It Is Time for a Multidisciplinary Rehabilitation Approach: A Scoping Review on Stomatognathic Diseases in Neurological Disorders
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. PICO Evaluation
2.3. Inclusion and Exclusion Criteria
2.4. Literature Selection
2.5. Study Risk of Bias Assessment
3. Results
4. Pathophysiology of Stomatognathic and Temporomandibular Joint Disorders in Neurological Disorders
5. Diagnostic Methods and Tools for TMD
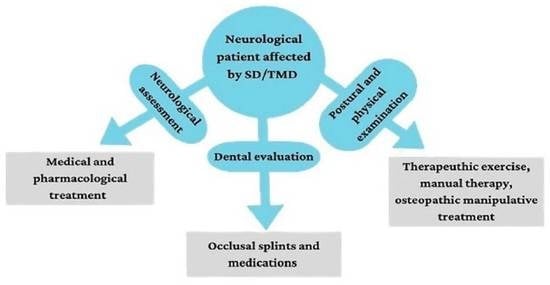
6. Cranial–Temporomandibular and Stomatognathic Rehabilitation Approach
7. Discussion
- -
- Parafunctional activities, such as bruxism and day clenching, have been found in PD and MS patients [11,19,22,23], causing muscle pain and harmful effects on tooth enamel. Currently, there are no treatment methods to make these alterations stop. Occlusal splint therapy can reduce bruxism and clenching symptoms, acting on a negative feedback mechanism that greatly decreases muscle activity, maintaining a normal activation threshold for the muscle protective reflex. In addition, MT and MFR could be useful to induce masseter, temporalis and neck muscle relaxation that are associated with these symptoms [65];
- -
- Reduction in TMJ movements due to painful muscle contractions is a common TMD feature in neurological conditions, especially in OMD, PD and SCA [21,24,29,35,37,38]. However, evidence-based treatments have been documented only for OMD subjects, suggesting the use of Botox injections in specific head and neck muscles, such as platysma, lateral pterygoid and temporalis, to reduce muscle spasms [38]. Coordination exercises of TMJ through opening and closing the mouth, using a mirror or fingers bilaterally, to promote symmetrical movements may also be of help [62,82,83]. Additionally, MT and/or OMT could induce muscle release and restore pain thanks to the discharge of endogenous opioids due to therapeutic touch [9,65];
- -
- Muscle weakness and reduced TMJ functions were found in post-stroke survivors as a result of the acute onset of the brain damage [25,26,27,28]. In this clinical condition, authors [26,27,60] suggested the administration of specific isometric and isotonic exercises to improve jaw muscle strength and postural programs [61,84], including exercises to increase TMJ and neck mobility. To restore oral muscle strength, some evidence supported the administration of specific exercises for labial, intrinsic tongue and masticatory muscles, which can be helpful in managing dysphagic symptoms [28].
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SS | Stomatognathic system |
| TMJ | Temporomandibular joint |
| TMD | Temporomandibular dysfunction |
| SD | Stomatognathic disease |
| PT | Physiotherapy |
| MT | Manual therapy |
| OMT | Osteopathic manipulative treatment |
| CPG | Central pattern generator |
| MS | Multiple sclerosis |
| SCA | Spinocerebellar ataxia |
| PD | Parkinson’s disease |
| OMD | Oromandibular dystonia |
| RoB | Revised Cochrane risk of bias |
| NOS | Newcastle–Ottawa Scale |
| MPQ | McGill Pain Questionnaire |
| SF-MPQ | Short form McGill Pain Questionnaire |
| PPI | Present pain intensity |
| VAS | Visual analogical scale |
| VRS | Verbal rating scale |
| NRS | Numerical rating scale |
| FPS | Faces pain scale |
| HCDI | Helkimo Clinical Dysfunction Index |
| OVD | Occlusal vertical dimension |
| RVD | Resting vertical dimension |
| MMO | Maximum mouth opening |
| LEP | Laser-evoked potential |
| CT | Computed tomography |
| MRI | Magnetic Resonance Imaging |
| CBCT | Cone-beam computed tomography |
| CTS-R | Cranial–temporomandibular and stomatognathic rehabilitation |
| MFR | Myofascial release |
| CST | Cranial–sacral therapy |
| BLT | Balanced ligamentous tension |
References
