ADONHERS (Aged DONor HEart Rescue by Stress Echo) National Protocol: Recipient’s Survival after 10-Year Follow-Up
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection and Data Collection
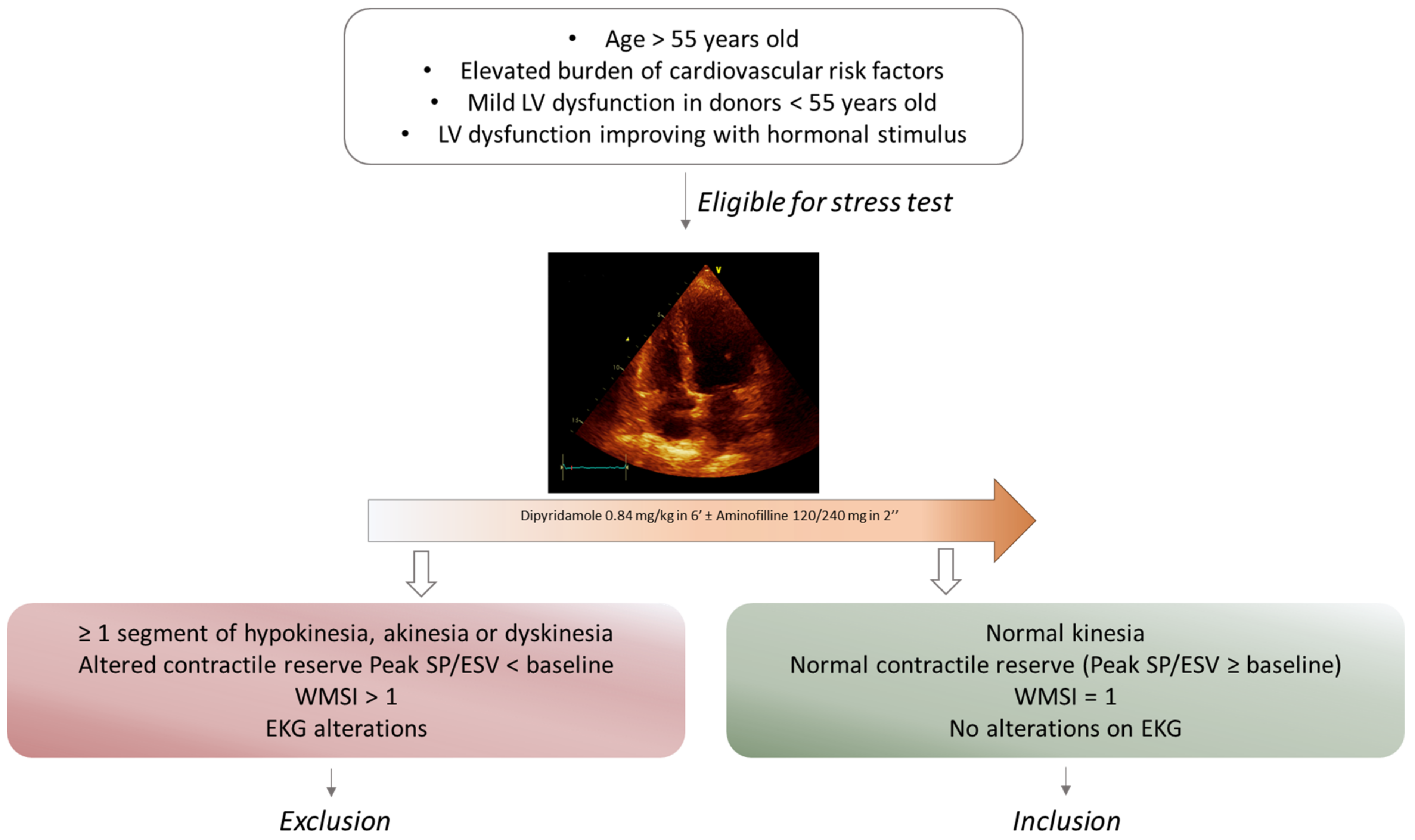
2.2. Marginal Hearts Selection
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
7. Clinical Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Tomasoni, D.; Vishram-Nielsen, J.K.; Pagnesi, M.; Adamo, M.; Lombardi, C.M.; Gustafsson, F.; Metra, M. Advanced heart failure: Guideline-directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022, 9, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Shah, P.; Lala, A.; McLean, R.C.; Pamboukian, S.; Horstmanshof, D.A.; Thibodeau, J.; Shah, K.; Teuteberg, J.; Gilotra, N.A.; et al. INTERMACS profiles and outcomes of ambulatory advanced heart failure patients: A report from the REVIVAL Registry. J. Heart Lung Transplant. 2020, 39, 16–26. [Google Scholar] [CrossRef]
- Samman-Tahhan, A.; Hedley, J.S.; McCue, A.A.; Bjork, J.B.; Georgiopoulou, V.V.; Morris, A.A.; Butler, J.; Kalogeropoulos, A.P. INTERMACS Profiles and Outcomes Among Non–Inotrope-Dependent Outpatients With Heart Failure and Reduced Ejection Fraction. JACC Heart Fail. 2018, 6, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Ambardekar, A.V.; Kittleson, M.M.; Palardy, M.; Mountis, M.M.; Forde-McLean, R.C.; DeVore, A.D.; Pamboukian, S.V.; Thibodeau, J.T.; Teuteberg, J.J.; Cadaret, L.; et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J. Heart Lung Transplant. 2019, 38, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.G. Only optimal donors should be accepted for heart transplantation: Protagonist. J. Heart Lung Transplant. 1995, 14 Pt 1, 1038–1042. [Google Scholar]
- Bifulco, O.; Bottio, T.; Caraffa, R.; Carrozzini, M.; Guariento, A.; Bejko, J.; Fedrigo, M.; Castellani, C.; Toscano, G.; Lorenzoni, G.; et al. Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. J. Clin. Med. 2022, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, T.; Wahlers, T. Marginal donor grafts in heart transplantation: Lessons learned from 25 years of experience. Transpl. Int. 2007, 21, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wheeldon, D.R.; Potter, C.D.; Oduro, A.; Wallwork, J.; Large, S.R. Transforming the “unacceptable” donor: Outcomes from the adoption of a standardized donor management technique. J. Heart Lung Transplant. 1995, 14, 734–742. [Google Scholar]
