Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Electroconvulsive Therapy
2.3. Image Data Acquisition
2.4. Rs-fMRI Preprocessing and Feature Extraction
2.5. Model Building and Validation
3. Results
3.1. Clinical Characteristics
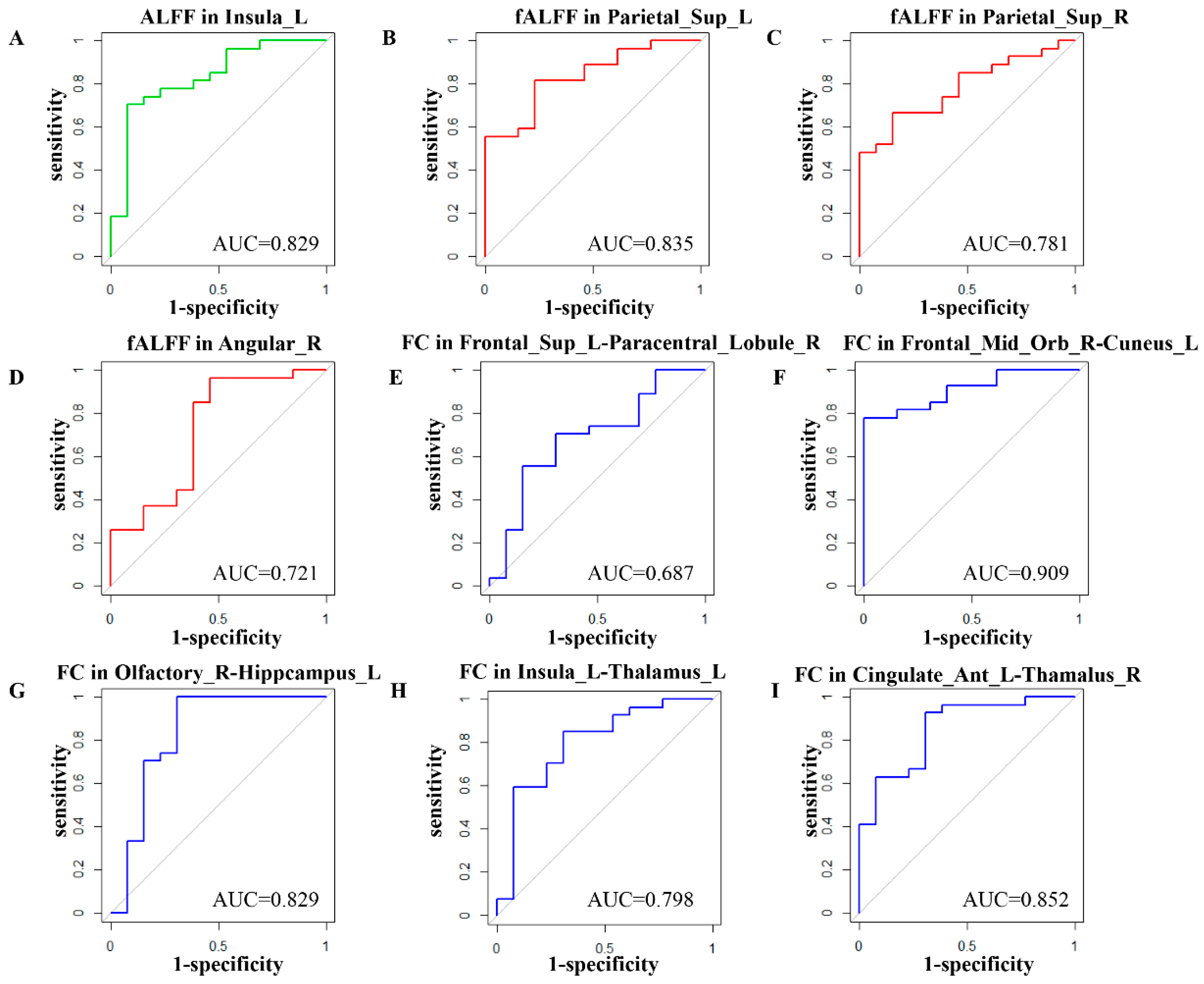
3.2. Feature Selection
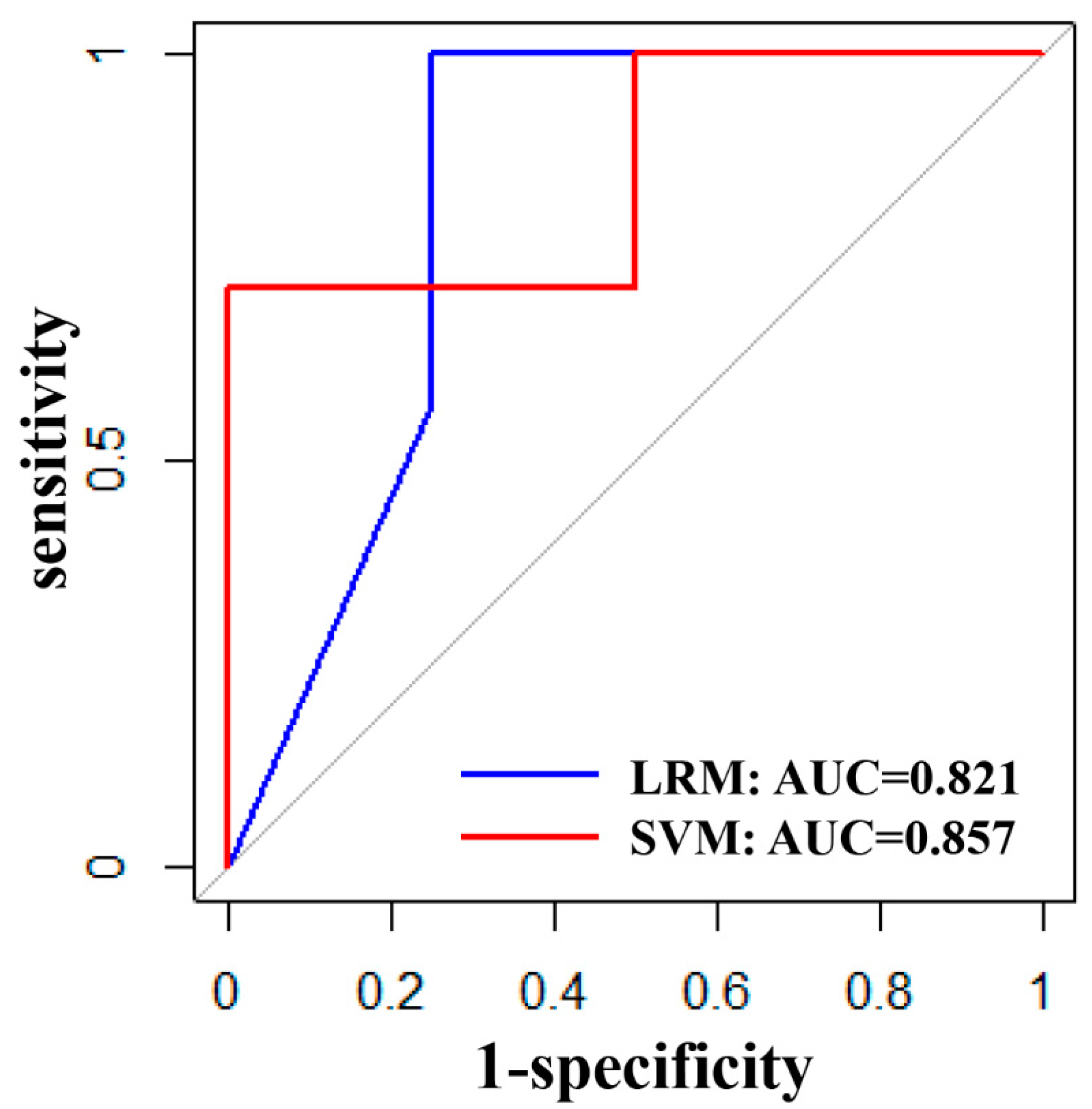
3.3. Validation Performance of LRM and SVM
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatham, A.N.; Shafi, H.; Hermida, A.P. The Use of ECT in the Elderly-Looking Beyond Depression. Curr. Psychiatry Rep. 2022, 24, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.H.; Greenberg, R.M.; Murrough, J.W.; Bryson, E.O.; Briggs, M.C.; Pasculli, R.M. ECT in treatment-resistant depression. Am. J. Psychiatry 2012, 169, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Ghaziuddin, N.; Kutcher, S.P.; Knapp, P. Practice parameter for use of electroconvulsive therapy with adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 1521–1539. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Espinoza, R.; Pirnia, T.; Shi, J.; Wang, Y.; Ayers, B.; Leaver, A.; Woods, R.P.; Naar, K.L. Structural Plasticity of the Hippocampus and Amygdala Induced by Electroconvulsive Therapy in Major Depression. Biol. Psychiatry 2016, 79, 282–292. [Google Scholar] [CrossRef]
- Qi, S.L.; Abbott, C.C.; Narr, K.L.; Jiang, R.; Upston, J.; McClintock, S.M.; Espinoza, R.; Jones, T.; Zhi, D.; Sun, H.; et al. Electroconvulsive therapy treatment responsive multimodal brain networks. Hum. Brain Mapp. 2020, 41, 1775–1785. [Google Scholar] [CrossRef]
- Long, Z.L.; Du, L.; Zhao, J.; Wu, S.; Zheng, Q.; Lei, X. Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J. Affect. Disord. 2020, 276, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.J.; Wei, Q.; Zhao, S.S.; Li, N.; Li, Z.; Lu, F.; Pang, J.; Zhang, R.; Wang, K.; Chu, C.; et al. Enhanced default mode network functional connectivity links with electroconvulsive therapy response in major depressive disorder. J. Affect. Disord. 2022, 306, 47–54. [Google Scholar] [CrossRef]