- Zieliński, G.; Filipiak, Z.; Ginszt, M.; Matysik-Woźniak, A.; Rejdak, R.; Gawda, P. The Organ of Vision and the Stomatognathic System—Review of Association Studies and Evidence-Based Discussion. Brain Sci. 2022, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Lam, O.L.; Lo, E.C.; Li, L.S.; Wen, Y.; McGrath, C. Orofacial functional impairments among patients following stroke: A systematic review. Oral. Dis. 2015, 21, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, M.; Boening, K.; Wiland, P.; Shiau, Y.-Y.; Paradowska-Stolarz, A. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J. Headache Pain 2015, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- McNeely, M.; Armijo Olivo, S.; Magee, D. A systematic review of physical therapy intervention for temporomandibular dis-orders. Phys. Ther. 2006, 86, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Pitance, L.; Singh, V.; Neto, F.; Thie, N.; Michelotti, A. Effectiveness of Manual Therapy and Therapeutic Exercise for Temporomandibular Disorders: Systematic Review and Meta-Analysis. Phys. Ther. 2016, 96, 9–25. [Google Scholar] [CrossRef]
- Easterbrook, S.; Keys, J.; Talsma, J.; Pierce-Talsma, S. Osteopathic Manipulative Treatment for Temporomandibular Disorders. J. Am. Osteopat. Assoc. 2019, 119, e29–e30. [Google Scholar] [CrossRef]
- Gesslbauer, C.; Vavti, N.; Keilani, M.; Mickel, M.; Crevenna, R. Effectiveness of osteopathic manipulative treatment versus osteopathy in the cranial field in temporomandibular disorders—A pilot study. Disabil. Rehabil. 2018, 40, 631–636. [Google Scholar] [CrossRef]
- McParlin, Z.; Cerritelli, F.; Friston, K.J.; Esteves, J.E. Therapeutic Alliance as Active Inference: The Role of Therapeutic Touch and Synchrony. Front. Psychol. 2022, 13, 783694. [Google Scholar] [CrossRef]
- Yin, Y.; He, S.; He, N.; Zhang, W.; Luo, L.; Chen, L.; Liu, T.; Tian, M.; Xu, J.; Chen, S.; et al. Brain alterations in sensorimotor and emotional regions associated with temporomandibular disorders. Oral. Dis. 2022. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Crimi, S.; Badnjević, A.; Cervino, G.; Bianchi, A.; Cicciù, M. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- An, J.-S.; Jeon, D.-M.; Jung, W.-S.; Yang, I.-H.; Lim, W.H.; Ahn, S.-J. Influence of temporomandibular joint disc displacement on craniocervical posture and hyoid bone position. Am. J. Orthod. Dentofac. Orthop. 2015, 147, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Paço, M.; Duarte, J.; Pinho, T. Orthodontic Treatment and Craniocervical Posture in Patients with Temporomandibular Disor-ders: An Observational Study. Int. J. Environ. Res. Public. Health 2021, 18, 3295. [Google Scholar] [CrossRef] [PubMed]
- Garstka, A.A.; Brzózka, M.; Bitenc-Jasiejko, A.; Ardan, R.; Gronwald, H.; Skomro, P.; Lietz-Kijak, D. Cause-Effect Relationships between Painful TMD and Postural and Functional Changes in the Musculoskeletal System: A Preliminary Report. Pain Res. Manag. 2022, 2022, 1429932. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. A Review of the PubMed PICO Tool: Using Evidence-Based Practice in Health Education. Health Promot. Pract. 2019, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Więckowska, B.; Kubiak, K.B.; Jóźwiak, P.; Moryson, W.; Stawińska-Witoszyńska, B. Cohen’s Kappa Coefficient as a Measure to Assess Classification Improvement following the Addition of a New Marker to a Regression Model. Int. J. Environ. Res. Public. Health 2022, 19, 10213. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Costa, C.; Santiago, H.; Pereira, S.; Castro, A.R.; Soares, S.C. Oral Health Status and Multiple Sclerosis: Classic and Non-Classic Manifestations—Case Report. Diseases 2022, 10, 62. [Google Scholar] [CrossRef]