- Hornby, K.; Ross, H.; Keshavjee, S.; Rao, V.; Shemie, S.D. Non-utilization of hearts and lungs after consent for donation: A Canadian multicentre study. Can. J. Anaesth. 2006, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Huckaby, L.V.; Hickey, G.; Sultan, I.; Kilic, A. Trends in the utilization of marginal donors for orthotopic heart transplantation. J. Card. Surg. 2021, 36, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Pellikka, P.A. Stress echo applications beyond coronary artery disease. Eur. Heart J. 2014, 35, 1033–1040. [Google Scholar] [CrossRef]
- Tryon, D.; Hasaniya, N.W.; Jabo, B.; Razzouk, A.J.; Bailey, L.L.; Rabkin, D.G. Effect of left ventricular dysfunction on utilization of donor hearts. J. Heart Lung Transplant. 2018, 37, 349–357. [Google Scholar] [CrossRef]
- Copeland, H.; Knezevic, I.; Baran, D.A.; Rao, V.; Pham, M.; Gustafsson, F.; Pinney, S.; Lima, B.; Masetti, M.; Ciarka, A.; et al. Donor heart selection: Evidence-based guidelines for providers. J. Heart Lung Transplant. 2023, 42, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Bombardini, T.; Arpesella, G.; Maccherini, M.; Procaccio, F.; Potena, L.; Bernazzali, S.; Leone, O.; Picano, E. Medium-term outcome of recipients of marginal donor hearts selected with new stress-echocardiographic techniques over standard criteria. Cardiovasc. Ultrasound 2014, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Zaroff, J.G.; Rosengard, B.R.; Armstrong, W.F.; Babcock, W.D.; D’alessandro, A.; Dec, G.; Edwards, N.M.; Higgins, R.S.; Jeevanandum, V.; Kauffman, M.; et al. Maximizing use of organs recovered from the cadaver donor: Cardiac recommendations1 1This article was originally published in Circulation. Copyright © 2002 American Heart Association, Inc. Reprinted with permission, Lippincott, Williams & Wilkins. J. Heart Lung Transplant. 2002, 21, 1153–1160. [Google Scholar] [CrossRef]
- Bombardini, T.; Gherardi, S.; Leone, O.; Sicari, R.; Picano, E. Transplant of stunned donor hearts rescued by pharmacological stress echocardiography: A “proof of concept” report. Cardiovasc. Ultrasound 2013, 11, 27. [Google Scholar] [CrossRef]
