Impact of COVID-19 on Sedation Requirements during Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome
Abstract
1. Introduction
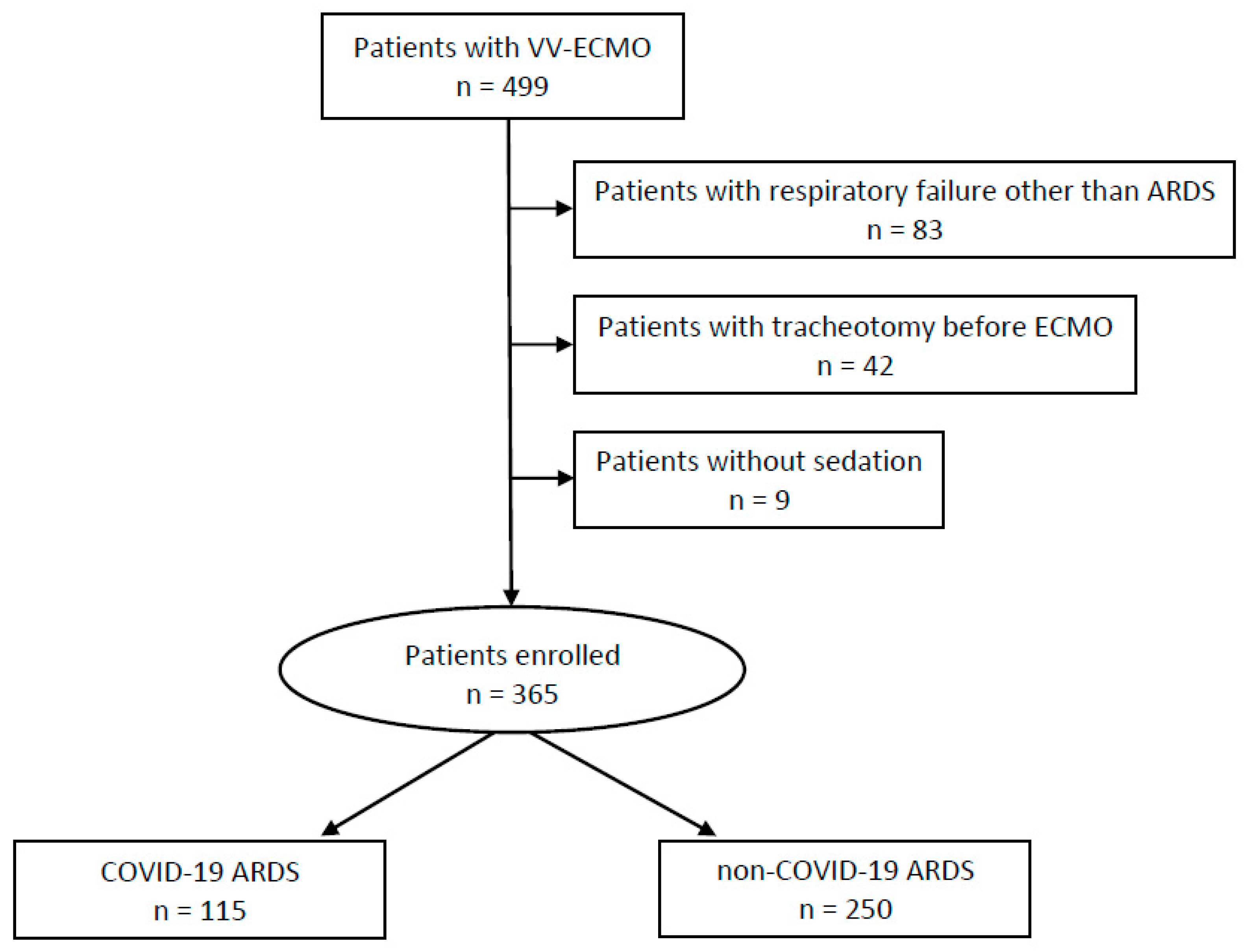
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Management of VV-ECMO for Acute Respiratory Distress Syndrome (C-ARDS and Non-C-ARDS)
2.3. Mechanical Ventilation Practices during VV-ECMO
2.4. Analgosedation Practice
2.5. Data Analysis
3. Results
3.1. Descriptive Cohort Characteristics
