Treckmann, J.; Lainka, E.; Paul, A.; Gerken, G.; Feldstein, A.E.; Hoyer, P.F.; Canbay, A. Endoscopic treatment of pediatric post-transplant biliary complications is safe and effective. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2015, 27, 505–511. [Google Scholar] [CrossRef]
- Abou-Rebyeh, H.; Veltzke-Schlieker, W.; Radke, C.; Steinmüller, T.; Wiedenmann, B.; Hintze, R.E. Complete bile duct sequestration after liver transplantation, caused by ischemic-type biliary lesions. Endoscopy 2003, 35, 616–620. [Google Scholar]
- Graziadei, I.W.; Schwaighofer, H.; Koch, R.; Nachbaur, K.; Koenigsrainer, A.; Margreiter, R.; Vogel, W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transplant. 2006, 12, 718–725. [Google Scholar] [CrossRef]
- Rizk, R.S.; McVicar, J.P.; Emond, M.J.; Rohrmann, C.A.; Kowdley, K.V.; Perkins, J.; Carithers, R.L.; Kimmey, M.B. Endoscopic management of biliary strictures in liver transplant recipients: Effect on patient and graft survival. Gastrointest. Endosc. 1998, 47, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Asham, E.H.; Goldstein, L.; Han, S.H.; Saab, S.; Tong, M.J.; Busuttil, R.W.; Durazo, F.A. Endoscopic treatment with multiple stents for post-liver-transplantation nonanastomotic biliary strictures. Gastrointest. Endosc. 2009, 69, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Zajko, A.B.; Campbell, W.L.; Logsdon, G.A.; Bron, K.M.; Tzakis, A.; Esquivel, C.O.; Starzl, T.E. Cholangiographic findings in hepatic artery occlusion after liver transplantation. AJR. Am. J. Roentgenol. 1987, 149, 485–489. [Google Scholar] [CrossRef]
- Sanchez-Urdazpal, L.; Gores, G.J.; Ward, E.M.; Hay, E.; Buckel, E.G.; Wiesner, R.H.; Krom, R.A.F. Clinical outcome of ischemic-type biliary complications after liver transplantation. Transplant. Proc. 1993, 25, 1107–1109. [Google Scholar] [PubMed]
- Hintze, R.E.; Abou-Rebyeh, H.; Adler, A.; Veltzke, W.; Langrehr, J.; Wiedenmann, B.; Neuhaus, P. Endoskopische Therapie ischämietypischer biliärer Läsionen (ITBL) bei Patienten nach orthotoper Lebertransplantation. Z. Gastroenterol. 1999, 37, 13–20. [Google Scholar] [PubMed]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.-H.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholangitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Beyer, G.; Hoffmeister, A.; Michl, P.; Gress, T.M.; Huber, W.; Algül, H.; Neesse, A.; Meining, A.; Seufferlein, T.W.; Rosendahl, J.; et al. S3-Leitlinie Pankreatitis—Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS)—September 2021—AWMF Registernummer 021-003. Z. Gastroenterol. 2022, 60, 419–521. [Google Scholar] [CrossRef]
- Dubbeld, J.; Hoekstra, H.; Farid, W.; Ringers, J.; Porte, R.J.; Metselaar, H.J.; Baranski, A.G.; Kazemier, G.; Berg, A.P.V.D.; van Hoek, B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 2010, 97, 744–753. [Google Scholar] [CrossRef]