- Cui, L.B.; Liu, L.; Wang, H.N.; Wang, L.X.; Guo, F.; Xi, Y.B.; Liu, T.T.; Li, C.; Tian, P.; Liu, K.; et al. Disease Definition for Schizophrenia by Functional Connectivity Using Radiomics Strategy. Schizophr. Bull. 2018, 44, 1053–1059. [Google Scholar] [CrossRef]
- Askland, K.D.; Garnaat, S.; Sibrava, N.J.; Boisseau, C.L.; Strong, D.; Mancebo, M.; Greenberg, M.; Rasmusse, S.; Eisen, J. Prediction of remission in obsessive compulsive disorder using a novel machine learning strategy. Int. J. Methods Psychiatr. Res. 2015, 24, 156–169. [Google Scholar] [CrossRef]
- Shimizu, Y.; Yoshimoto, J.; Toki, S.; Takamura, M.; Yoshimura, S.; Okamoto, Y.; Yamawaki, S.; Doya, K. Toward Probabilistic Diagnosis and Understanding of Depression Based on Functional MRI Data Analysis with Logistic Group LASSO. PLoS ONE 2015, 10, e0123524. [Google Scholar] [CrossRef]
- Davidson, R.J.; Jackson, D.C.; Kalin, N.H. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol. Bull. 2000, 126, 890–909. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp. Brain Res. 2000, 133, 44–54. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Harrison, B.J.; Davey, C.G.; Yücel, M.; Pantelis, C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012, 42, 671–681. [Google Scholar] [CrossRef]
- Bora, E.; Fornito, A.; Pantelis, C.; Yücel, M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel-based morphometry studies. J. Affect. Disord. 2012, 138, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.X.; Yang, C.X.; Li, G.Z.; Wang, Y.; Liu, P.; Liu, Z.; Sun, N.; Zhang, K. Functional connectivity of the prefrontal cortex and amygdala is related to depression status in major depressive disorder. J. Affect. Disord. 2020, 274, 897–902. [Google Scholar] [CrossRef]
- Porta-Casteràs, D.; Cano, M.; Camprodon, J.; Loo, C.; Palao, D.; Soriano-Mas, C.; Cardoner, N. A multimetric systematic review of fMRI findings in patients with MDD receiving ECT. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110178. [Google Scholar] [CrossRef]