- Williams, D.E.; Lynch, J.E.; Doshi, V.; Singh, G.D.; Hargens, A.R. Bruxism and Temporal Bone Hypermobility in Patients with Multiple Sclerosis. Cranio® 2011, 29, 178–186. [Google Scholar] [CrossRef]
- Ferreira, B.; Palinkas, M.; Gonçalves, L.; Da Silva, G.; Arnoni, V.; Regalo, I.H.; Vasconcelos, P.; Júnior, W.; Hallak, J.; Regalo, S.C.H.; et al. Spinocerebellar ataxia: Functional analysis of the stomatognathic system. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e165–e171. [Google Scholar] [CrossRef]
- Choi, H.-G.; Yoon, J.-H.; Chung, T.-H.; Min, C.; Yoo, D.-M.; Wee, J.-H.; Kang, S.-Y.; Choi, Y.; Hong, S.-J.; Byun, S.-H. Association between Temporomandibular Joint Disorder and Parkinson’s Disease. Brain Sci. 2021, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Beers, E.; Van Grootheest, A.C. Bruxisme als bijwerking van serotonineheropnameremmers. Ned. Tijdschr. Tandheelkd. 2007, 114, 388–390. [Google Scholar] [PubMed]
- Handa, S.; Shaefer, J.R.; Keith, D.A. Oromandibular dystonia and temporomandibular disorders. J. Am. Dent. Assoc. 2022, 153, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.; Moura, B.G.; Araujo, C.C.; Del Antonio, T.T.; Da Silva, J.K.M. Temporomandibular dysfunction in patients with a history of stroke. Man. Ther. Posturol. Rehabil. J. 2020, 17, 1–5. [Google Scholar] [CrossRef]
- Choi, J.B.; Jung, Y.J.; Park, J. Comparison of 2 types of therapeutic exercise: Jaw opening exercise and head lift exercise for dysphagic stroke: A pilot study. Medicine 2020, 99, e22136. [Google Scholar] [CrossRef]
- Oh, D.-W.; Kang, T.-W.; Kim, S.-J. Effect of Stomatognathic Alignment Exercise on Temporomandibular Joint Function and Swallowing Function of Stroke Patients with Limited Mouth Opening. J. Phys. Ther. Sci. 2013, 25, 1325–1329. [Google Scholar] [CrossRef]
- Umay, E.K.; Yilmaz, V.; Gundogdu, I.; Ozturk, E.; Gurcay, E.; Karaahmet, O.; Saylam, G.; Ceylan, T.; Cakci, A. What happens to swallowing muscles after stroke? A prospective randomized controlled electrophysiological study. Neurol. India 2019, 67, 1459–1466. [Google Scholar] [CrossRef]
- Verhoeff, M.C.; Lobbezoo, F.; Wetselaar, P.; Aarab, G.; Koutris, M. Parkinson’s disease, temporomandibular disorders and bruxism: A pilot study. J. Oral. Rehabil. 2018, 45, 854–863. [Google Scholar] [CrossRef]
- Minervini, G.; Mariani, P.; Fiorillo, L.; Cervino, G.; Cicciù, M.; Laino, L. Prevalence of temporomandibular disorders in people with multiple sclerosis: A systematic review and meta-analysis. Cranio® 2022, 1–9. [Google Scholar] [CrossRef]
- Manchery, N.; Henry, J.D.; Nangle, M.R. A systematic review of oral health in people with multiple sclerosis. Community Dent. Oral. Epidemiol. 2020, 48, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Lee, K.-E.; Suh, B.-J. Influence of psychological factors on the prognosis of temporomandibular disorders pain. J. Dent. Sci. 2021, 16, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.; Matta, A.; Nascimento, O. Temporomandibular disorders (TMD) and multiple sclerosis (MS)(P1. 120). Cranio 2015, 84, 120. [Google Scholar]
- Liang, L.; Chen, T.; Wu, Y. The electrophysiology of spinocerebellar ataxias. Neurophysiol. Clin. 2016, 46, 27–34. [Google Scholar] [CrossRef]
- Velázquez-Pérez, L.C.; Rodríguez-Labrada, R.; Fernandez-Ruiz, J. Spinocerebellar Ataxia Type 2: Clinicogenetic Aspects, Mechanistic Insights, and Management Approaches. Front. Neurol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.C.; Koutris, M.; Berendse, H.W.; van Dijk, K.D.; Lobbezoo, F. Parkinson’s disease, temporomandibular disorder pain and bruxism and its clinical consequences: A protocol of a single-centre observational outpatient study. BMJ Open. 2022, 12, e052329. [Google Scholar] [CrossRef]