- Bombardini, T.; Gherardi, S.; Arpesella, G.; Maccherini, M.; Serra, W.; Magnani, G.; Del Bene, R.; Picano, E. Favorable Short-Term Outcome of Transplanted Hearts Selected from Marginal Donors by Pharmacological Stress Echocardiography. J. Am. Soc. Echocardiogr. 2011, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.W.; Khush, K.K. New Approaches to Donor Selection and Preparation in Heart Transplantation. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Leone, O.; Gherardi, S.; Targa, L.; Pasanisi, E.; Mikus, P.; Tanganelli, P.; Maccherini, M.; Arpesella, G.; Picano, E.; Bombardini, T. Stress Echocardiography as a Gatekeeper to Donation in Aged Marginal Donor Hearts: Anatomic and Pathologic Correlations of Abnormal Stress Echocardiography Results. J. Heart Lung Transplant. 2009, 28, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Zaroff, J.G.; Babcock, W.D.; Shiboski, S.C.; Solinger, L.L.; Rosengard, B.R. Temporal changes in left ventricular systolic function in heart donors: Results of serial echocardiography. J. Heart Lung Transplant. 2003, 22, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, P.S.; Aneman, A.; Bhonagiri, D.; Jones, D.; O’callaghan, G.; Silvester, W.; Watson, A.; Dobb, G. A systematic review and meta-analysis of clinical trials of thyroid hormone administration to brain dead potential organ donors. Crit. Care Med. 2012, 40, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Bombardini, T.; Dokollari, A.; Sassi, C.; Losito, M.; Sparla, S.; Lisi, G.; Bernazzali, S.; Davoli, G.; Capannini, G.; et al. Longitudinal Strain Stress-Echo Evaluation of Aged Marginal Donor Hearts: Feasibility in the Adonhers Project. Transplant. Proc. 2016, 48, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Franchi, D.; Cini, D.; Arpesella, G.; Gherardi, S.; Calamai, I.; Barletta, G.; Valente, S.; Pasanisi, E.; Sansoni, S.; Ricci, C.; et al. Second-opinion stress tele-echocardiography for the Adonhers (Aged donor heart rescue by stress echo) project. Cardiovasc. Ultrasound 2010, 8, 20. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.-U.; Zamorano, J.L. On behalf of the European Association of Echocardiography Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. J. Echocardiogr. 2008, 9, 415–437. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.B.; Lyster, H.; Lindenfeld, J.; Doligalski, C.; Baran, D.; Yost, C.; Shullo, M.; Schweiger, M.; Weill, D.; Stuckey, L.; et al. Report from the 2018 consensus conference on immunomodulating agents in thoracic transplantation: Access, formulations, generics, therapeutic drug monitoring, and special populations. J. Heart Lung Transplant. 2020, 39, 1050–1069. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_365_allegato.pdf (accessed on 1 December 2022).