- Chen, D.N.; Lei, X.; Du, L.; Long, Z. Use of machine learning in prdicting the efficacy of repetitive transcranial magnetic stimulation on treating depression based on functional and structural thalamo-prefrontal connectivity: A pilot study. J. Psychiatr. Res. 2022, 148, 88–94. [Google Scholar] [CrossRef]
- Bouckaert, F.; Winter, F.L.D.; Emsell, L.; Dols, A.; Rhebergen, D.; Wampers, M.; Sunaert, S.; Stek, M.; Sienaert, P.; Vandenbulcke, M. Grey matter volume increase following electroconvulsive therapy in patients with late life depression: A longitudinal MRI study. J. Psychiatry Neurosci. JPN 2015, 40, 140322. [Google Scholar] [CrossRef]
- Wang, Y.L.; Yang, S.Z.; Sun, W.L.; Shi, Y.Z.; Duan, H.F. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav. Brain Res. 2015, 298, 301–309. [Google Scholar] [CrossRef]
- Wang, L.J.; Wei, Q.; Wang, C.; Xu, J.; Wang, K.; Tian, Y.; Wang, J. Altered functional connectivity patterns of insular subregions in major depressive disorder after electroconvulsive therapy. Brain Imaging Behav. 2020, 14, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Hayden, B.Y.; Pearson, J.M.; Platt, M.L. Fictive reward signals in the anterior cingulate cortex. Science 2009, 324, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H. Gray matter volume in major depressive disorder: A meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013, 211, 37–46. [Google Scholar] [CrossRef]
- Auer, D.P.; Putz, B.; Kraft, E.; Lipinski, B.; Schill, J.; Holsboer, F. Reduced glutamate in the anterior cingulate cortex in depression: An in vivo proton magnetic resonance spectroscopy study. Biol. Psychiatry 2000, 47, 305–313. [Google Scholar] [CrossRef]
- Connolly, C.G.; Wu, J.; Ho, T.C.; Hoeft, F.; Wolkowitz, O.; Eisendrath, S.; Frank, G.; Hendren, R.; Max, J.E.; Paulus, M.P.; et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry 2013, 74, 898–907. [Google Scholar] [CrossRef]