- Albanese, A.; Bhatia, K.; Bressman, S.B.; DeLong, M.R.; Fahn, S.; Fung, V.S.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Klein, C.; et al. Phenomenology and classification of dystonia: A consensus update. Mov. Disord. 2013, 28, 863–873. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins 2022, 14, 282. [Google Scholar] [CrossRef]
- Yoshida, K. Clinical Characteristics of Functional Movement Disorders in the Stomatognathic System. Front. Neurol. 2020, 11, 123. [Google Scholar] [CrossRef]
- Schimmel, M.; Leemann, B.; Christou, P.; Kiliaridis, S.; Herrmann, F.R.; Müller, F. Quantitative assessment of facial muscle impairment in patients with hemispheric stroke. J. Oral. Rehabil. 2011, 38, 800–809. [Google Scholar] [CrossRef]
- Lau, K.T.; Cheung, K.Y.; Chan, K.B.; Chan, M.H.; Lo, K.Y.; Chiu, T.T.W. Relationships between sagittal postures of thoracic and cervical spine, presence of neck pain, neck pain severity and disability. Man. Ther. 2010, 15, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, E.C.; Bérzin, F. Temporomandibular disorder and dysfunctional breathing. Braz. J. Oral. Sci. 2004, 3, 498–502. [Google Scholar]
- Saran, S.; Saccomanno, S.; Petricca, M.T.; Carganico, A.; Bocchieri, S.; Mastrapasqua, R.F.; Caramaschi, E.; Levrini, L. Physiotherapists and Osteopaths’ Attitudes: Training in Management of Temporomandibular Disorders. Dent. J. 2022, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.D. Assessment of the Orofacial Pain Patient. Dent. Clin. 2018, 62, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Sirintawat, N.; Sawang, K.; Chaiyasamut, T.; Wongsirichat, N. Pain measurement in oral and maxillofacial surgery. J. Dent. Anesth. Pain Med. 2017, 17, 253–263. [Google Scholar] [CrossRef]
- Alonso-Royo, R.; Sánchez-Torrelo, C.M.; Ibáñez-Vera, A.J.; Zagalaz-Anula, N.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Rodríguez-Almagro, D.; Lomas-Vega, R. Validity and Reliability of the Helkimo Clinical Dysfunction Index for the Diagnosis of Temporomandibular Disorders. Diagnostics 2021, 11, 472. [Google Scholar] [CrossRef]
- De Tommaso, M. Laser-evoked potentials in primary headaches and cranial neuralgias. Expert. Rev. Neurother. 2008, 8, 1339–1345. [Google Scholar] [CrossRef]
- Małgorzata, P.; Małgorzata, K.-M.; Karolina, C.; Gala, A. Diagnostic of Temporomandibular Disorders and Other Facial Pain Conditions—Narrative Review and Personal Experience. Medicina 2020, 56, 472. [Google Scholar] [CrossRef]
- Krasińska-Mazur, M.; Homel, P.; Gala, A.; Stradomska, J.; Pihut, M. Differential diagnosis of temporomandibular disorders—A review of the literature. Folia Med. Cracov. 2022, 62, 121–137. [Google Scholar] [CrossRef]
- Okeson, J.P.; de Leeuw, R. Differential Diagnosis of Temporomandibular Disorders and Other Orofacial Pain Disorders. Dent. Clin. 2011, 55, 105–120. [Google Scholar] [CrossRef]
- Mehta, S.B.; Rizzo, D.; Paulose, B.; Botbol, A.; Vijay, S.; Arjuna, A.; Banerji, S. An evaluation of dental practitioner habits with occlusal assessment and the clinical application of practical techniques in occlusion, amongst a cohort of participants based in the UK, South Africa, Malta, and Malaysia. J. Oral. Rehabil. 2022, 49, 944–953. [Google Scholar] [CrossRef] [PubMed]
- de Kanter, R.J.A.M.; Battistuzzi, P.G.F.C.M.; Truin, G.-J. Temporomandibular Disorders: “Occlusion” Matters! Pain Res. Manag. 2018, 2018, 8746858. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, S.S.; Stern, I.; Sollecito, T.P. Disorders of the Masticatory Muscles. Dent. Clin. 2013, 57, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H. Associations Among Temporomandibular Joint Osteoarthritis, Airway Dimensions, and Head and Neck Posture. J. Oral. Maxillofac. Surg. 2020, 78, 2183.e1–2183.e12. [Google Scholar] [CrossRef]