- Chan, E.Y.; Olson, L.C.; Kisthard, J.A.; Perkins, J.D.; Bakthavatsalam, R.; Halldorson, J.B.; Reyes, J.D.; Larson, A.M.; Levy, A.E. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transplant. 2008, 14, 604–610. [Google Scholar] [CrossRef]
- Pine, J.K.; Aldouri, A.; Young, A.L.; Davies, M.H.; Attia, M.; Toogood, G.J.; Pollard, S.G.; Lodge, J.P.A.; Prasad, K.R. Liver transplantation following donation after cardiac death: An analysis using matched pairs. Liver Transplant. 2009, 15, 1072–1082. [Google Scholar] [CrossRef]
- Zoepf, T.; de Dechêne, E.J.M.; Dechêne, A.; Malágo, M.; Beckebaum, S.; Paul, A.; Gerken, G.; Hilgard, P. Optimized endoscopic treatment of ischemic-type biliary lesions after liver transplantation. Gastrointest. Endosc. 2012, 76, 556–563. [Google Scholar] [CrossRef]
- Williams, E.D.; Draganov, P.V. Endoscopic management of biliary strictures after liver transplantation. World J. Gastroenterol. 2009, 15, 3725–3733. [Google Scholar] [CrossRef]
- Guichelaar, M.M.J.; Benson, J.T.; Malinchoc, M.; Krom, R.A.F.; Wiesner, R.H.; Charlton, M.R. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am. J. Transplant. 2003, 3, 885–890. [Google Scholar] [CrossRef]
- Dobrindt, E.M.; Eurich, D.; Veltzke-Schlieker, W.; Pratschke, J.; Sauer, I.; Öllinger, R.; Schmuck, R.B. Ischemic-Type Biliary Lesions After Liver Transplant: Factors Causing Early-Onset Versus Late-Onset Disease. Exp. Clin. Transplant. 2020, 18, 591–597. [Google Scholar] [CrossRef]
- Macías-Gómez, C.; Dumonceau, J.-M. Endoscopic management of biliary complications after liver transplantation: An evidence-based review. World J. Gastrointest. Endosc. 2015, 7, 606–616. [Google Scholar] [CrossRef]
- Hintze, R.E.; Adler, A.; Veltzke, W.; Abou-Rebyeh, H.; Felix, R.; Neuhaus, P. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepato-Gastroenterol. 1997, 44, 258–262. [Google Scholar]
- Hu, B.; Sun, B.; Cai, Q.; Lau, J.Y.W.; Ma, S.; Itoi, T.; Moon, J.H.; Yasuda, I.; Zhang, X.; Wang, H.-P.; et al. Asia-Pacific consensus guidelines for endoscopic management of benign biliary strictures. Gastrointest. Endosc. 2017, 86, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Dumonceau, J.-M.; Tringali, A.; Papanikolaou, I.S.; Blero, D.; Mangiavillano, B.; Schmidt, A.; Vanbiervliet, G.; Costamagna, G.; Devière, J.; García-Cano, J.; et al. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline—Updated October 2017. Endoscopy 2018, 50, 910–930. [Google Scholar] [CrossRef]
- Chathadi, K.V.; Chandrasekhara, V.; Acosta, R.D.; Decker, G.A.; Early, D.S.; Eloubeidi, M.A.; Evans, J.A.; Faulx, A.L.; Fanelli, R.D.; Fisher, D.A.; et al. The role of ERCP in benign diseases of the biliary tract. Gastrointest. Endosc. 2015, 81, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.T.G.B.; Ribeiro, I.B.; de Moura, D.T.H.; McCarty, T.R.; Neto, A.M.D.P.; Farias, G.F.A.; Neto, A.A.D.M.; de Oliveira, P.V.A.G.; Bernardo, W.M.; de Moura, E.G.H. Stent versus Balloon Dilation for the Treatment of Dominant Strictures in Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Clin. Endosc. 