- Gunten, A.; Ron, M. Hippocampal volume and subjective memory impairment in depressed patients. Eur. Psychiatry 2004, 19, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, D.; Enneking, V.; Meinert, S.; Förster, K.; Bürger, C.; Dohm, K.; Grotegert, D.; Redlich, R.; Dietsche, B.; Krug, A. Effects of cumulative illness severity on hippocampal gray matter volume in major depression: A voxel-based morphometry study. Psychol. Med. 2018, 48, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Vakili, K.; Pillay, S.; Lafer, B.; Fava, M.; Renshaw, P.F.; Bonello-Cintron, C.M.; Yurgelun-Todd, D.A. Hippocampal volume in primary unipolar major depression: A magnetic resonance imaging study. Biol. Psychiatry 2000, 47, 1087–1090. [Google Scholar] [CrossRef]
- Yrondi, A.; Nemmi, F.; Billoux, S.; Giron, A.; Sporer, M.; Taib, S.; Salles, J.; Pierre, D.; Thalmas, C.; Schmitt, L.; et al. Significant Decrease in Hippocampus and Amygdala Mean Diffusivity in Treatment-Resistant Depression Patients Who Respond to Electroconvulsive Therapy. Front Psychiatry 2019, 10, 694. [Google Scholar] [CrossRef]
- Peng, H.; Wu, K.; Li, J.; Qi, H.; Guo, S.; Chi, M.; Wu, X.; Guo, Y.; Ynag, Y.; Ning, Y. Increased suicide attempts in young depressed patients with abnormal temporal-parietal-limbic gray matter volume. J. Affect. Disord. 2014, 165, 69–73. [Google Scholar] [CrossRef]
- Giakoumatos, C.I.; Tandon, N.; Shah, J.; Mathew, I.T.; Brady, R.O.; Clementz, B.A.; Pearlson, G.D.; Thaker, G.K.; Tamminga, C.A.; Sweeney, J.A.; et al. Are structural brain abnormalities associated with suicidal behavior in patients with psychotic disorders? J. Psychiatr. Res. 2013, 47, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Van Heeringen, K.; Wu, G.R.; Vervaet, M.; Vanderhasselt, M.A.; Baeken, C. Decreased resting state metabolic activity in frontopolar and parietal brain regions is associated with suicide plans in depressed individuals. J. Psychiatr. Res. 2017, 84, 243–248. [Google Scholar] [CrossRef] [PubMed]

| Feature | Responder (n = 27) | Non-Responder (n = 13) | p |
|---|---|---|---|
| Male (M/F) | 8/19 | 5/8 | 0.635 # |
| Age (y) | 16.36 (1.53) | 15.04 (1.35) | 0.36 * |
| Education (y) | 9.95 (1.73) | 8.35 (1.64) | 0.165 * |