- Opris, H.; Baciut, M.; Bran, S.; Onisor, F.; Almasan, O.; Manea, A.; Tamas, T.; Stoia, S.; Gabriel, A.; Baciut, G.; et al. Lateral Cephalometric Analytical Uses for Temporomandibular Joint Disorders: The Importance of Cervical Posture and Hyoid Position. Int. J. Environ. Res. Public. Health 2022, 19, 11077. [Google Scholar] [CrossRef]
- Manfredini, D.; Lobbezoo, F. Role of psychosocial factors in the etiology of bruxism. J. Orofac. Pain 2009, 23, 153–166. [Google Scholar]
- Ferreira, L.A.; Grossmann, E.; Januzzi, E.; de Paula, M.V.Q.; Carvalho, A.C.P. Diagnosis of temporomandibular joint disorders: Indication of imaging exams. Braz. J. Otorhinolaryngol. 2016, 82, 341–352. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar]
- Chan, N.H.Y.; Ip, C.K.; Li, D.T.S.; Leung, Y.Y. Diagnosis and Treatment of Myogenous Temporomandibular Disorders: A Clinical Update. Diagnostics 2022, 12, 2914. [Google Scholar] [CrossRef]
- Zapata-Soria, M.; Cabrera-Martos, I.; López-López, L.; Ortiz-Rubio, A.; Granados-Santiago, M.; Ríos-Asín, I.; Valenza, M.C. Clinical Characteristics and Rehabilitation Strategies for the Stomatognathic System Disturbances in Patients with Stroke: A Systematic Review. Int. J. Environ. Res. Public. Health 2023, 20, 657. [Google Scholar] [CrossRef]
- Carini, F.; Mazzola, M.; Fici, C.; Palmeri, S.; Messina, M.; Damiani, P.; Tomasello, G. Posture and posturology, anatomical and physiological profiles: Overview and current state of art. Acta Bio Med. Atenei Parm. 2017, 88, 11–16. [Google Scholar] [CrossRef]
- Haketa, T.; Kino, K.; Sugisaki, M.; Takaoka, M.; Ohta, T. Randomized Clinical Trial of Treatment for TMJ Disc Displacement. J. Dent. Res. 2010, 89, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, A.B.; Ergun, N.; Tuncer, A.H.; Karahan, S. Effectiveness of manual therapy and home physical therapy in patients with temporomandibular disorders: A randomized controlled trial. J. Bodyw. Move Ther. 2013, 17, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ishigaki, S.; Matsuka, Y.; Komiyama, O.; Torisu, T.; Oono, Y.; Sato, H.; Naganawa, T.; Mine, A.; Yamazaki, Y.; et al. Effects of exercise therapy on painful temporomandibular disorders. J. Oral. Rehabil. 2019, 46, 475–481. [Google Scholar] [CrossRef]
- Roberts, A.; Harris, K.; Outen, B.; Bukvic, A.; Smith, B.; Schultz, A.; Bergman, S.; Mondal, D. Osteopathic Manipulative Medicine: A Brief Review of the Hands-On Treatment Approaches and Their Therapeutic Uses. Medicines 2022, 9, 33. [Google Scholar] [CrossRef]
- Brantingham, J.W.; Cassa, T.K.; Bonnefin, D.; Pribicevic, M.; Robb, A.; Pollard, H.; Tong, V.; Korporaal, C. Manipulative and Multimodal Therapy for Upper Extremity and Temporomandibular Disorders: A Systematic Review. J. Manip. Physiol. Ther. 2013, 36, 143–201. [Google Scholar] [CrossRef]
- Bordoni, B.; Zanier, E. Sutherland’s legacy in the new millennium: The osteopathic cranial model and modern osteopathy. Adv. Mind Body Med. 2015, 29, 15–21. [Google Scholar]
- Zhang, L.; Xu, L.; Wu, D.; Yu, C.; Fan, S.; Cai, B. Effectiveness of exercise therapy versus occlusal splint therapy for the treatment of painful temporomandibular disorders: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 6122–6132. [Google Scholar] [CrossRef]
- de Paula Gomes CA, F.; El Hage, Y.; Amaral, A.P.; Politti, F.; Biasotto-Gonzalez, D.A. Effects of massage therapy and occlusal splint therapy on electromyographic activity and the intensity of signs and symptoms in individuals with temporomandibular disorder and sleep bruxism: A randomized clinical trial. Chiropr. Man. Ther. 2014, 22, 43. [Google Scholar] [CrossRef]
- Penlington, C.; Bowes, C.; Taylor, G.; Otemade, A.A.; Waterhouse, P.; Durham, J.; Ohrbach, R. Psychological therapies for temporomandibular disorders (TMDs). Cochrane Database Syst. Rev. 2022, 8, CD013515. [Google Scholar] [CrossRef]
