Lipoprotein(a) Does Not Predict Thrombotic Events and In-Hospital Outcomes in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
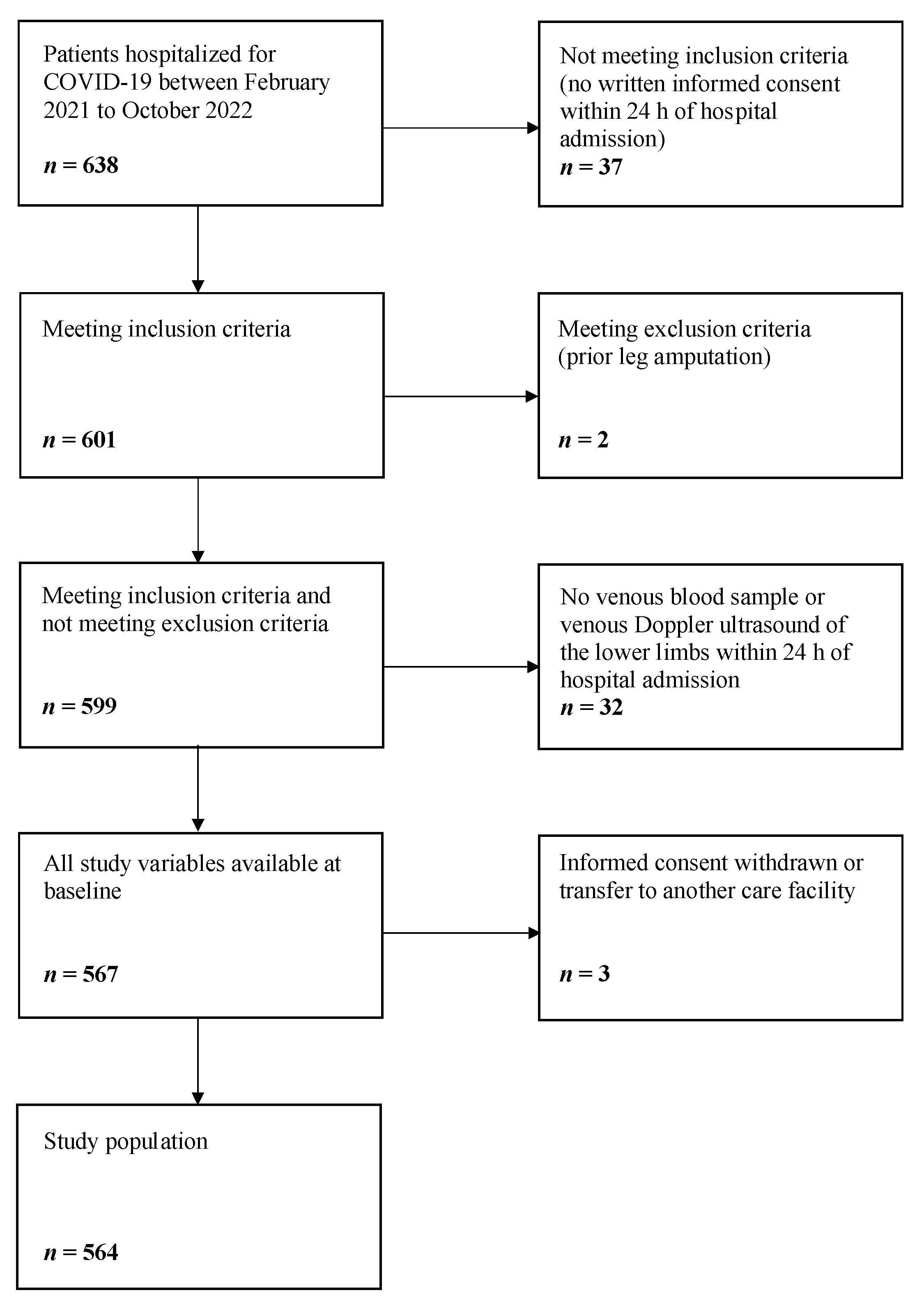
2.1. Enrollment of the Study Population
2.2. Baseline Data Collection
2.3. Clinical End Points
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Clinical Course
3.3. Lp(a) and Biomarkers of Thrombo-Inflammation
3.4. Lp(a) and Thrombotic Events
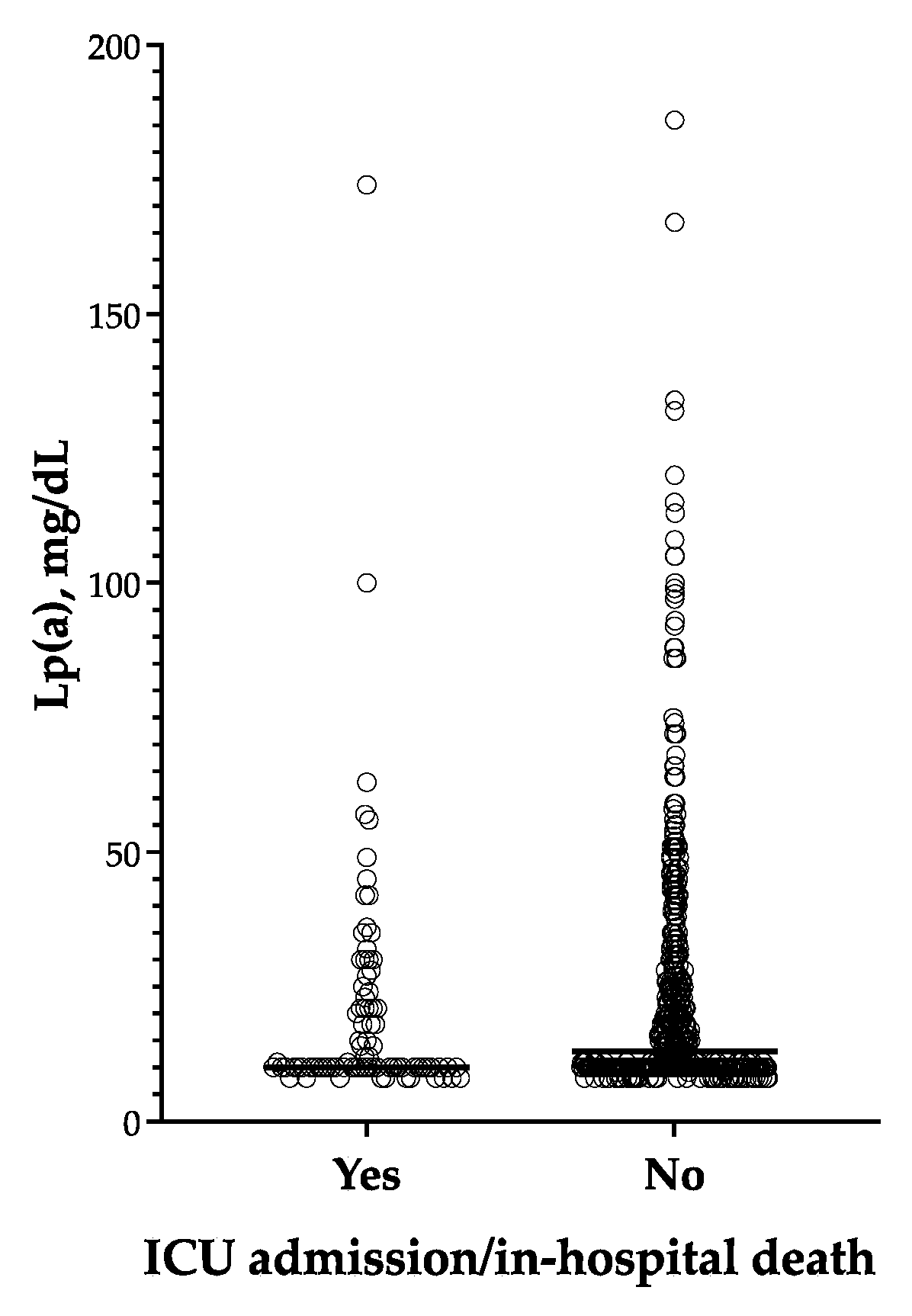
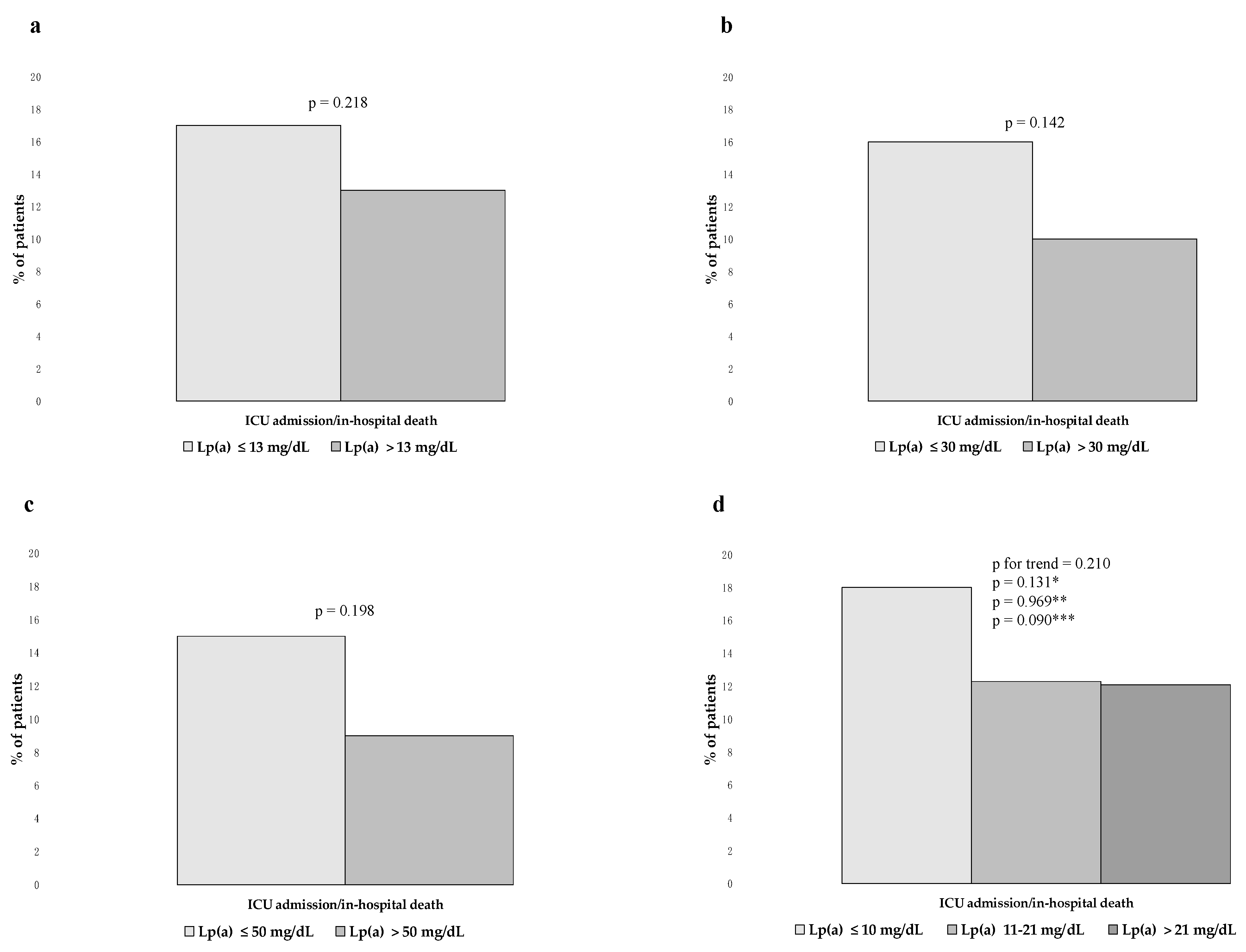
3.5. Lp(a) and the Composite End Point of ICU Admission/in-Hospital Death
3.6. Exploratory Analyses in the Subgroup of Patients with Severe COVID-19
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 March 2023).
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Song, H.; Yip, S.; Zhang, T.; He, D. Impact of low vaccine coverage on the resurgence of COVID-19 in Central and Eastern Europe. One Health 2022, 14, 100402. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Harun, M.G.D.; Sumon, S.A.; Mohona, T.M.; Abdullah, S.A.H.M.; Khan, M.N.H.; Gazi, M.I.; Islam, M.S.; Anwar, M.M.U. Hospitalization and Mortality by Vaccination Status among COVID-19 Patients Aged ≥ 25 Years in Bangladesh: Results from a Multicenter Cross-Sectional Study. Vaccines 2022, 10, 1987. [Google Scholar] [CrossRef] [PubMed]