2021, 54, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Wellhöner, F.; Plumeier, I.; Kahl, S.; Chhatwal, P.; Vital, M.; Voigtländer, T.; Pieper, D.H.; Manns, M.P.; Lenzen, H.; et al. The biliary microbiome in ischaemic-type biliary lesions can be shaped by stenting but is resilient to antibiotic treatment. Liver Int. 2022, 42, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
| ITBL (n = 28) | IBL (n = 7) | Total (n = 35) | p-Value | |
|---|---|---|---|---|
| Liver disease before transplantation | n.s. | |||
| Viral, n (%) | 15 (54%) | 5 (71%) | 19 (54%) | |
| Alcohol, n (%) | 4 (14%) | 0 (0%) | 4 (11%) | |
| NASH, n (%) | 1 (4%) | 0 (0%) | 1 (3%) | |
| Autoimmune, n (%) | 4 (14%) | 1 (14%) | 5 (14%) | |
| PSC, n (%) | 2 (7%) | 1 (14%) | 3 (9%) | |
| Acute liver failure, n (%) | 2 (7%) | 1 (14%) | 3 (9%) | |
| Other, n (%) | 3 (11%) | 0 (0%) | 3 (9%) | |
| Age (years) * | 63 (53.25/67.25) | 63 (55.5/64.5) | 63 (54.5/66.5) | n.s. |
| Male sex, n (%) | 21 (75%) | 3 (43%) | 24 (69%) | n.s. |
| MELD at liver transplantation * | 15 (11.5/29) | 19 (11.5/33.5) | 16.5 (11.25/29.5) | n.s. |
| Hypertension, n (%) | 14 (50%) | 4 (57%) | 18 (51%) | n.s. |
| Heart disease, n (%) | 4 (14%) | 0 (0%) | 4 (11%) | n.s. |
| Arteriosclerosis, n (%) | 4 (14%) | 0 (0%) | 4 (11%) | n.s. |
| Diabetes mellitus, n (%) | 10 (36%) | 1 (14%) | 11 (31%) | n.s. |
| Kidney disease, n (%) | 13 (46%) | 3 (43%) | 16 (46%) | n.s. |
| CMV reactivation, n (%) | 8 (29%) | 3 (43%) | 11 (31%) | n.s. |
| Cold ischemic time (h:min) * | 8:41 (7:56/10:23) | 9:52 (7:36/10:57) | 8:45 (7:54/10:47) | n.s. |
| Warm ischemic time (min) * | 43 (36/47) | 52 (45/57) | 45 (36/49) | n.s. |
| Surgical revision of the bile duct anastomosis, n (%) | 4 (14%) | 1 (14%) | 5 (14%) | n.s. |
| Surgical revision of the artery, n (%) | (0%) | 7 (100%) | 7 (24%) | <0.001 |
| Variables | Only Endoscopic Therapeutic Approach | Multidisciplinary Therapy | p-Value of All Four Values | p-Value of Endoscopic Therapy Only | HR (95% CI) of Endoscopic Therapy Only | ||
|---|---|---|---|---|---|---|---|
| Alive (n = 16) | Dead (n = 9) | Alive (n = 4) | Dead (n = 1) | ||||
| ITBL, n (%) | 13 (81%) | 6 (67%) | 3 (75%) | 1 (100%) | n.s. | n.s. | 0.46 (0.71–2.99) |
| NAS locus intrahepatic, n (%) | 10 (62%) | 8 (89%) | 2 (50%) | 1 (100%) | n.s. | n.s. | 0.21 (0.21–2.10) |
| Male sex, n (%) | 9 (56%) | 7 (77%) | 4 (100%) | 1 (100%) | n.s. | n.s. | 2.72 (0.43–17.4) |
| Age * | 64 (57/68) | 63 (42/65) | 63 (47/72) | 51 | n.s. | n.s. | n.a. |
| MELD * | 15 (10/31) | 19 (10/29) | 13 (9/26) | 40 | n.s. | n.s. | n.a. |
| Hypertension, n (%) | 11 (69%) | 3 (33%) | 3 (75%) | 0 (0%) | n.s. | n.s. | 4.40 (0.77–25.1) |