- Urbański, P.; Trybulec, B.; Pihut, M. The Application of Manual Techniques in Masticatory Muscles Relaxation as Adjunctive Therapy in the Treatment of Temporomandibular Joint Disorders. Int. J. Environ. Res. Public. Health 2021, 18, 12970. [Google Scholar] [CrossRef] [PubMed]
- De Melo, L.A.; Medeiros, A.; Campos, M.D.F.T.P.; De Resende, C.M.B.M.; Barbosa, G.A.S.; De Almeida, E.O. Manual Therapy in the Treatment of Myofascial Pain Related to Temporomandibular Disorders: A Systematic Review. J. Oral. Facial Pain. Headache 2020, 34, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martinez, A.; Paris-Alemany, A.; López-De-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. J. Pain Res. 2018, 11, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Kütük, S.G.; Özkan, Y.; Kütük, M.; Özdaş, T. Comparison of the Efficacies of Dry Needling and Botox Methods in the Treatment of Myofascial Pain Syndrome Affecting the Temporomandibular Joint. J. Craniofacial Surg. 2019, 30, 1556–1559. [Google Scholar] [CrossRef]
- Ouanounou, A.; Goldberg, M.; Haas, D.A. Pharmacotherapy in Temporomandibular Disorders: A Review. J. Canadian Dent. Assoc. 2017, 83, h7. [Google Scholar]
- Bae, Y.; Park, Y. The Effect of Relaxation Exercises for the Masticator Muscles on Temporomandibular Joint Dysfunction (TMD). J. Phys. Ther. Sci. 2013, 25, 583–586. [Google Scholar] [CrossRef]
- Cuccia, A.M.; Caradonna, C.; Caradonna, D. Manual therapy of the mandibular accessory ligaments for the man-agement of temporomandibular joint disorders. J. Osteopath. Med. 2011, 111, 102–112. [Google Scholar]
- Yin, C.S.; Lee, Y.J. Neurological influences of the temporomandibular joint. J. Bodyw. Mov. Ther. 2007, 11, 285–294. [Google Scholar] [CrossRef]
- Schleip, R.; Klingler, W.; Lehmann-Horn, F. Active fascial contractility: Fascia may be able to contract in a smooth muscle-like manner and thereby influence musculoskeletal dynamics. Med. Hypotheses 2005, 65, 273–277. [Google Scholar] [CrossRef]
- Schleip, R.; Gabbiani, G.; Wilke, J.; Naylor, I.; Hinz, B.; Zorn, A.; Jäger, H.; Breul, R.; Schreiner, S.; Klingler, W. Fascia Is Able to Actively Contract and May Thereby Influence Musculoskeletal Dynamics: A Histochemical and Mechanographic Investigation. Front. Physiol. 2019, 10, 336. [Google Scholar] [CrossRef]
- Stecco, A.; Giordani, F.; Fede, C.; Pirri, C.; De Caro, R.; Stecco, C. From Muscle to the Myofascial Unit: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 4527. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.T.; Akashi, P.M.H.; Sacco, I.D.C.N. Global Body Posture Evaluation in Patients with Temporomandibular Joint Disorder. Clinics 2009, 64, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Moraes, A.D.R.; Sanches, M.L.; Ribeiro, E.C.; Guimarães, A.S. Therapeutic exercises for the control of temporomandibular disorders. Dent. Press J. Orthod. 2013, 18, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Craane, B.; Dijkstra, P.U.; Stappaerts, K.; De Laat, A. Randomized Controlled Trial on Physical Therapy for TMJ Closed Lock. J. Dent. Res. 2012, 91, 364–369. [Google Scholar] [CrossRef] [PubMed]




| Neurological Disorders | Temporomandibular/Stomatognathic Diseases | Conservative and Rehabilitative Approaches |
|---|---|---|
| “Parkinson’s disease” OR “Multiple sclerosis” OR “Spino-cerebellar ataxia” OR “stroke” OR “oro-mandibular dystonia” OR “movement disorders” | “Temporomandibular joint disorders” OR “temporomandibular joint dysfunctions” OR “stomatognathic disease” OR “bruxism” OR “disc displacement” OR “temporomandibular myofascial pain” OR “orofacial pain” | “Physical exercise therapy” OR “manual therapy” OR “osteopathic manipulative treatment” OR “cranial-sacral therapy” OR “physical therapy” OR “occlusal splint therapy” |
