Comparison of Different Ex-Vivo Preservation Strategies on Cardiac Metabolism in an Animal Model of Donation after Circulatory Death
Abstract
:1. Introduction
2. Materials and Methods
2.1. Heart Procurement
2.2. Preservation
2.3. Cardiac Metabolism
2.4. Myocardial Edema
2.5. Statistical Analysis
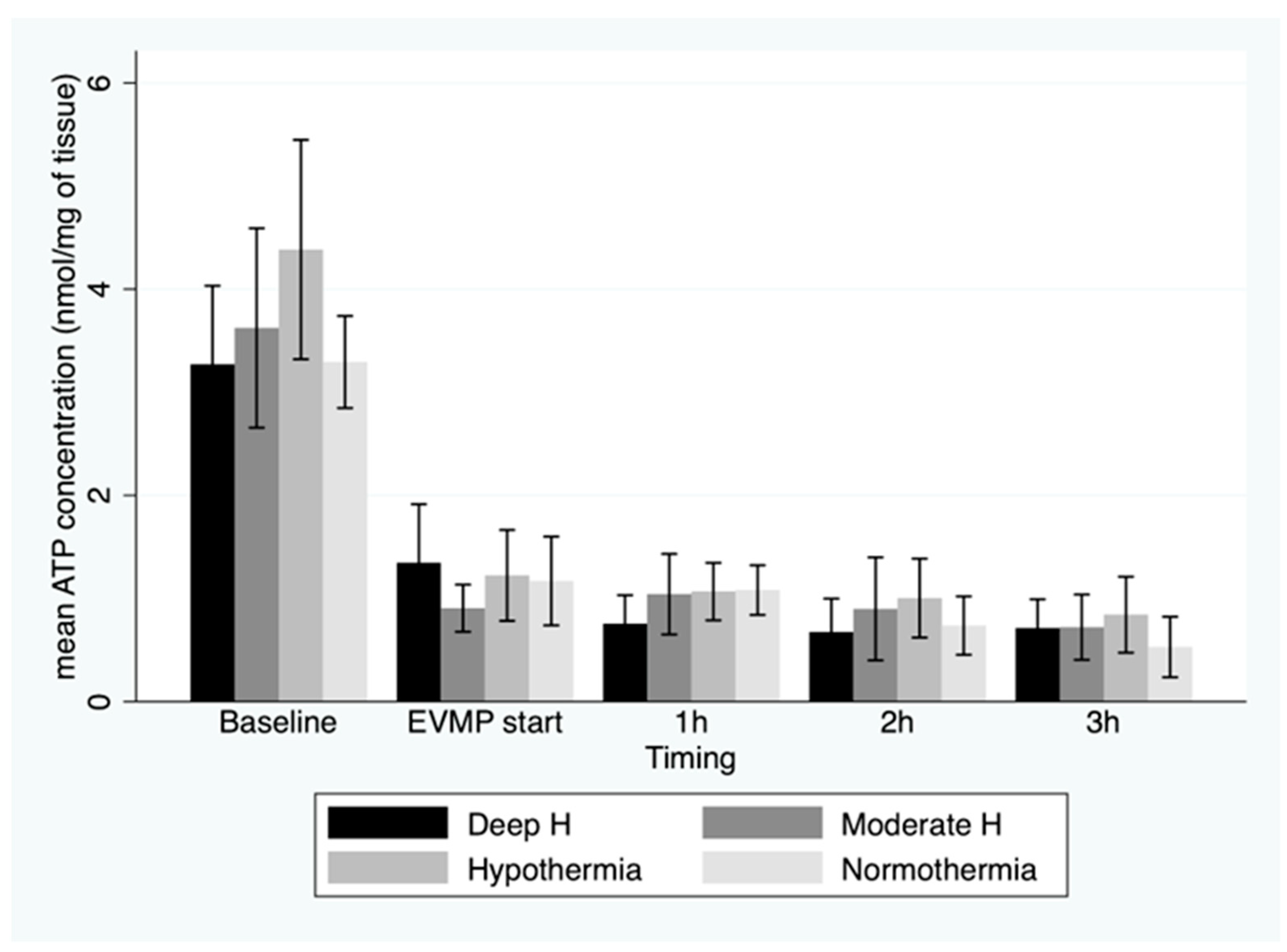
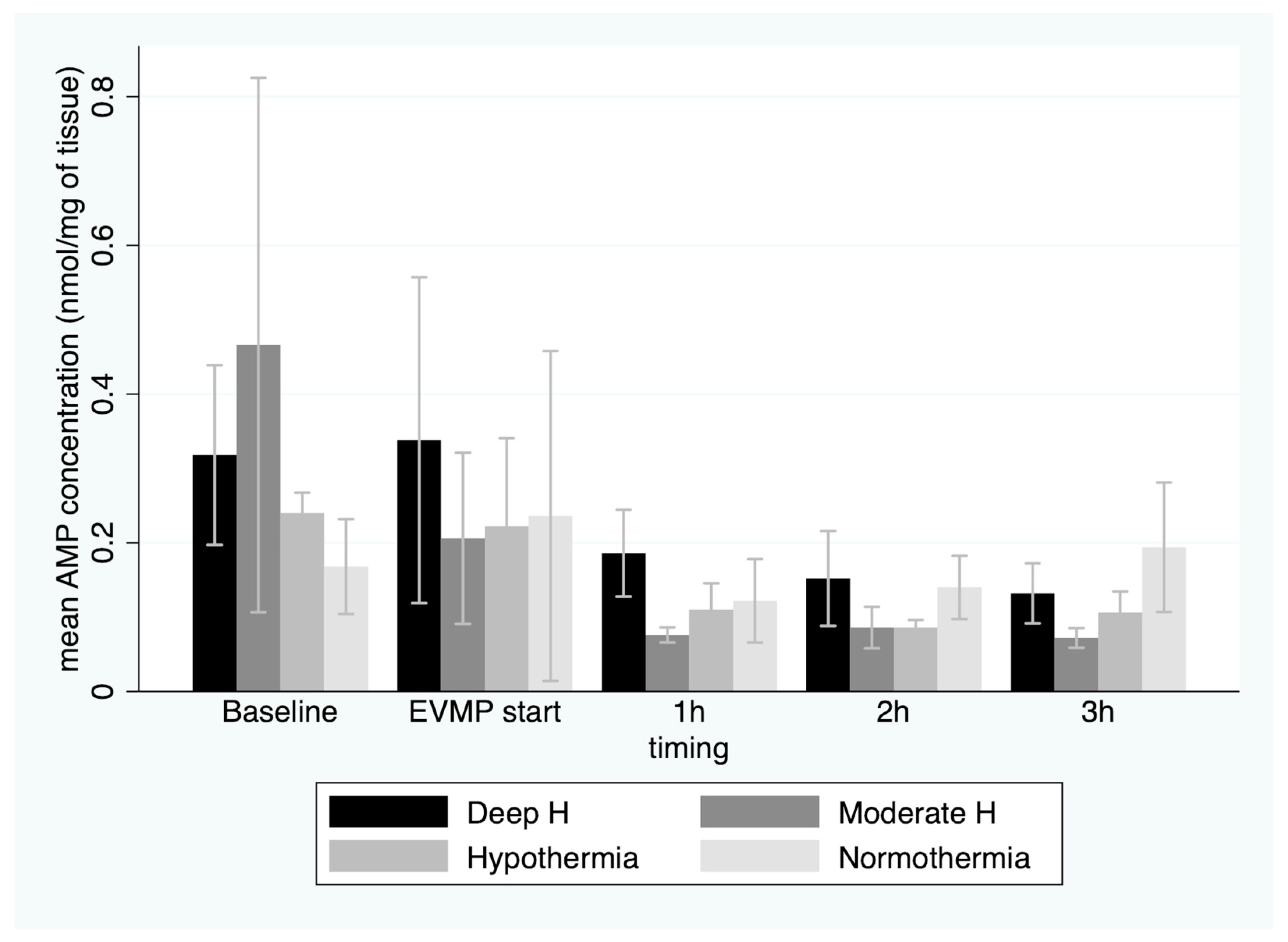
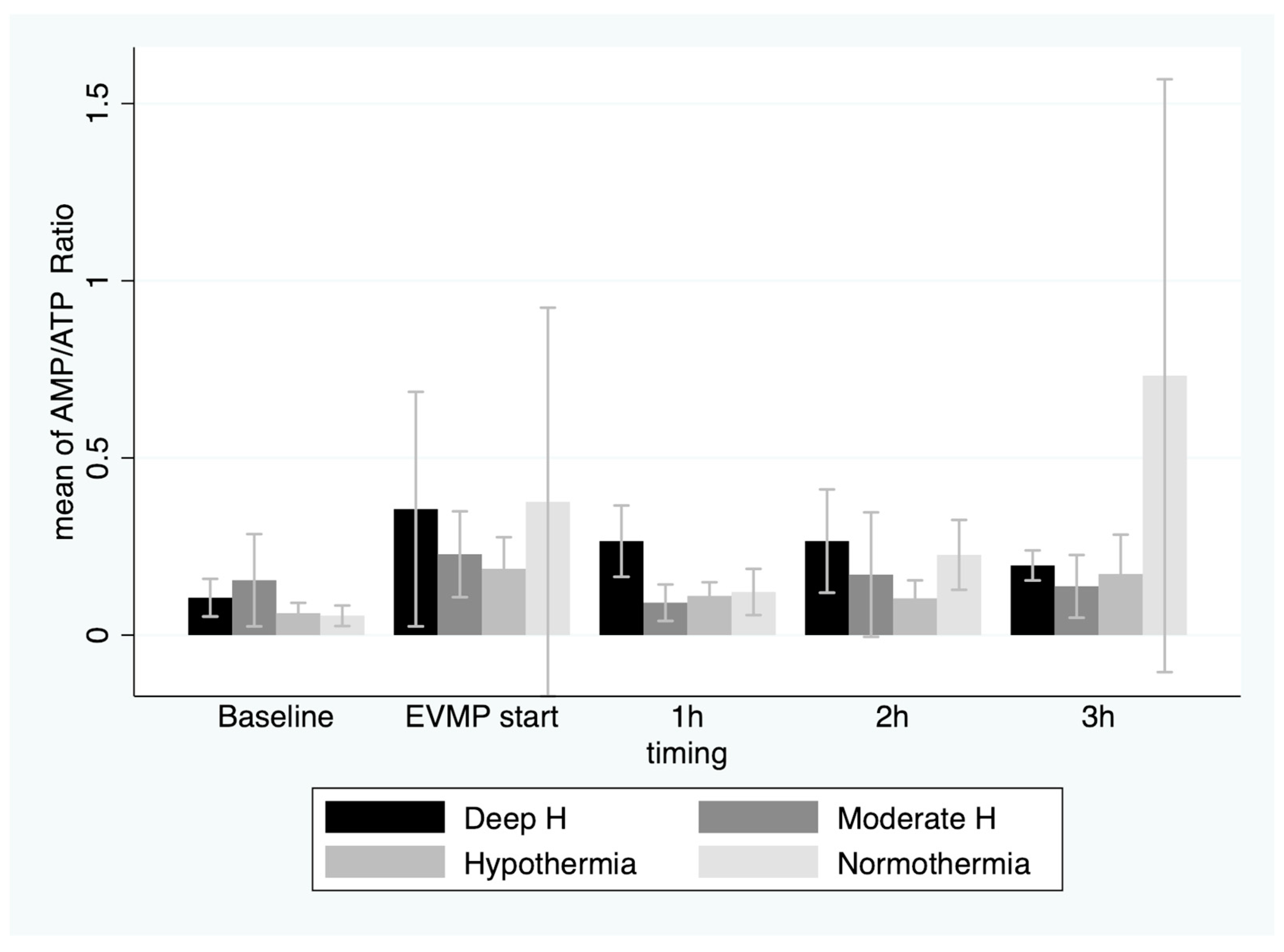
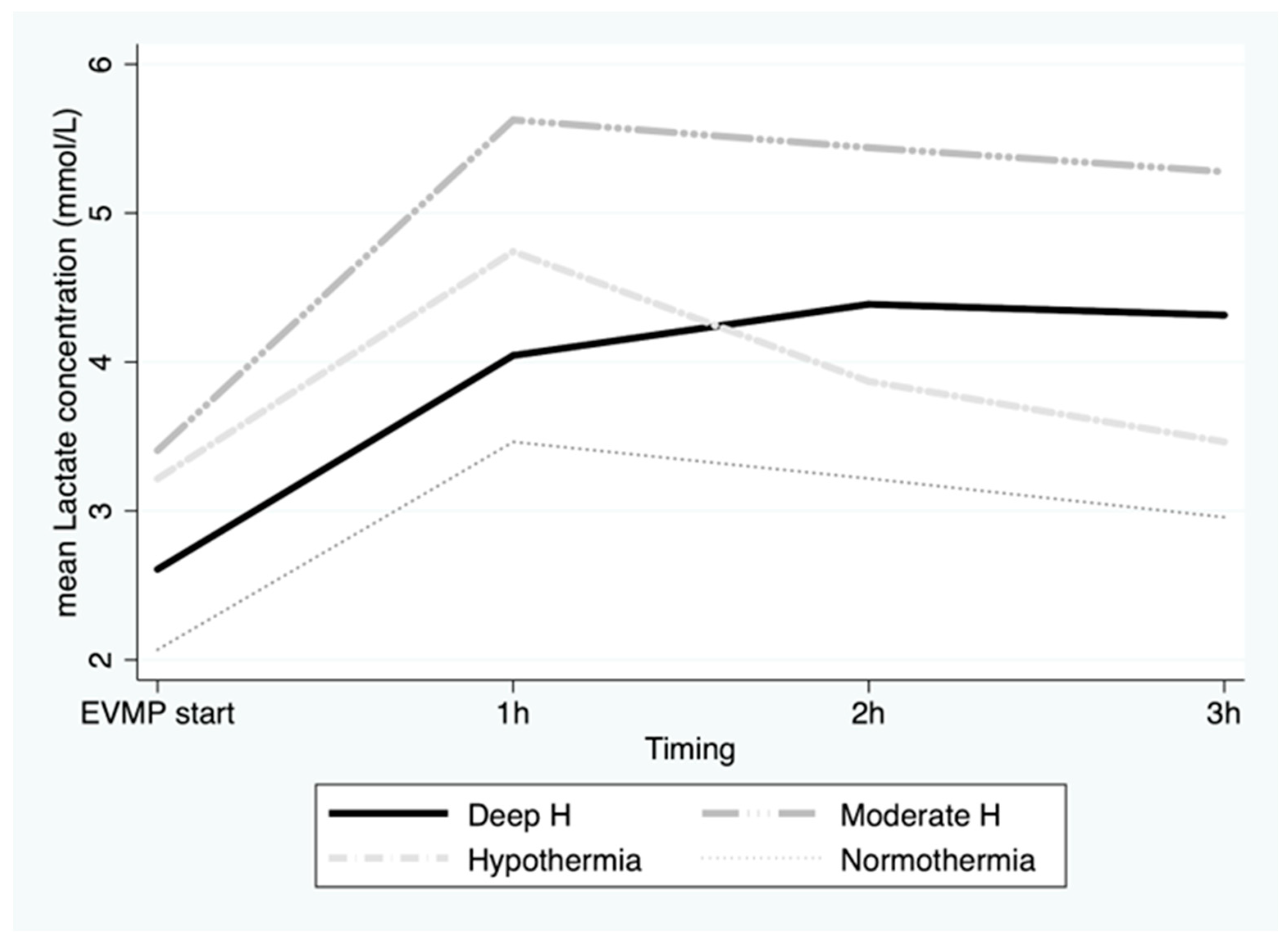
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messer, S.; Cernic, S.; Page, A.; Berman, M.; Kaul, P.; Colah, S.; Ali, J.; Pavlushkov, E.; Baxter, J.; Quigley, R.; et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J. Heart Lung Transplant. 2020, 39, 1463–1475. [Google Scholar] [CrossRef]
- Hamed, A.; Tsui, S.; Huber, J.; Lin, R.; Poggio, E.; Ardehali, A. 19: Serum Lactate Is a Highly Sensitive and Specific Predictor of Post Cardiac Transplant Outcomes Using the Organ Care System. J. Heart Lung Transplant. 2009, 28, S71. [Google Scholar] [CrossRef]
- Messer, S.J.; Axell, R.G.; Colah, S.; White, P.A.; Ryan, M.; Page, A.A.; Parizkova, B.; Valchanov, K.; White, C.W.; Freed, D.H.; et al. Functional assessment and transplantation of the donor heart following circulatory death. J. Heart. Lung Transplant. 