- Havers, F.P.; Pham, H.; Taylor, C.A.; Whitaker, M.; Patel, K.; Anglin, O.; Kambhampati, A.K.; Milucky, J.; Zell, E.; Moline, H.L.; et al. COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022. JAMA Intern. Med. 2022, 182, 1071–1081. [Google Scholar] [CrossRef]
- Mannarino, M.R.; Bianconi, V.; Cosentini, E.; Figorilli, F.; Natali, C.; Cellini, G.; Colangelo, C.; Giglioni, F.; Braca, M.; Pirro, M. The HACOR Score Predicts Worse in-Hospital Prognosis in Patients Hospitalized with COVID-19. J. Clin. Med. 2022, 11, 3509. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P.; Vestri, A.R.; Spagnoli, A.; Cipollone, F.; Ceccarelli, G.; Oliva, A.; Amitrano, M.; Pirro, M.; Taliani, G.; et al. The ADA (Age-D-Dimer-Albumin) Score to Predict Thrombosis in SARS-CoV-2. Thromb. Haemost. 2022, 122, 1567–1572. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M.; et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Schiaroli, E.; Cosentini, E.; Batori, G.; Marini, E.; Sahebkar, A.; Grignani, F.; Gidari, A.; et al. Low Brachial Artery Flow-Mediated Dilation Predicts Worse Prognosis in Hospitalized Patients with COVID-19. J. Clin. Med. 2021, 10, 5456. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Cosentini, E.; Figorilli, F.; Colangelo, C.; Cellini, G.; Braca, M.; Lombardini, R.; Paltriccia, R.; Sahebkar, A.; et al. The impact of statin therapy on in-hospital prognosis and endothelial function of patients at high-to-very high cardiovascular risk admitted for COVID-19. J. Med. Virol. 2023, 95, e28678. [Google Scholar] [CrossRef]