| Heart disease, n (%) | 2 (13%) | 1 (11%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 1.14 (0.09–14.7) |
| Atherosclerosis, n (%) | 2 (13%) | 0 (0%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 8.00 (0.80–79.7) |
| Diabetes mellitus, n (%) | 5 (31%) | 3 (33%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 0.91 (0.16–5.20) |
| Kidney disease, n (%) | 10 (63%) | 3 (33%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 3.33 (0.60–18.5) |
| CMV infection, n (%) | 5 (31%) | 3 (33%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 0.93 (0.15–5.61) |
| Cold ischemic time in h * | 8 (7/11) | 8 (7/10) | 9 (7/12) | 10 | n.s. | n.s. | n.a. |
| Warm ischemic time in min * | 44 (41/48) | 48 (44/51) | 40 (33/48) | 45 | n.s. | n.s. | n.a. |
| Median duration until diagnosis in months * | 6 (3/19) | 5 (3/15) | 1 (1/3) | 7 | 0.04 | 0.05 | n.a. |
| Median duration of stent program in months * | 19 (12/44) | 14 (4/59) | 41 (9/48) | 41 | 0.02 | n.s. | n.a. |
| Number of ERCPs since diagnosis of NAS * | 13 (9/14) | 11 (6/26) | 13 (0/31) | 29 | 0.06 | n.s. | n.a. |
| Number of ERCPs in total * | 13 (9/15) | 13 (7/31) | 13 (0/31) | 30 | 0.05 | n.s. | n.a. |
| Cast extraction, n (%) | 13 (81%) | 7 (78%) | 0 (0%) | 1 (100%) | 0.01 | n.s. | 1.24 (0.17–9.25) |
| Bile stone extraction, n (%) | 7 (78%) | 11 (69%) | 0 (0%) | 1 (100%) | 0.04 | n.s. | 0.63 (0.10–4.18) |
| Balloon dilatation, n (%) | 6 (38%) | 5 (56%) | 1 (25%) | 0 (0%) | n.s. | n.s. | 0.48 (0.09–2.52) |
| Cholangitis, n (%) | 14 (88%) | 6 (67%) | 3 (25%) | 0 (0%) | n.s. | n.s. | 3.50 (0.46–26.6) |
| Pancreatitis, n (%) | 6 (38%) | 0 (0%) | 1 (25%) | 0 (0%) | n.s. | 0.07 | n.a. |
| Bleeding after ERCP, n (%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | n.s. | n.s. | n.a. |
| Perforation, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | n.s. | n.s. | n.a. |
| ITBL | IBL | Total | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Extrahepatic Strictures | Intrahepatic Strictures | Combined Strictures | Total ITBL | Extrahepatic Strictures | Intrahepatic Strictures | Combined Strictures | Total IBL | Comparison ITBL vs. IBL | |||
| Number | 8 (29%) | 6 (21%) | 14 (50%) | 28 (80%) | 2 (29%) | 1 (14%) | 4 (57%) | 7 (20%) | 35 (100%) | ||
| Procedure-related data | Successful termination of ERCP-based stent program | 4 (50%) | 2 (33%) | 7 (50%) | 13 (46%) | 2 (100%) | 0 (0%) | 1 (25%) | 3 (43%) | 16 (46%) | n.s. |
| Still part of ERCP-based stent program | 1 (13%) | 2 (33%) | 2 (14%) | 5 (18%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (14%) | n.s. | |
| Switch to surgical approach | 2 (25%) | 0 (0%) | 2 (14%) | 4 (14%) | 0 (0%) | 0 (0%) | 1 (25%) | 1 (14%) | 5 (14%) | n.s. | |
| Outcome analysis | Alive at end of follow-up period | 6 (75%) | 2 (33%) | 9 (64%) | 17 (61%) | 2 (100%) | 0 (0%) | 2 (50%) | 4 (57%) | 21 (60%) | n.s. |
| Deaths during the ERCP-based stent program | 1 (13%) | 2 (33%) | 3 (21%) | 6 (21%) | 0 (0%) | 1 (100%) | 2 (50%) | 3 (43%) | 9 (26%) | n.s. | |