| Duration of illness (y) | 1.3 (0.9) | 1.2 (1.1) | 0.376 * |
| HAMD score pre-ECT | 29.45 (5.72) | 30.21 (6.03) | 0.662 * |
| HAMD score post-ECT | 11.36 (5.43) | 18.32 (4.53) | <0.001 + |
| BSSI score pre-ECT | 23.88 (6.34) | 22.94 (5.63) | 0.532 * |
| BSSI score post-ECT | 5.56 (3.72) | 14.32 (4.86) | <0.001 + |
| Number of ECT | 8.3 (2.0) | 8.1 (2.9) | 0.67 * |
| Antidepressants dose (mg/d) a | 49.3 (7.9) | 51.8 (6.4) | 0.54 * |
| Number | Brain Region | Feature | Coefficient |
|---|---|---|---|
| 1 | INS.L | ALFF | 0.289 |
| 2 | SPG.L | fALFF | 0.209 |
| 3 | SPG.R | fALFF | 0.136 |
| 4 | ANG.R | fALFF | 0.001 |
| 5 | SFGdor.L–PCL.R | FC | −0.007 |
| 6 | ORBmid.R–CUN.L | FC | 0.478 |
| 7 | OLF.R–HIP.L | FC | 0.127 |
| 8 | INS.L–THA.L | FC | −0.348 |
| 9 | ACG.L–HIP.R | FC | 0.161 |
| ACC | SPENC | SPEC | PPV | NPV | AUC | |
|---|---|---|---|---|---|---|
| LRM | 81.8% | 100% | 50% | 77.8% | 100% | 0.821 |
| SVM | 81.8% | 100% | 50% | 77.8% | 100% | 0.857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Guo, J.; Chen, X.; Yu, R.; Chen, W.; Zheng, A.; Yu, Y.; Zhou, D.; Dai, L.; Kuang, L. Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI. J. Clin. Med. 2023, 12, 3556. https://doi.org/10.3390/jcm12103556
Li X, Guo J, Chen X, Yu R, Chen W, Zheng A, Yu Y, Zhou D, Dai L, Kuang L. Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI. Journal of Clinical Medicine. 2023; 12(10):3556. https://doi.org/10.3390/jcm12103556
Chicago/Turabian StyleLi, Xiao, Jiamei Guo, Xiaolu Chen, Renqiang Yu, Wanjun Chen, Anhai Zheng, Yanjie Yu, Dongdong Zhou, Linqi Dai, and Li Kuang. 2023. "Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI" Journal of Clinical Medicine 12, no. 10: 3556. https://doi.org/10.3390/jcm12103556
APA StyleLi, X., Guo, J., Chen, X., Yu, R., Chen, W., Zheng, A., Yu, Y., Zhou, D., Dai, L., & Kuang, L. (2023). Predicting Responses to Electroconvulsive Therapy in Adolescents with Treatment-Refractory Depression Based on Resting-State fMRI. Journal of Clinical Medicine, 12(10), 3556. https://doi.org/10.3390/jcm12103556