2016, 35, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Tolboom, H.; Olejníčková, V.; Reser, D.; Rosser, B.; Wilhelm, M.J.; Gassmann, M.; Bogdanova, A.; Falk, V. Moderate hypothermia during ex vivo machine perfusion promotes recovery of hearts donated after cardiocirculatory death. Eur. J. Cardio Thorac. Surg. 2016, 49, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.; Isaac, J.R.; Clavien, P.-A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef]
- Patel, K.; Nath, J.; Hodson, J.; Inston, N.; Ready, A. Outcomes of donation after circulatory death kidneys undergoing hypothermic machine perfusion following static cold storage: A UK population-based cohort study. Am. J. Transplant. 2018, 18, 1408–1414. [Google Scholar] [CrossRef]
- Choong, J.W.; Ou, R.; Lim, Y.W.; Rosenfeldt, F.L. Cold Crystalloid Perfusion Provides Cardiac Preservation Superior to Cold Storage for Donation After Circulatory Death. Transplantation 2016, 100, 546–553. [Google Scholar] [CrossRef]
- Rosenfeldt, F.; Ou, R.; Salamonsen, R.; Marasco, S.; Zimmet, A.; Byrne, J.; Cosic, F.; Saxena, P.; Esmore, D. A novel combination technique of cold crystalloid perfusion but not cold storage facilitates transplantation of canine hearts donated after circulatory death. J. Heart Lung Transplant. 2016, 35, 1358–1364. [Google Scholar] [CrossRef]
- Van Caenegem, O.; Beauloye, C.; Bertrand, L.; Horman, S.; Lepropre, S.; Sparavier, G.; Vercruysse, J.; Bethuyne, N.; Poncelet, A.J.; Gianello, P.; et al. Hypothermic continuous machine perfusion enables preservation of energy charge and functional recovery of heart grafts in an ex vivo model of donation following circulatory death. Eur. J. Cardiothorac. Surg. 2016, 49, 1348–1353. [Google Scholar] [CrossRef]