- Wagner, D.D.; Heger, L.A. Thromboinflammation: From Atherosclerosis to COVID-19. Arter. Thromb. Vasc. Biol. 2022, 42, 1103–1112. [Google Scholar] [CrossRef]
- Connors, J.M.; Ridker, P.M. Thromboinflammation and Antithrombotics in COVID-19: Accumulating Evidence and Current Status. JAMA 2022, 327, 1234–1235. [Google Scholar] [CrossRef]
- Bianconi, V.; Violi, F.; Fallarino, F.; Pignatelli, P.; Sahebkar, A.; Pirro, M. Is Acetylsalicylic Acid a Safe and Potentially Useful Choice for Adult Patients with COVID-19? Drugs 2020, 80, 1383–1396. [Google Scholar] [CrossRef]
- Violi, F.; Ceccarelli, G.; Cangemi, R.; Cipollone, F.; D’Ardes, D.; Oliva, A.; Pirro, M.; Rocco, M.; Alessandri, F.; D’Ettorre, G.; et al. Arterial and venous thrombosis in coronavirus 2019 disease (COVID-19): Relationship with mortality. Int. Emerg. Med. 2021, 16, 1231–1237. [Google Scholar] [CrossRef]
- Ganjali, S.; Bianconi, V.; Penson, P.E.; Pirro, M.; Banach, M.; Watts, G.F.; Sahebkar, A. Commentary: Statins, COVID-19, and coronary artery disease: Killing two birds with one stone. Metabolism 2020, 113, 154375. [Google Scholar] [CrossRef]