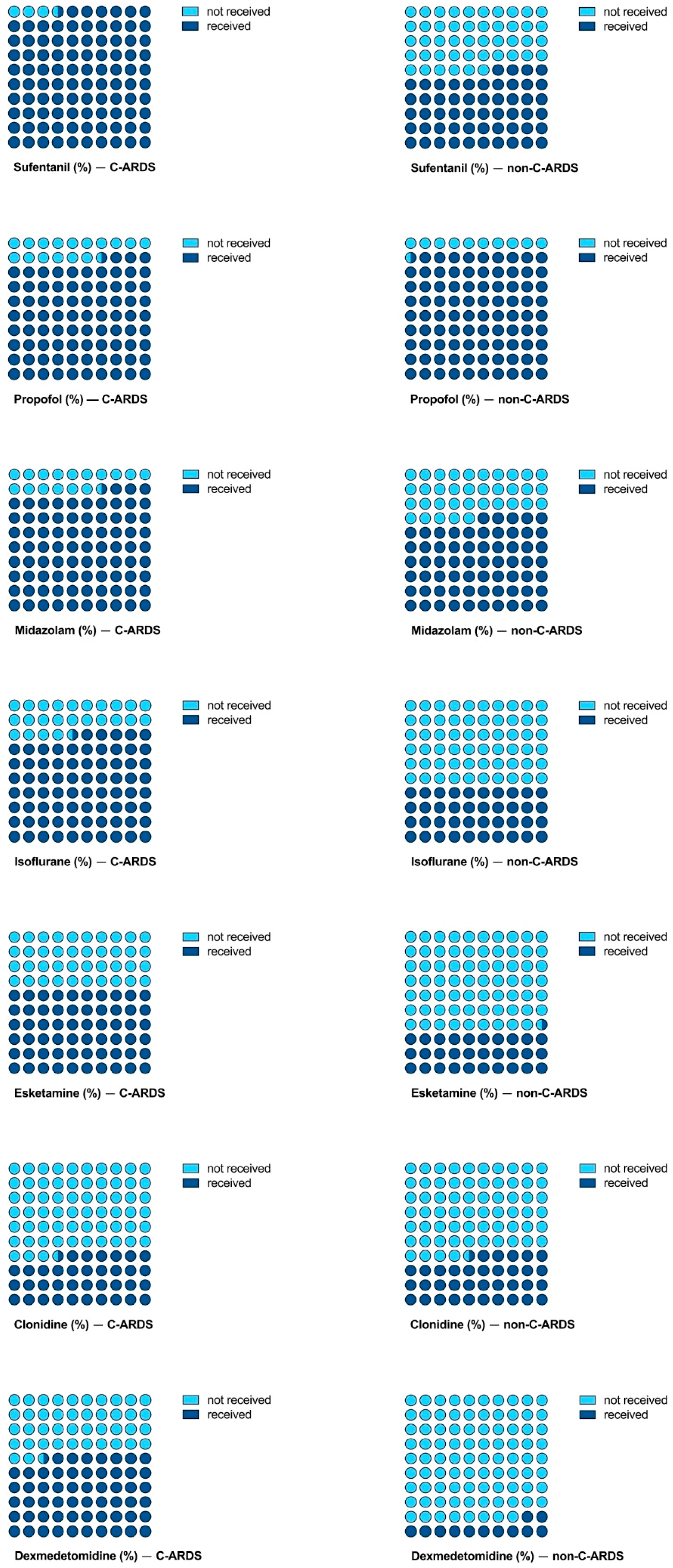
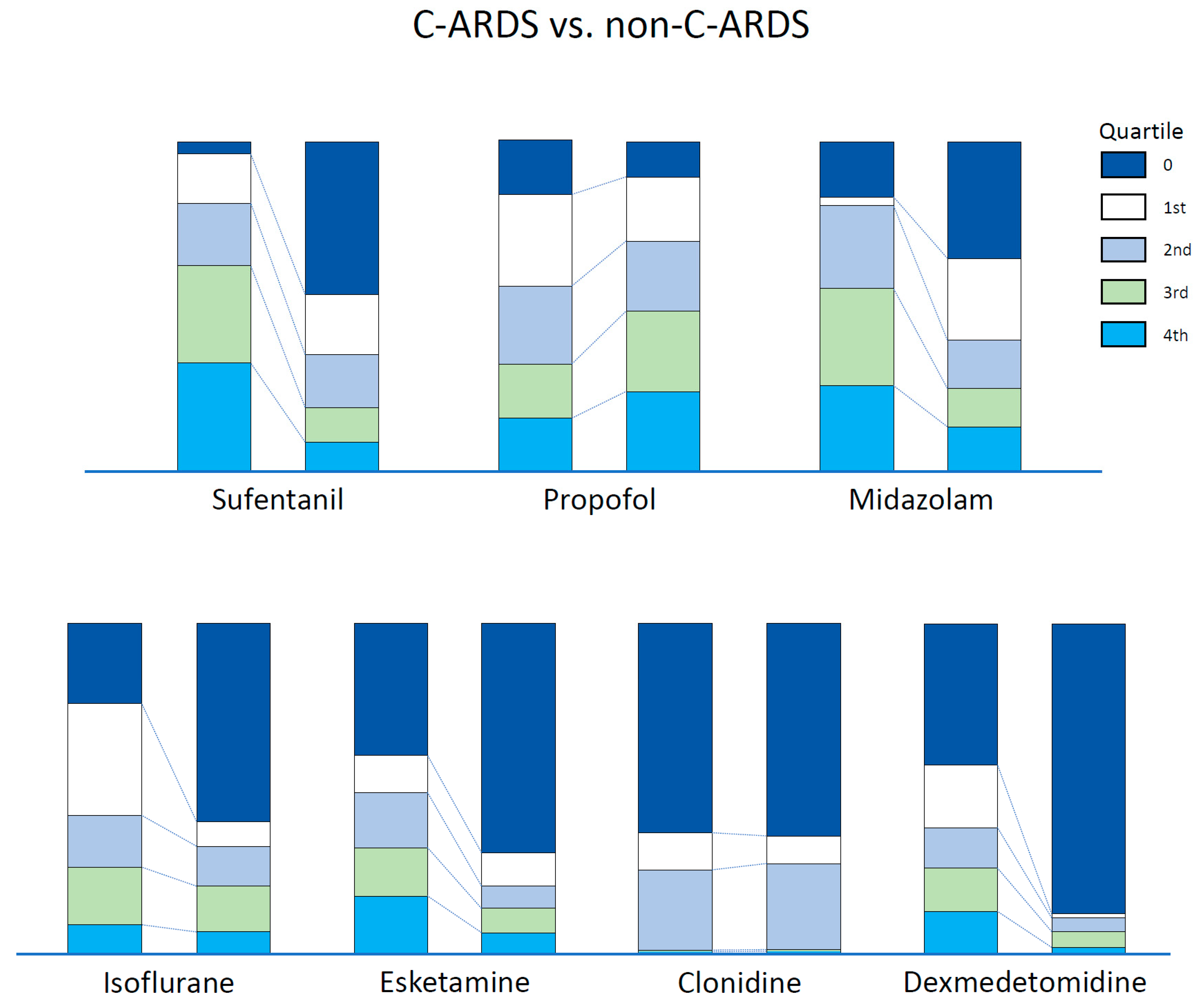
3.2. Analgosedation Needs
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Jarczak, D.; Thasler, L.; Bachmann, M.; Schulte, F.; Bein, B.; Weber, C.F.; Schäfer, U.; Veit, C.; Hauber, H.-P.; et al. Mechanical Ventilation and Mortality among 223 Critically Ill Patients with Coronavirus Disease 2019: A Multicentric Study in Germany. Aust. Crit. Care 2021, 34, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Kahn, A.; Jarczak, D.; Fischer, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Nierhaus, A.; et al. Clinical Characteristics, Complications and Outcomes of Patients with Severe Acute Respiratory Distress Syndrome Related to COVID-19 or Influenza Requiring Extracorporeal Membrane Oxygenation—A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5440. [Google Scholar] [CrossRef]
- WHO. WHO Coronavirus (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 28 March 2023).