- Colvin, M.; Smith, J.; Ahn, Y.; Skeans, M.; Messick, E.; Bradbrook, K.; Gauntt, K.; Israni, A.; Snyder, J.; Kasiske, B. OPTN/SRTR 2020 Annual Data Report: Heart. Am. J. Transplant. 2022, 22, 350–437. [Google Scholar] [CrossRef]
- Fonarow, G.C. How old is too old for heart transplantation? Curr. Opin. Cardiol. 2000, 15, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Rodriguez, J.A.; Portela, F.; Paniagua, M.J.; Muñiz, J.; Hermida, L.F.; Vazquez, N.; Cuenca, J.J.; Juffé-Stein, A.; Castro-Beiras, A. Coronary artery disease transmitted by donors older than 40 years: Prevalence and prognosis. Transplant. Proc. 1999, 31, 2542–2543. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Naftel, D.C.; Pritzker, M.R.; Heilman, J.K.; Boehmer, J.; Brozena, S.C.; Dec, G.W.; Ventura, H.O.; Kirklin, J.K.; Bourge, R.C.; et al. Heart transplant coronary artery disease detected by coronary angiography: A multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J. Heart Lung Transplant. 1998, 17, 744–753. [Google Scholar]
- Grauhan, O.; Patzurek, J.; Hummel, M.; Lehmkuhl, H.; Dandel, M.; Pasic, M.; Weng, Y.; Hetzer, R. Donor-transmitted coronary atherosclerosis. J. Heart Lung Transplant. 2003, 22, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Mercer, P.; Sharpies, L.; Edmunds, J.; Gittins, R.; Baines, J.; Wallwork, J.; Large, S.; Parameshwar, J. Evaluating the donor pool: Impact of using hearts from donors over the age of 49 years. Transplant. Proc. 1997, 29, 3293–3296. [Google Scholar] [CrossRef]
- Potapov, E.V.; Loebe, M.; Hübler, M.; Musci, M.; Hummel, M.; Weng, Y.; Hetzer, R. MEDIUM-TERM results of heart transplantation using donors over 63 years of age1. Transplantation 1999, 68, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Blanche, C.; Kamlot, A.; Blanche, D.A.; Kearney, B.; Magliato, K.E.; Czer, L.S.; Trento, A. Heart transplantation with donors fifty years of age and older. J. Thorac. Cardiovasc. Surg. 2002, 123, 810–815. [Google Scholar] [CrossRef]
- Arpesella, G.; Gherardi, S.; Bombardini, T.; Picano, E. Recruitment of aged donor heart with pharmacological stress echo. A case report. Cardiovasc. Ultrasound 2006, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2021 Annual Data Report. 2023. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9970342/ (accessed on 20 February 2023).
- Copeland, H.; Hayanga, J.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transplant. 2020, 39, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Gongora, E. Role of cardiovascular imaging in selection of donor hearts. World J. Transplant. 2015, 5, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, M.; Bombardini, T.; Simion, D.; Gaspari, M.G.; Procaccio, F. Wait, treat and see: Echocardiographic monitoring of brain-dead potential donors with stunned heart. Cardiovasc. Ultrasound 2012, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S. Allograft Vasculopathy. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy—2010. J. Heart Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Amarelli, C.; De Santo, L.S.; Marra, C.; Maiello, C.; Bancone, C.; Della Corte, A.; Nappi, G.; Romano, G. Early graft failure after heart transplant: Risk factors and implications for improved donor-recipient matching. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Vallakati, A.; Reddy, S.; Dunlap, M.E.; Taylor, D.O. Impact of Statin Use After Heart Transplantation. Circ. Heart Fail. 2016, 9, e003265. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Moainie, S.L.; Griffith, B.P.; Poston, R.S. Preserving and evaluating hearts with ex vivo machine perfusion: An avenue to improve early graft performance and expand the donor pool. Eur. J. Cardio-Thoracic Surg. 2008, 34, 318–325. [Google Scholar] [CrossRef]