| Reference Number | Association between SD/TMD and Neurological Disorder (Yes or No) | Stomatognathic Disease | Diagnostic Tools | Musculoskeletal Structures Involved | Conservative and Complementary Treatments | Major Findings |
|---|---|---|---|---|---|---|
| Multiple sclerosis (MS) | ||||||
| Costa et al. [19] | Yes | TMD, bruxism, tooth hypersensitivity and hyposalivation | Clinical intra- and extra-oral examination | Suboccipital and cervical muscles | Endodontic intervention, occlusal adjustment and behavioral education | The endodontic treatment met the aesthetic pleasing of the patient |
| Williams et al. [20] | Yes | Jaw clenching/bruxism | Ultrasonic pulsed phase-locked loop (PPLL) and change in acoustic pathlength (∆L) as the measure of intracranial distance | Masticatory muscles, temporal bones and TMJ | NA | Jaw clenching/bruxism was associated with the displacement of the temporal bones and expansion of the cranial cavity in MS patients compared to healthy control |
| Spinocerebellar ataxia (SCA) | ||||||
| Ferreira et al. [21] | Yes | Increased masticatory muscle activity and reduction of maximal molar bite force | RDCTMD, electromyographic activity, muscle thickness and maximum bite force | TMJ structures, masseter and temporalis | NA | SCA is characterized by functional and electromyographic alterations in SS, especially in chewing and bite force |
| Parkinson’s disease (PD) | ||||||
| Choi et al. [22] | Yes | Jaw tremor, bruxism amd TMJ rigidity | Diagnosis of TMD was considered using ICD-10 code K07.6 | Masticatory muscles, TMJ structures and cervical spine | NA | The authors stated that PD patients have a high risk to develop TMD; conversely, individuals affected by TMD have more risk to develop PD in the future |
| Verhoeff et al. [23] | Yes | Bruxism (both sleep and awake bruxism), TMD and orofacial pain | The authors created an 18-item questionnaire, reporting: (i) chronic pain; (ii) the DC/TMD; (iii) oral behavior; (iv) DC/TMD symptom questionnaire; (v) TMD pain screener | Masticatory muscles, TMJ structures and cervical spine | NA | There are correlations between PD and bruxism, and PD with TMJ pain |
| Oromandibular dystonia (OMD) | ||||||
| Handa et al. [24] | Yes | Myofascial pain in masticatory muscles and dental problems | Differential diagnosis between OMD and TMD by clinical examination and ICD-10 | Masticatory muscles, TMJ structures and dental arches | NA | Since OMD shares clinical features with TMD, they are often misdiagnosed with the risk to receive unnecessary treatments |
| Stroke | ||||||
| Alvater Ramos et al. [25] | Yes | Disc displacement and myogenous TMD | TMD diagnosis was performed using RDCTMD; physical mechanical pressure on trigger points was tested using the algometer Wagner PAIN TES and Pressure Pain Threshold Test, while cervical ROM was assessed using a Sanny Fleximeter | Cervical lateral-flexors muscles, TMJ and masticatory muscles | NA | The authors found that post-stroke patients manifested augmented muscle tone and reduced cervical ROM on the affected side, suggesting that the musculoskeletal alterations caused by a stroke can predispose to TMD |
| Choi et al. [26] | Yes | Dysphagia | Dysphagia was confirmed by a video-fluoroscopic swallowing study | Suprahyoid muscles (digastric and mylohyoid muscles) and hyoid bone movements | Jaw opening exercise (isometric and isotonic) and head lift exercise | JOE and HLE were useful to improve supra-hyoid muscle strength and thickness. However, JOE required less effort than HLF |