- Van Caenegem, O.; Beauloye, C.; Vercruysse, J.; Horman, S.; Bertrand, L.; Bethuyne, N.; Poncelet, A.J.; Gianello, P.; Demuylder, P.; Legrand, E.; et al. Hypothermic continuous machine perfusion improves metabolic preservation and functional recovery in heart grafts. Transplant. Int. 2015, 28, 224–231. [Google Scholar] [CrossRef]
- Wahlberg, J.A.; Southard, J.H.; Belzer, F.O. Development of a cold storage solution for pancreas preservation. Cryobiology 1986, 23, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.C.; Iyer, A.; Connellan, M.; Scheuer, S.; Villanueva, J.; Gao, L.; Hicks, M.; Harkness, M.; Soto, C.; Dinale, A.; et al. Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J. Am. Coll. Cardiol. 2019, 73, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Gao, L.; Doyle, A.; Rao, P.; Jayewardene, D.; Wan, B.; Kumarasinghe, G.; Jabbour, A.; Hicks, M.; Jansz, P.C.; et al. Increasing the Tolerance of DCD Hearts to Warm Ischemia by Pharmacological Postconditioning. Am. J. Transplant. 2014, 14, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Messer, S.; Page, A.; Axell, R.; Berman, M.; Hernández-Sánchez, J.; Colah, S.; Parizkova, B.; Valchanov, K.; Dunning, J.; Pavlushkov, E.; et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J. Heart Lung Transplant. 