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. High Sedation Needs of Critically Ill COVID-19 ARDS Patients—A Monocentric Observational Study. PLoS ONE 2021, 16, e0253778. [Google Scholar] [CrossRef] [PubMed]
- Tapaskar, N.; Colon Hidalgo, D.; Koo, G.; Shingada, K.; Rao, S.; Rodriguez, R.; Alcantar, D.; Espinoza Barrera, D.; Lee, R.; Rameshkumar, N.; et al. Sedation Usage in COVID-19 Acute Respiratory Distress Syndrome: A Multicenter Study. Ann. Pharmacother. 2022, 56, 117–123. [Google Scholar] [CrossRef]
- Hanidziar, D.; Bittner, E.A. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth. Analg. 2020, 131, e40–e41. [Google Scholar] [CrossRef] [PubMed]
- Kapp, C.M.; Zaeh, S.; Niedermeyer, S.; Punjabi, N.M.; Siddharthan, T.; Damarla, M. The Use of Analgesia and Sedation in Mechanically Ventilated Patients with COVID-19 Acute Respiratory Distress Syndrome. Anesth. Analg. 2020, 131, e198–e200. [Google Scholar] [CrossRef]
- Madhok, J.; Mihm, F.G. Rethinking Sedation during Prolonged Mechanical Ventilation for COVID-19 Respiratory Failure. Anesth. Analg. 2020, 131, e123–e124. [Google Scholar] [CrossRef]
- Balakrishna, A.; Walsh, E.C.; Hamidi, A.; Berg, S.; Austin, D.; Pino, R.M.; Hanidziar, D.; Chang, M.G.; Bittner, E.A. An Examination of Sedation Requirements and Practices for Mechanically Ventilated Critically Ill Patients with COVID-19. Am. J. Health Syst. Pharm. 2021, 78, 1952–1961. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. Associated Factors of High Sedative Requirements within Patients with Moderate to Severe COVID-19 ARDS. J. Clin. Med. 2022, 11, 588. [Google Scholar] [CrossRef]
- Bohman, J.K.; Nei, S.D.; Mellon, L.N.; Ashmun, R.S.; Guru, P.K. Physical Therapy and Sedation While on Extracorporeal Membrane Oxygenation for COVID-19–Associated Acute Respiratory Distress Syndrome. J. Cardiothorac. Vasc. Anesth. 2022, 36, 524–528. [Google Scholar] [CrossRef]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Rubio Mateo-Sidron, J.A.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Ex-tracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.C.; Vasilevskis, E.E.; Olsen, K.M.; Schmid, K.K.; Shostrom, V.; Cohen, M.Z.; Peitz, G.; Gannon, D.E.; Sisson, J.; Sullivan, J.; et al. Effectiveness and Safety of the Awakening and Breathing Coordination, Delirium Monitoring/Management, and Early Exercise/Mobility Bundle. Crit. Care Med. 2014, 42, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Malin, J.J.; Spinner, C.D.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Gastmeier, P.; Langer, F.; Wepler, M.; Westhoff, M.; et al. Key Summary of German National Treatment Guidance for Hospitalized COVID-19 Patients: Key Pharmacologic Recommendations from a National German Living Guideline Using an Evidence to Decision Framework (Last Updated 17.05.2021). Infection 2022, 50, 93–106. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Marx, G.; Karagiannidis, C. German Recommendations for Critically Ill Patients with COVID 19. Med. Klin. Intensivmed. Notfmed. 2020, 115 (Suppl. S3), 111–114. [Google Scholar] [CrossRef]
- Chanques, G.; Constantin, J.-M.; Devlin, J.W.; Ely, E.W.; Fraser, G.L.; Gélinas, C.; Girard, T.D.; Guérin, C.; Jabaudon, M.; Jaber, S.; et al. Analgesia and Sedation in Patients with ARDS. Intensive Care Med. 2020, 46, 2342–2356. [Google Scholar] [CrossRef]
- Dreucean, D.; Harris, J.E.; Voore, P.; Donahue, K.R. Approach to Sedation and Analgesia in COVID-19 Patients on Venovenous Extracorporeal Membrane Oxygenation. Ann. Pharmacother. 2022, 56, 73–82. [Google Scholar] [CrossRef]