- Lampart, M.; Zellweger, N.; Bassetti, S.; Tschudin-Sutter, S.; Rentsch, K.M.; Siegemund, M.; Bingisser, R.; Osswald, S.; Kuster, G.M.; Twerenbold, R. Clinical utility of inflammatory biomarkers in COVID-19 in direct comparison to other respiratory infections-A prospective cohort study. PLoS ONE 2022, 17, e0269005. [Google Scholar] [CrossRef]
- Gorog, D.A.; Storey, R.F.; Gurbel, P.A.; Tantry, U.S.; Berger, J.S.; Chan, M.Y.; Duerschmied, D.; Smyth, S.S.; Parker, W.A.E.; Ajjan, R.A.; et al. Current and novel biomarkers of thrombotic risk in COVID-19: A Consensus Statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat. Rev. Cardiol. 2022, 19, 475–495. [Google Scholar] [CrossRef]
- Karimi, A.; Shobeiri, P.; Kulasinghe, A.; Rezaei, N. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front. Immunol. 2021, 12, 741061. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, C.; Fei, Y.; Shen, M.; Zhou, Y.; Zhang, Y.; Zeng, X.; Zhang, S. Anti-inflammatory and immune therapy in severe coronavirus disease 2019 (COVID-19) patients: An update. Clin. Immunol. 2022, 239, 109022. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Connors, J.M.; Douketis, J.D.; Goldin, M.; Hunt, B.J.; Kotila, T.R.; Lopes, R.D.; Schulman, S. Good practice statements for antithrombotic therapy in the management of COVID-19: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2022, 20, 2226–2236. [Google Scholar] [CrossRef]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 1 March 2023).