2017, 36, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Joachimbauer, A.; Segiser, A.; Fiedler, G.M.; Carrel, T.P.; Stahel, H.T.T.; Longnus, S.L. Differential effects of ischemia/reperfusion on endothelial function and contractility in donation after circulatory death. J. Heart Lung Transplant. 2019, 38, 767–777. [Google Scholar] [CrossRef]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef]
- Ko, T.; Otani, H.; Imamura, H.; Omori, K.; Inagaki, C. Role of sodium pump activity in warm induction of cardioplegia combined with reperfusion of oxygenated cardioplegic solution. J. Thorac. Cardiovasc. Surg. 1995, 110, 103–110. [Google Scholar] [CrossRef]
- Parolari, A.; Rubini, P.; Cannata, A.; Bonati, L.; Alamanni, F.; Tremoli, E.; Biglioli, P. Endothelial damage during myocardial preservation and storage. Ann. Thorac. Surg. 2002, 73, 682–690. [Google Scholar] [CrossRef]
- White, C.W.; Avery, E.; Müller, A.; Li, Y.; Le, H.; Thliveris, J.; Arora, R.C.; Lee, T.W.; Dixon, I.M.C.; Tian, G.; et al. Avoidance of Profound Hypothermia During Initial Reperfusion Improves the Functional Recovery of Hearts Donated After Circulatory Death. Am. J. Transplant. 2016, 16, 773–782. [Google Scholar] [CrossRef]
- Watson, A.J.; Gao, L.; Sun, L.; Tsun, J.; Doyle, A.; Faddy, S.C.; Jabbour, A.; Orr, Y.; Dhital, K.; Hicks, M.; et al. Enhanced Preservation of Pig Cardiac Allografts by Combining Erythropoietin With Glyceryl Trinitrate and Zoniporide. Am. J. Transplant. 2013, 13, 1676–1687. [Google Scholar] [CrossRef]