- Sigala, M.I.; Dreucean, D.; Harris, J.E.; Donahue, K.R.; Bostan, F.; Voore, P.; Cuevas, J.; Morton, C. Comparison of Sedation and Analgesia Requirements in Patients with SARS-CoV-2 versus Non-SARS-CoV-2 Acute Respiratory Distress Syndrome on Veno-Venous ECMO. Ann. Pharmacother. 2023; ahead of print. [Google Scholar]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Chang, S.H.; Smith, D.E.; Carillo, J.A.; Sommer, P.M.; Geraci, T.C.; Williams, D.; Paone, D.; Goldenberg, R.; Chan, J.; Kon, Z.N.; et al. Efficacy of Proning in Acute Respiratory Distress Syndrome on Extracorporeal Membrane Oxygenation. JTCVS Tech. 2022, 16, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Voelker, M.T.; Jahn, N.; Bercker, S.; Becker-Rux, D.; Köppen, S.; Kaisers, U.X.; Laudi, S. Bauchlagerung von Patienten an der venovenösen ECMO ist möglich und sicher. Anaesthesist 2016, 65, 250–257. [Google Scholar] [CrossRef]
- Zaaqoq, A.M.; Barnett, A.G.; Griffee, M.J.; MacLaren, G.; Jacobs, J.P.; Heinsar, S.; Suen, J.Y.; Bassi, G.L.; Fraser, J.F.; Dalton, H.J.; et al. Beneficial Effect of Prone Positioning during Venovenous Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019. Crit. Care Med. 2022, 50, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Demeulemeester, F.; de Punder, K.; van Heijningen, M.; van Doesburg, F. Obesity as a Risk Factor for Severe COVID-19 and Complications: A Review. Cells 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Helvaci, N.; Eyupoglu, N.D.; Karabulut, E.; Yildiz, B.O. Prevalence of Obesity and Its Impact on Outcome in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 598249. [Google Scholar] [CrossRef]
- Weaver, L.; Das, A.; Saffaran, S.; Yehya, N.; Scott, T.E.; Chikhani, M.; Laffey, J.G.; Hardman, J.G.; Camporota, L.; Bates, D.G. High Risk of Patient Self-Inflicted Lung Injury in COVID-19 with Frequently Encountered Spontaneous Breathing Patterns: A Computational Modelling Study. Ann. Intensive Care 2021, 11, 109. [Google Scholar] [CrossRef] [PubMed]
| Parameters | C-ARDS n = 115 | Non-C-ARDS n = 250 | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (years) | 56 (48–62.8) | 54 (44.3–63) | 0.359 |
| Sex | 0.788 | ||
| Male | 78 (67.8%) | 166 (66.4%) | |
| Female | 37 (32.2%) | 84 (33.6%) | |
| Height (cm) | 176 (167.5–182) | 176 (168–180) | 0.934 |
| Weight (kg) | 95 (82–110) | 82.5 (70–95.8) | <0.001 |
| BMI (kg/m2) | 31.3 (27.4–35.8) | 26.6 (23.5–31.3) | <0.001 |
| Comorbidities | |||
| Arterial hypertension | 55 (47.8%) | 57 (22.8%) | <0.001 |
| Chronic heart failure | 5 (4.3%) | 111 (44.4%) | <0.001 |
| Diabetes mellitus | 36 (31.3%) | 48 (19.2%) | 0.011 |
| Chronic kidney disease (any stage) | 4 (3.5%) | 12 (4.8%) | 0.784 |
| Liver cirrhosis (any stage) | 1 (0.9%) | 0 (0%) | 1.000 |
| Chronic lung disease | 17 (14.8%) | 139 (55.6%) | <0.001 |
| Immunosuppression | 11 (9.6%) | 68 (27.2%) | <0.001 |
| Sum of comorbidities | 0.003 | ||
| 0 | 34 (29.6%) | 58 (23.2%) | |
| 1 | 52 (45.2%) | 84 (33.6%) | |
| 2 | 20 (17.4%) | 50 (20%) | |
| 3 | 9 (7.8%) | 45 (18.0%) | |
| 4 | 0 (0%) | 13 (5.2%) | |
| Reasons for VV-ECMO | --- | ||
| COVID-19 | 115 (100%) | 0 (0%) | |
| Bacterial pneumonia | 0 (0%) | 163 (65.2%) | |
| Influenza | 0 (0%) | 37 (14.8%) | |
| Other | 0 (0%) | 50 (20.0%) | |
| ICU characteristics | |||
| SAPS II on admission | 40 (33–48) | 41 (34–49) | 0.583 |
| ICU survival | 47 (40.9%) | 100 (40.0%) | 0.875 |
| ICU length of stay (days) | 35 (18–55) | 24 (13–39) | <0.001 |
| Parameters | C-ARDS n = 115 | Non-C-ARDS n = 250 | p-Value |
|---|---|---|---|