- Moriarty, P.M.; Gorby, L.K.; Stroes, E.S.; Kastelein, J.P.; Davidson, M.; Tsimikas, S. Lipoprotein(a) and Its Potential Association with Thrombosis and Inflammation in COVID-19: A Testable Hypothesis. Curr. Atheroscler. Rep. 2020, 22, 48. [Google Scholar] [CrossRef]
- Vuorio, A.; Kaste, M.; Kovanen, P.T. Elevated Lipoprotein(a) and Cerebral Venous Sinus Thrombosis in COVID-19. J. Stroke Cerebrovasc. Dis. 2021, 30, 105865. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Bianconi, V.; Paciullo, F.; Mannarino, M.R.; Bagaglia, F.; Sahebkar, A. Lipoprotein(a) and inflammation: A dangerous duet leading to endothelial loss of integrity. Pharmacol. Res. 2017, 119, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Johnston, T.P.; Banach, M.; Pirro, M.; Sahebkar, A. Lipoprotein(a): A missing culprit in the management of athero-thrombosis? J. Cell. Physiol. 2018, 233, 2966–2981. [Google Scholar] [CrossRef] [PubMed]
- Pawlos, A.; Gorzelak-Pabiś, P.; Staciwa, M.; Broncel, M. Elevated Lp(a) and course of COVID-19: Is there a relationship? PLoS ONE 2022, 17, e0266814. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Szergyuk, I.; de Oliveira, M.H.S.; Benoit, S.W.; Benoit, J.L.; Favaloro, E.J.; Henry, B.M. The role of lipoprotein(a) in coronavirus disease 2019 (COVID-19) with relation to development of severe acute kidney injury. J. Thromb. Thrombolysis 2022, 53, 581–585. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Collard, D.; Reeskamp, L.F.; Kaiser, Y.; Kroon, J.; Tromp, T.R.; van den Born, B.H.; Coppens, M.; Vlaar, A.P.J.; Beudel, M.; et al. Lipoprotein(a), venous thromboembolism and COVID-19: A pilot study. Atherosclerosis 2022, 341, 43–49. [Google Scholar] [CrossRef]
- Kaltoft, M.; Glavind, K.S.; Nielsen, S.F.; Langsted, A.; Iversen, K.K.; Nordestgaard, B.G.; Kamstrup, P.R. Lipoprotein(a) during COVID-19 hospitalization: Thrombosis, inflammation, and mortality. Atherosclerosis 2022, 357, 33–40. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Iodice, S.; Tersalvi, G.; Rota, I.; Ghidini, S.; Terranova, L.; Valenti, L.; Amati, F.; Aliberti, S.; et al. Prognostic parameters of in-hospital mortality in COVID-19 patients-An Italian experience. Eur. J. Clin. Investig. 2021, 51, e13629. [Google Scholar] [CrossRef]
- Di Maio, S.; Lamina, C.; Coassin, S.; Forer, L.; Würzner, R.; Schönherr, S.; Kronenberg, F. Lipoprotein(a) and SARS-CoV-2 infections: Susceptibility to infections, ischemic heart disease and thromboembolic events. J. Intern. Med. 2022, 291, 101–107. [Google Scholar] [CrossRef]
- Mannarino, M.R.; Bianconi, V.; Cosentini, E.; Figorilli, F.; Colangelo, C.; Giglioni, F.; Lombardini, R.; Paltriccia, R.; Pirro, M. Thyroid-Stimulating Hormone Predicts Total Cholesterol and Low-Density Lipoprotein Cholesterol Reduction during the Acute Phase of COVID-19. J. Clin. Med. 2022, 11, 3347. [Google Scholar] [CrossRef]
- Pasqualini, L.; Cortese, C.; Marchesi, S.; Siepi, D.; Pirro, M.; Vaudo, G.; Liberatoscioli, L.; Gnasso, A.; Schillaci, G.; Mannarino, E. Paraoxonase-1 activity modulates endothelial function in patients with peripheral arterial disease. Atherosclerosis 2005, 183, 349–354. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Ministrini, S.; Fallarino, F.; Lupattelli, G.; Bianconi, V.; Bagaglia, F.; Mannarino, E. Effects of a nutraceutical combination on lipids, inflammation and endothelial integrity in patients with subclinical inflammation: A randomized clinical trial. Sci. Rep. 2016, 6, 23587. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Banach, M.; Sahebkar, A.; Pirro, M. The detrimental impact of elevated Ferritin to Iron ratio on in-hospital prognosis of patients with COVID-19. Expert Rev. Mol. Diagn. 2022, 22, 469–478. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Barbar, S.; Noventa, F.; Rossetto, V.; Ferrari, A.; Brandolin, B.; Perlati, M.; De Bon, E.; Tormene, D.; Pagnan, A.; Prandoni, P. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: The Padua Prediction Score. J. Thromb. Haemos 2010, 8, 2450–2457. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: A European Atherosclerosis Society consensus statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef]
- von Depka, M.; Nowak-Göttl, U.; Eisert, R.; Dieterich, C.; Barthels, M.; Scharrer, I.; Ganser, A.; Ehrenforth, S. Increased lipoprotein(a) levels as an independent risk factor for venous thromboembolism. Blood 2000, 96, 3364–3368. [Google Scholar] [CrossRef]
- Helgadottir, A.; Gretarsdottir, S.; Thorleifsson, G.; Holm, H.; Patel, R.S.; Gudnason, T.; Jones, G.T.; van Rij, A.M.; Eapen, D.J.; Baas, A.F.; et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J. Am. Coll. Cardiol. 2012, 60, 722–729. [Google Scholar] [CrossRef]
- Larsson, S.C.; Gill, D.; Mason, A.M.; Jiang, T.; Bäck, M.; Butterworth, A.S.; Burgess, S. Lipoprotein(a) in Alzheimer, Atherosclerotic, Cerebrovascular, Thrombotic, and Valvular Disease: Mendelian Randomization Investigation. Circulation 2020, 141, 1826–1828. [Google Scholar] [CrossRef]
- Chen, R.; Lan, Z.; Ye, J.; Pang, L.; Liu, Y.; Wu, W.; Qin, X.; Guo, Y.; Zhang, P. Cytokine Storm: The Primary Determinant for the Pathophysiological Evolution of COVID-19 Deterioration. Front. Immunol. 2021, 12, 589095. [Google Scholar] [CrossRef] [PubMed]
- Chelariu, A.C.; Coman, A.E.; Lionte, C.; Gorciac, V.; Sorodoc, V.; Haliga, R.E.; Petris, O.R.; Bologa, C.; Puha, G.; Stoica, A.; et al. The Value of Early and Follow-Up Elevated Scores Based on Peripheral Complete Blood Cell Count for Predicting Adverse Outcomes in COVID-19 Patients. J. Pers. Med. 2022, 12, 2037. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, A.; Elgendy, I.Y.; Sahai, A.; Bhandari, R.; McCarthy, M.; Gomes, M.; Bishop, G.J.; Bartholomew, J.R.; Kapadia, S.; Cameron, S.J. Incidence and Outcomes of Thrombotic Events in Symptomatic Patients With COVID-19. Arter. Thromb. Vasc. Biol. 2021, 41, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Caplice, N.M.; Panetta, C.; Peterson, T.E.; Kleppe, L.S.; Mueske, C.S.; Kostner, G.M.; Broze, G.J., Jr.; Simari, R.D. Lipoprotein(a) binds and inactivates tissue factor pathway inhibitor: A novel link between lipoproteins and thrombosis. Blood 2001, 98, 2980–2987. [Google Scholar] [CrossRef]
- Boffa, M.B. Beyond fibrinolysis: The confounding role of Lp(a) in thrombosis. Atherosclerosis 2022, 349, 72–81. [Google Scholar] [CrossRef]
- Simantiris, S.; Antonopoulos, A.S.; Papastamos, C.; Benetos, G.; Koumallos, N.; Tsioufis, K.; Tousoulis, D. Lipoprotein(a) and inflammation- pathophysiological links and clinical implications for cardiovascular disease. J. Clin. Lipidol. 2023, 17, 55–63. [Google Scholar] [CrossRef]