- Schroder, J.N.; D’Alessandro, D.; Esmailian, F.; Boeve, T.; Tang, P.; Liao, K.; Wang, I.; Anyanwu, A.; Shah, A.; Mudy, K.; et al. Successful Utilization of Extended Criteria Donor (ECD) Hearts for Transplantation-Results of the OCSTM Heart EXPAND Trial to Evaluate the Effectiveness and Safety of the OCS Heart System to Preserve and Assess ECD Hearts for Transplantation. J. Heart Lung Transplant. 2019, 38, S42. [Google Scholar] [CrossRef]
- Jawitz, O.K.; Devore, A.D.; Patel, C.B.; Bryner, B.S.; Schroder, J.N. EXPANDing the Donor Pool: Quantifying the Potential Impact of a Portable Organ-Care System for Expanded Criteria Heart Donation. J Card Fail. 2021, 27, 1462–1465. [Google Scholar] [CrossRef] [PubMed]



| Acceptable Heart Donor | |
|---|---|
| Age | <55 years old |
| Heart morphology and function | EF 55–65% Normal segmental kinesia No valvular or congenital defects Posterior wall or Interventricular septum < 12 mm |
| Weight | Maximum 20% donor–receiver weight mismatch |
| Size | In case of female donor heart for a man: female > 10% larger in height and weight |
| Marginal heart donor | |
| Age | >55 years old |
| CV risk factors | High risk (diabetes, arterial hypertension, dyslipidemia, smoking history) |
| Heart morphology and function | Left ventricular hypertrophy Valvular and/or congenital defects Pre-mature coronary artery disease |
| Serology | HBV/HCV positive |
| Substances | Substance intoxication (e.g., CO, cyclic antidepressants) Drug abuse (e.g., cocaine or amphetamine) |
| Ischemic time | >3–4 h |
| Size | Elevated donor–receiver size discrepancy (>20%) |
| Total | Marginal | Acceptable | |
|---|---|---|---|
| Population | 22 | 11 | 11 |
| Baseline characteristics | |||
| Age | 54 ± 10 | 55 ± 10 | 54 ± 9 |
| Age of donors | 41 ± 23 | 45 ± 29 | 36 ± 12 |
| Sex | M 14 (63.6%) | M 7 (63.6%) | M 7 (63.6%) |
| Sex of donors | M 12 (54.5%) | M 5 (45.4%) | M 7 (63.6%) |
| NYHA III/IV | 3 (13.6%) | 0 | 3 (27.3%) |
| Arterial hypertension | 10 (58.8%) | 4 (36.4%) | 6 (54.5%) |
| Diabetes | 4 (18.2%) | 1 (9.1%) | 3 (27.3%) |
| Dyslipidemia | 6 (27.3%) | 1 (9.1%) | 5 (45.4%) |
| CKD | 11 (50%) | 4 (36.4%) | 7 (63.6%) |
| Follow-up | |||
| Rejection | 5 (22.7%) | 1 (9.1%) | 4 (36.4%) |
| Anti-HLA Ab | 7 (31.8%) | 2 (18.2%) | 5 (45.4%) |
| CAV | 7 (31.8%) | 1 (9.1%) | 6 (54.5%) |
| Death | 4 (18.2%) | 1 (9.1%) | 3 (27.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandoli, G.E.; Barilli, M.; Soviero, D.; Ghionzoli, N.; Landra, F.; Maccherini, M.; Bernazzali, S.; Natali, B.M.; Focardi, M.; Cavigli, L.; et al. ADONHERS (Aged DONor HEart Rescue by Stress Echo) National Protocol: Recipient’s Survival after 10-Year Follow-Up. J. Clin. Med. 2023, 12, 3505. https://doi.org/10.3390/jcm12103505
Mandoli GE, Barilli M, Soviero D, Ghionzoli N, Landra F, Maccherini M, Bernazzali S, Natali BM, Focardi M, Cavigli L, et al. ADONHERS (Aged DONor HEart Rescue by Stress Echo) National Protocol: Recipient’s Survival after 10-Year Follow-Up. Journal of Clinical Medicine. 2023; 12(10):3505. https://doi.org/10.3390/jcm12103505
Chicago/Turabian StyleMandoli, Giulia Elena, Maria Barilli, Davide Soviero, Nicolò Ghionzoli, Federico Landra, Massimo Maccherini, Sonia Bernazzali, Benedetta Maria Natali, Marta Focardi, Luna Cavigli, and et al. 2023. "ADONHERS (Aged DONor HEart Rescue by Stress Echo) National Protocol: Recipient’s Survival after 10-Year Follow-Up" Journal of Clinical Medicine 12, no. 10: 3505. https://doi.org/10.3390/jcm12103505