| Oh et al. [27] | Yes | Decreased TMJ function | Clinical examination with craniomandibular index and limited range in opening mouth; swallowing function was assessed using MASA | TMJ structures, masticatory muscles, neck and shoulder muscles | Stomatognathic alignment exercise program (exercises to increase the mobility of the neck and TMJ), head and neck posture exercises and anterior chest stretching exercise) | Stomatognathic alignment exercises were useful to improve TMJ and swallowing functions |
| Umay et al. [28] | Yes | Swallowing dysfunction, masticatory and swallowing muscles weakness | Swallowing intervals and motor action potentials (MAPs) of trigeminal, facial and hypoglossal nerves were measured | Swallowing muscles, masticatory muscles, hyoid bone and neck structures | Thermal stimulation (to radix of tongue, palate, tonsillar plica, and oral mucosa); oral motor strength exercises for labial, intrinsic tongue and masticatory muscles; intermittent galvanic stimulation | After four weeks of treatment, significant recovery in swallowing, motor and general functional levels of the patients was provided |
| Reference | Randomization Process | Effect of Assignment on Intervention | Effect of Adhering to Intervention | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Results |
|---|---|---|---|---|---|---|
| Choi et al. [26] | SC | SC | L | SC | SC | SC |
| Oh et al. [27] | L | L | L | L | SC | SC |
| Umay et al. [28] | L | L | L | L | L | L |
| Reference | NOS |
|---|---|
| Williams et al. [20] | 4 |
| Ferreira et al. [21] | 4 |
| Choi et al. [22] | 6 |
| Verhoeff et al. [23] | 7 |
| Handa et al. [24] | 5 |
| Alvater Ramos [25] | 3 |
| Main Occlusal Physiological Parameters | Description |
|---|---|
| Centric occlusion | Consists of a full occlusal contact between upper and lower teeth in habitual occlusion. |
| Incisal guidance | Consists of the influence of the contacting surfaces of the mandibular and maxillary anterior teeth on mandibular movements. |
| Canine guidance | Vertical displacement of the mandible due to gliding contact of the canine teeth, preventing potential damages. |
| Overjet | Defined as the horizontal overlap of the incisors, which can be augmented in the second occlusion class or reduced in the third class. |
| Overbite | Defined as the vertical distance between the incisal margins of the upper incisors and the incisal margins of the lower incisors. It can be increased in case of a deep bite or reduced in an open bite. |
| Occlusal vertical dimension (OVD) | Also known as the vertical dimension of occlusion and indicates the occlusion position of teeth in maximum intercuspation. A common trick is to ask the patient to say the word “Emma”, and after completing the word, the clinician has an estimate of OVD. |
| Resting vertical dimension (RVD) | Refers to a resting position of the mandibula. It happens when the maxillary and mandibular arches are not in contact with each other. |
| Freeway space | Defined as the neutral position attained by the mandibula as it is involuntarily suspended by the reciprocal coordination of masticatory muscles, with the maxillary and mandibular teeth separated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militi, A.; Bonanno, M.; Calabrò, R.S. It Is Time for a Multidisciplinary Rehabilitation Approach: A Scoping Review on Stomatognathic Diseases in Neurological Disorders. J. Clin. Med. 2023, 12, 3528. https://doi.org/10.3390/jcm12103528
Militi A, Bonanno M, Calabrò RS. It Is Time for a Multidisciplinary Rehabilitation Approach: A Scoping Review on Stomatognathic Diseases in Neurological Disorders. Journal of Clinical Medicine. 2023; 12(10):3528. https://doi.org/10.3390/jcm12103528
Chicago/Turabian StyleMiliti, Angela, Mirjam Bonanno, and Rocco Salvatore Calabrò. 2023. "It Is Time for a Multidisciplinary Rehabilitation Approach: A Scoping Review on Stomatognathic Diseases in Neurological Disorders" Journal of Clinical Medicine 12, no. 10: 3528. https://doi.org/10.3390/jcm12103528