- Salama, C.; Han, J.; Yau, L.; Reiss, W.G.; Kramer, B.; Neidhart, J.D.; Criner, G.J.; Kaplan-Lewis, E.; Baden, R.; Pandit, L.; et al. Tocilizumab in Patients Hospitalized with COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 20–30. [Google Scholar] [CrossRef]
- Malik, M.I.; Zafar, S.A.F.; Qayyum, F.; Malik, M.; Asghar, M.S.; Tahir, M.J.; Arshad, A.; Khalil, F.; Naz, H.S.; Aslam, M.; et al. Tocilizumab in severe COVID-19—A randomized, double-blind, placebo-controlled trial. Infect. Med. 2022, 1, 88–94. [Google Scholar] [CrossRef]
- Navarese, E.P.; Podhajski, P.; Gurbel, P.A.; Grzelakowska, K.; Ruscio, E.; Tantry, U.; Magielski, P.; Kubica, A.; Niezgoda, P.; Adamski, P.; et al. PCSK9 Inhibition During the Inflammatory Stage of SARS-CoV-2 Infection. J. Am. Coll. Cardiol. 2023, 81, 224–234. [Google Scholar] [CrossRef]
- Schultz, O.; Oberhauser, F.; Saech, J.; Rubbert-Roth, A.; Hahn, M.; Krone, W.; Laudes, M. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein(a) levels in human subjects with rheumatoid diseases. PLoS ONE 2010, 5, e14328. [Google Scholar] [CrossRef]
- Gaudet, D.; Watts, G.F.; Robinson, J.G.; Minini, P.; Sasiela, W.J.; Edelberg, J.; Louie, M.J.; Raal, F.J. Effect of Alirocumab on Lipoprotein(a) Over ≥1.5 Years (from the Phase 3 ODYSSEY Program). Am. J. Cardiol. 2017, 119, 40–46. [Google Scholar] [CrossRef]
- Kronenberg, F. Lipoprotein(a) measurement issues: Are we making a mountain out of a molehill? Atherosclerosis 2022, 349, 123–135. [Google Scholar] [CrossRef]


| Lp(a) ≤ 13 mg/dL | Lp(a) > 13 mg/dL | p | |
|---|---|---|---|
| Age, years | 73 ± 17 | 76 ± 16 | 0.069 |
| Male sex, % | 51 | 51 | 0.881 |
| BMI, Kg/m2 | 26 ± 5 | 26 ± 4 | 0.102 |
| Current smoking, % | 7 | 6 | 0.623 |
| Hypertension, % | 62 | 71 | 0.033 |
| Type 2 diabetes, % | 21 | 24 | 0.360 |
| CKD, % | 16 | 19 | 0.366 |
| ASCVD, % | 19 | 28 | 0.012 |
| AF, % | 19 | 19 | 0.959 |
| Previous VTE, % | 6 | 7 | 0.711 |
| ACE inhibitors, % | 28 | 25 | 0.497 |
| ARBs, % | 12 | 15 | 0.198 |
| BBs, % | 29 | 37 | 0.049 |
| CCBs, % | 22 | 25 | 0.506 |
| Diuretics, % | 36 | 44 | 0.068 |
| Oral anticoagulants, % | 16 | 17 | 0.572 |
| Antiplatelets, % | 22 | 32 | 0.006 |
| Oral hypoglycemic drugs, % | 12 | 11 | 0.804 |
| Insulin, % | 11 | 13 | 0.503 |
| Statins, % | 19 | 30 | 0.002 |
| Anti-SARS-CoV-2 vaccine, % | 60 | 65 | 0.287 |
| PaO2/FiO2 < 300, % | 53 | 48 | 0.310 |
| Total cholesterol, mg/dL | 149 ± 40 | 157 ± 42 | 0.043 |
| LDL cholesterol, mg/dL | 87 ± 32 | 94 ± 34 | 0.016 |
| HDL cholesterol, mg/dL | 40 ± 17 | 40 ± 14 | 0.729 |
| Triglycerides, mg/dL | 105 (73–144) | 101 (79–130) | 0.676 |
| CCI | 5 (3–7) | 5 (4–7) | 0.053 |
| SOFA score | 2 (2–4) | 2 (1–4) | 0.636 |
| PP score | 5 (3–6) | 5 (3–7) | 0.257 |
| Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|
| Dependent variable: thrombotic events | Lp(a), mg/dL | 1.006 (95%CI 0.997–1.016) | 1.007 (95%CI 0.997–1.017) | * 1.010 (95%CI 0.999–1.021) | ° 1.009 (95%CI 0.998–1.020) |
| Lp(a) > 13 mg/dL | 1.648 (95%CI 0.971–2.797) | 1.568 (95%CI 0.914–2.689) | * 1.641 (95%CI 0.925–2.910) | ° 1.561 (95%CI 0.862–2.827) | |
| Lp(a) > 30 mg/dL | 1.111 (95%CI 0.600–2.058) | 1.066 (95%CI 0.569–1.997) | * 1.192 (95%CI 0.613–2.317) | ° 1.153 (95%CI 0.586–2.267) | |
| Lp(a) > 50 mg/dL | 1.828 (95%CI 0.872–3.830) | 1.841 (95%CI 0.862–3.935) | * 2.092 (95%CI 0.921–4.750) | ° 2.015 (95%CI 0.879–4.623) | |
| Lp(a) tertiles | 1.264 (95%CI 0.935–1.707) | 1.040 (95%CI 1.018–1.063) | * 1.305 (95%CI 0.935–1.822) | ° 1.251 (95%CI 0.889–1.760) | |