| ECMO therapy | |||
| Time with ECMO and MV (hours) | 442.0 (174.0–735.2) | 170.1 (100.0–288.5) | <0.001 |
| Days with ECMO (days) | 19.7 (7.6–32.2) | 10.1 (5.8–17.0) | <0.001 |
| ECMO proning | 37 (32.2%) | 25 (10.0%) | <0.001 |
| Tracheotomy | 12 (12.2%) | 124 (49.6%) | <0.001 |
| CRRT | 44 (38.3%) | 154 (61.6%) | <0.001 |
| Sedation | |||
| Sufentanil (µg/kg/h ECMO) | 0.58 (0.43–0.75) | 0.41 (0.31–0.60) | <0.001 |
| Propofol (mg/kg/h ECMO) | 1.05 (0.26–1.92) | 1.49 (0.67–2.20) | 0.008 |
| Midazolam (µg/kg/h ECMO) | 42.22 (21.37–62.29) | 8.60 (0.07–50.00) | <0.001 |
| Isoflurane (ml/kg/h ECMO) | 0.03 (0.02–0.05) | 0.04 (0.03–0.05) | 0.002 |
| Esketamine (mg/kg/h ECMO) | 0.51 (0.29–0.76) | 0.45 (0.16–0.70) | 0.139 |
| Clonidine (µg/kg/h ECMO) | 0.75 (0.41–1.12) | 0.68 (0.51–0.92) | 0.528 |
| Dexmedetomidine (µg/kg/h ECMO) | 0.45 (0.25–0.70) | 0.58 (0.47–0.70) | 0.061 |
| Sum score of quartiles incl. sufentanil | 12 (9–15) | 7 (4–11) | <0.001 |
| Year of ECMO | --- | ||
| 2009 | 3 (1.2%) | ||
| 2010 | 2 0.8%) | ||
| 2011 | 6 (2.4%) | ||
| 2012 | 3 (1.2%) | ||
| 2013 | 18 (7.2%) | ||
| 2014 | 24 (9.6%) | ||
| 2015 | 39 (11.6%) | ||
| 2016 | 51 (20.4%) | ||
| 2017 | 32 (12.8%) | ||
| 2018 | 29 (11.6%) | ||
| 2019 | 31 (12.4%) | ||
| 2020 | 42 (36.5%) | 12 (4.8%) | |
| 2021 | 62 (53.9%) | 0 (0%) | |
| 2022 (until April 30th) | 11 (9.6%) | 0 (0%) |
| Variable | B | 95% CI | p |
|---|---|---|---|
| Univariable model | |||
| COVID-19 (ref. non-COVID-19) | 0.468 | 0.372, 0.563 | <0.001 |
| Multivariable model, first step | |||
| COVID-19 (ref. non-COVID-19) | −0.135 | −0.297, 0.026 | 0.099 |
| BMI | −0.006 | −0.010, −0.002 | 0.005 |
| chronic comorbidities | −0.027 | −0.080, 0.026 | 0.323 |
| CHF | −0.070 | −0.215, 0.074 | 0.337 |
| AKI requiring CRRT | −0.034 | −0.135, 0.068 | 0.514 |
| year of VV-ECMO | 0.125 | 0.096, 0.154 | <0.001 |
| prone positioning on VV-ECMO | 0.122 | 0.016, 0.228 | 0.024 |
| SAPS II on admission | −0.006 | −0.010, −0.002 | 0.002 |
| Multivariable model, final step | |||
| COVID-19 (ref. non-COVID-19) | −0.054 | −0.195, 0.086 | 0.445 |
| BMI | −0.007 | −0.011, −0.003 | <0.001 |
| year of VV-ECMO | 0.119 | 0.090, 0.148 | <0.001 |
| prone positioning on VV-ECMO | 0.119 | 0.013, 0.225 | 0.028 |
| SAPS II on admission | −0.007 | −0.010, −0.003 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paparoupa, M.; Fischer, M.; Pinnschmidt, H.O.; Grensemann, J.; Roedl, K.; Kluge, S.; Jarczak, D. Impact of COVID-19 on Sedation Requirements during Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. J. Clin. Med. 2023, 12, 3515. https://doi.org/10.3390/jcm12103515
Paparoupa M, Fischer M, Pinnschmidt HO, Grensemann J, Roedl K, Kluge S, Jarczak D. Impact of COVID-19 on Sedation Requirements during Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Journal of Clinical Medicine. 2023; 12(10):3515. https://doi.org/10.3390/jcm12103515
Chicago/Turabian StylePaparoupa, Maria, Marlene Fischer, Hans O. Pinnschmidt, Jörn Grensemann, Kevin Roedl, Stefan Kluge, and Dominik Jarczak. 2023. "Impact of COVID-19 on Sedation Requirements during Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome" Journal of Clinical Medicine 12, no. 10: 3515. https://doi.org/10.3390/jcm12103515
APA StylePaparoupa, M., Fischer, M., Pinnschmidt, H. O., Grensemann, J., Roedl, K., Kluge, S., & Jarczak, D. (2023). Impact of COVID-19 on Sedation Requirements during Veno-Venous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Journal of Clinical Medicine, 12(10), 3515. https://doi.org/10.3390/jcm12103515