- White, C.W.; Ali, A.; Hasanally, D.; Xiang, B.; Li, Y.; Mundt, P.; Lytwyn, M.; Colah, S.; Klein, J.; Ravandi, A.; et al. A cardioprotective preservation strategy employing ex vivo heart perfusion facilitates successful transplant of donor hearts after cardiocirculatory death. J. Heart Lung Transplant. 2013, 32, 734–743. [Google Scholar] [CrossRef] [PubMed]
- White, C.W.; Messer, S.J.; Large, S.R.; Conway, J.; Kim, D.H.; Kutsogiannis, D.J.; Nagendran, J.; Freed, D.H. Transplantation of Hearts Donated after Circulatory Death. Front. Cardiovasc. Med. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Carmona, N.; Wyss, R.K.; Arnold, M.; Segiser, A.; Kalbermatter, N.; Joachimbauer, A.; Carrel, T.P.; Longnus, S.L. Effects of graft preservation conditions on coronary endothelium and cardiac functional recovery in a rat model of donation after circulatory death. J. Heart Lung Transplant. 2021, 40, 1396–1407. [Google Scholar] [CrossRef] [PubMed]


| Group A (4 °C) | Group B (18 °C) | Group C (25 °C) | Group D (35 °C) | p | |
|---|---|---|---|---|---|
| Time from WLS to cardiac arrest (min) | 8 ± 4 | 7 ± 3 | 6 ± 3 | 7 ± 5 | ns |
| Time from cardiac arrest to cardioplegia (FWIT) (min) | 20 | 20 | 20 | 20 | ns |
| Time from WLS to cardioplegia (WIT) (min) | 27.8 ± 4.2 | 26.6 ± 2.5 | 26.2 ± 2.6 | 26.0 ± 4.6 | ns |