| Dependent variable: arterial thrombotic events | Lp(a), mg/dL | 1.009 (95%CI 0.997–1.022) | 1.011 (95%CI 0.998–1.024) | ** 1.012 (95%CI 0.998–1.026) | °° 1.011 (95%CI 0.997–1.026) |
| Lp(a) > 13 mg/dL | 1.866 (95%CI 0.839–4.150) | 1.767 (95%CI 0.785–3.979) | ** 1.702 (95%CI 0.740–3.912) | °° 1.528 (95%CI 0.631–3.697) | |
| Lp(a) > 30 mg/dL | 1.270 (95%CI 0.524–3.078) | 1.219 (95%CI 0.497–2.994) | ** 1.240 (95%CI 0.492–3.125) | °° 1.276 (95%CI 0.494–3.299) | |
| Lp(a) > 50 mg/dL | 2.783 (95%CI 1.073–7.216) | 2.853 (95%CI 1.072–7.593) | ** 2.974 (95%CI 1.082–8.173) | °° 3.228 (95%CI 1.148–9.079) | |
| Lp(a) tertiles | 1.291 (95%CI 0.825–2.020) | 1.265 (95%CI 0.799–2.002) | ** 1.245 (95%CI 0.772–2.006) | °° 1.138 (95%CI 0.688–1.881) | |
| Dependent variable: venous thrombotic events | Lp(a), mg/dL | 1.002 (95%CI 0.989–1.015) | 1.002 (95%CI 0.989–1.015) | *** 0.997 (95%CI 0.978–1.017) | °°° 0.672 (95%CI 0.976–1.016) |
| Lp(a) > 13 mg/dL | 1.264 (95%CI 0.658–2.427) | 1.202 (95%CI 0.623–2.322) | *** 1.068 (95%CI 0.477–2.392) | °°° 1.082 (95%CI 0.474–2.474) | |
| Lp(a) > 30 mg/dL | 0.920 95%CI 0.412–2.056) | 0.888 (95%CI 0.395–1.995) | *** 0.971 (95%CI 0.372–2.537) | °°° 0.905 (95%CI 0.339–2.418) | |
| Lp(a) > 50 mg/dL | 1.040 (95%CI 0.355–3.041) | 1.023 (95%CI 0.347–3.015) | *** 0.798 (95%CI 0.205–3.110) | °°° 0.699 (95%CI 0.174–2.809) | |
| Lp(a) tertiles | 1.291 (95%CI 0.825–2.020) | 1.145 (95%CI 0.783–1.676) | *** 1.055 (95%CI 0.661–1.685) | °°° 1.056 (95%CI 0.656–1.699) |
| Model 1 | Model 2 | Model 3 | Model 4 | ||
|---|---|---|---|---|---|
| Dependent variable: ICU admission/in-hospital death | Lp(a), mg/dL | 0.997 (95%CI 0.986–1.009) | 0.997 (95%CI 0.986–1.008) | 1.006 (95%CI 0.992–1.019) | 1.004 (95%CI 0.990–1.019) |
| Lp(a) > 13 mg/dL | 0.817 (95%CI 0.526–1.269) | 0.798 (95%CI 0.514–1.239) | 1.212 (95%CI 0.602–2.439) | 1.123 (95%CI 0.548–2.298) | |
| Lp(a) > 30 mg/dL | 0.773 (95%CI 0.426–1.403) | 0.746 (95%CI 0.410–1.355) | 0.744 (95%CI 0.311–1.781) | 0.654 (95%CI 0.261–1.639) | |
| Lp(a) > 50 mg/dL | 0.748 (95%CI 0.301–1.858) | 0.735 (95%CI 0.296–1.827) | 1.060 (95%CI 0.310–3.624) | 1.019 (95%CI 0.292–3.559) | |
| Lp(a) tertiles | 0.865 (95%CI 0.666–1.122) | 0.855 (95%CI 0.657–1.114) | 1.037 (95%CI 0.699–1.538) | 1.021 (95%CI 0.684–1.525) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianconi, V.; Mannarino, M.R.; Ramondino, F.; Fusaro, J.; Giglioni, F.; Braca, M.; Ricciutelli, F.; Lombardini, R.; Paltriccia, R.; Greco, A.; et al. Lipoprotein(a) Does Not Predict Thrombotic Events and In-Hospital Outcomes in Patients with COVID-19. J. Clin. Med. 2023, 12, 3543. https://doi.org/10.3390/jcm12103543
Bianconi V, Mannarino MR, Ramondino F, Fusaro J, Giglioni F, Braca M, Ricciutelli F, Lombardini R, Paltriccia R, Greco A, et al. Lipoprotein(a) Does Not Predict Thrombotic Events and In-Hospital Outcomes in Patients with COVID-19. Journal of Clinical Medicine. 2023; 12(10):3543. https://doi.org/10.3390/jcm12103543
Chicago/Turabian StyleBianconi, Vanessa, Massimo R. Mannarino, Federica Ramondino, Jessica Fusaro, Francesco Giglioni, Marco Braca, Federica Ricciutelli, Rita Lombardini, Rita Paltriccia, Alessia Greco, and et al. 2023. "Lipoprotein(a) Does Not Predict Thrombotic Events and In-Hospital Outcomes in Patients with COVID-19" Journal of Clinical Medicine 12, no. 10: 3543. https://doi.org/10.3390/jcm12103543