| Death after surgical approach | 0 (0%) | 0 (0%) | 1 (7%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) | n.s. | |
| Cause of death other than NAS | 1 (13%) | 2 (33%) | 1 (7%) | 4 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (11%) | n.s. | |
| Overall Mortality | Successful Termination of the ERCP-Based Stent Program | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Patient characteristics | Age | 1.13 (1.01–1.26) | 0.03 | 0.99 (0.94–1.05) | n.s. |
| Male sex | 2.38 (0.92–1.09) | n.s. | 1.16 (0.44–3.07) | n.s. | |
| MELD | 1.00 (0.91–1.10) | n.s. | 1.03 (0.98–1.07) | n.s. | |
| Hypertension | 0.13 (0.01–1.46) | n.s. | 0.68 (0.25–1.85) | n.s. | |
| Heart disease | 47.7 (0.85–2680) | 0.06 | 0.24 (0.03–1.81) | n.s. | |
| Arteriosclerosis | 2.01 (0.31–129) | n.s. | 0.67 (0.15–2.97) | n.s. | |
| Diabetes mellitus | 14.2 (0.77–261) | 0.08 | 0.37 (0.12–1.16) | n.s. | |
| Kidney disease | 0.23 (0.19–2.76) | n.s. | 0.64 (0.23–1.81) | n.s. | |
| CMV infection | 0.52 (0.11–2.50) | n.s. | 0.42 (0.13–1.33) | n.s. | |
| Surgical characteristics (two-sided p-values) | Surgical revision of the bile duct anastomosis | 0.53 (0.06–4.58) | n.s. | 0.60 (0.08–4.76) | n.s. |
| Surgical revision of the arterial anastomosis | 4.62 (0.94–22.69) | 0.06 | 1.53 (0.20–11.8) | n.s. | |
| Cold ischemic time | 0.99 (0.99–1.004) | n.s. | 0.99 (0.99–1.003) | n.s. | |
| Warm ischemic time | 0.99 (0.97–1.03) | n.s. | 1.01 (0.99–1.03) | n.s. | |
| Laboratory values (one-sided p-values) | log (Bilirubin) | 5.22 (2.22–12.31) | <0.0001 | 1.61 (0.87–2.97) | n.s. |
| log (ALT) | 1.97 (1.22–3.19) | 0.006 | 0.58 (0.30–1.15) | n.s. | |
| log (AST) | 2.77 (1.60–4.80) | 0.0003 | 0.33 (0.10–1.09) | n.s. | |
| log (GGT) | 0.50 (0.26–0.96) | n.s. | 0.54 (0.33–0.90) | n.s. | |
| log (AP) | 0.33 (0.10–1.16) | n.s. | 0.39 (0.17–0.85) | n.s. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael, F.A.; Friedrich-Rust, M.; Erasmus, H.-P.; Graf, C.; Ballo, O.; Knabe, M.; Walter, D.; Steup, C.D.; Mücke, M.M.; Mücke, V.T.; et al. Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy? J. Clin. Med. 2023, 12, 3491. https://doi.org/10.3390/jcm12103491
Michael FA, Friedrich-Rust M, Erasmus H-P, Graf C, Ballo O, Knabe M, Walter D, Steup CD, Mücke MM, Mücke VT, et al. Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy? Journal of Clinical Medicine. 2023; 12(10):3491. https://doi.org/10.3390/jcm12103491
Chicago/Turabian StyleMichael, Florian A., Mireen Friedrich-Rust, Hans-Peter Erasmus, Christiana Graf, Olivier Ballo, Mate Knabe, Dirk Walter, Christoph D. Steup, Marcus M. Mücke, Victoria T. Mücke, and et al. 2023. "Treatment of Non-Anastomotic Biliary Strictures after Liver Transplantation: How Effective Is Our Current Treatment Strategy?" Journal of Clinical Medicine 12, no. 10: 3491. https://doi.org/10.3390/jcm12103491