| Time from cardiac arrest to EVMP (min) | 39 ± 4 * | 41 ± 4 | 46 ± 5 * | 39 ± 4 | 0.06 * |
| Allograft weight before EVMP (grams) | 279 ± 46 | 281 ± 16 | 364 ± 26 | 284 ± 53 | 0.01 |
| Allograft weight after EVMP (grams) | 299 ± 43 * | 394 ± 42 | 428 ± 45 * | 383 ± 100 | 0.02 * |
| Allograft weight wet–dry ratio | 1.1 ± 0.0 * | 1.4 ± 0.2 * | 1.2 ± 0.1 | 1.3 ± 0.1 | 0.01 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastrobuoni, S.; Johanns, M.; Vergauwen, M.; Beaurin, G.; Rider, M.; Gianello, P.; Poncelet, A.; Van Caenegem, O. Comparison of Different Ex-Vivo Preservation Strategies on Cardiac Metabolism in an Animal Model of Donation after Circulatory Death. J. Clin. Med. 2023, 12, 3569. https://doi.org/10.3390/jcm12103569
Mastrobuoni S, Johanns M, Vergauwen M, Beaurin G, Rider M, Gianello P, Poncelet A, Van Caenegem O. Comparison of Different Ex-Vivo Preservation Strategies on Cardiac Metabolism in an Animal Model of Donation after Circulatory Death. Journal of Clinical Medicine. 2023; 12(10):3569. https://doi.org/10.3390/jcm12103569
Chicago/Turabian StyleMastrobuoni, Stefano, Manuel Johanns, Martial Vergauwen, Gwen Beaurin, Mark Rider, Pierre Gianello, Alain Poncelet, and Olivier Van Caenegem. 2023. "Comparison of Different Ex-Vivo Preservation Strategies on Cardiac Metabolism in an Animal Model of Donation after Circulatory Death" Journal of Clinical Medicine 12, no. 10: 3569. https://doi.org/10.3390/jcm12103569





