Association between Cerebrospinal Fluid Soluble TREM2, Alzheimer’s Disease and Other Neurodegenerative Diseases
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
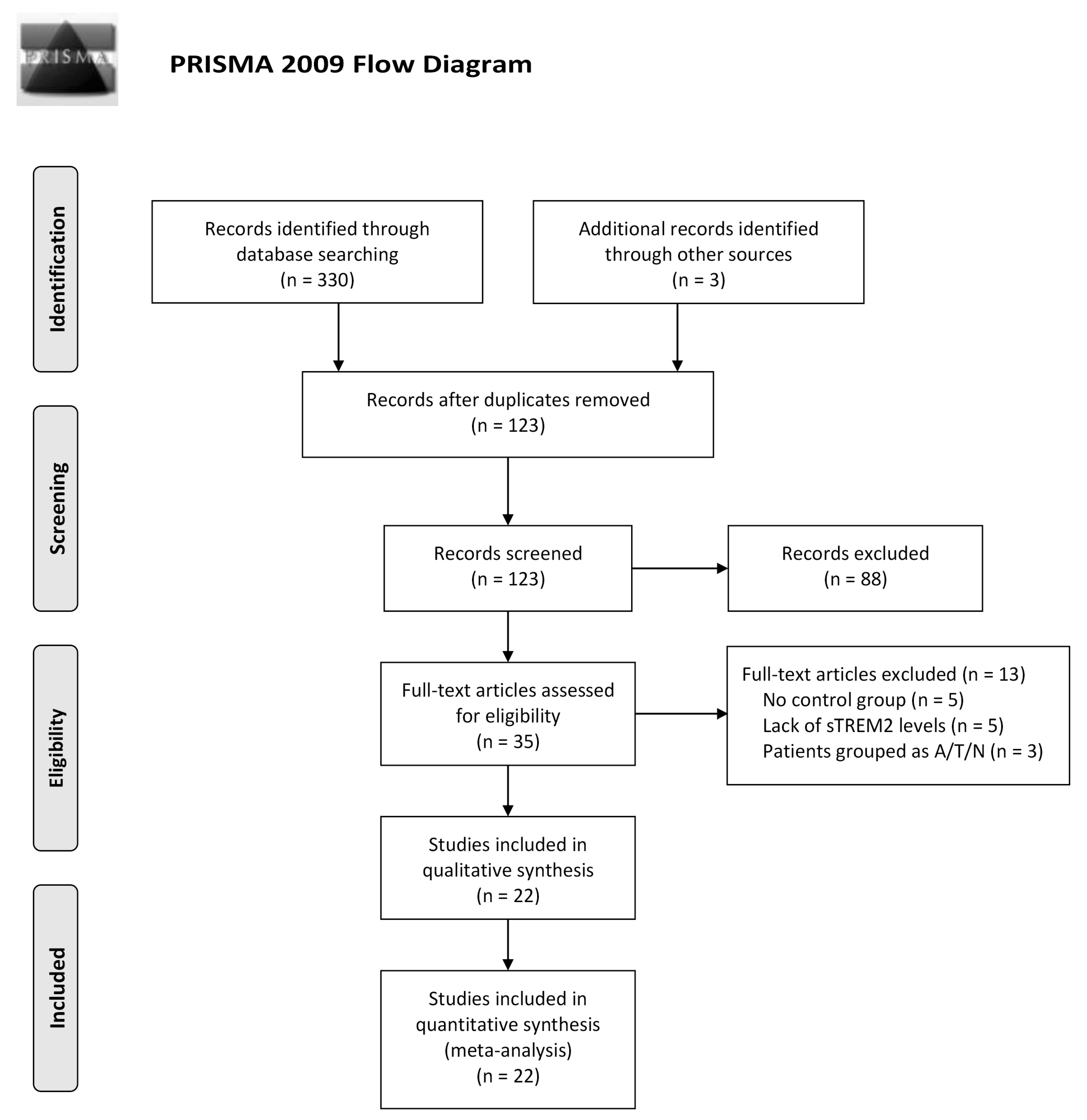
3.1. Study Characteristics and Quality Assessment
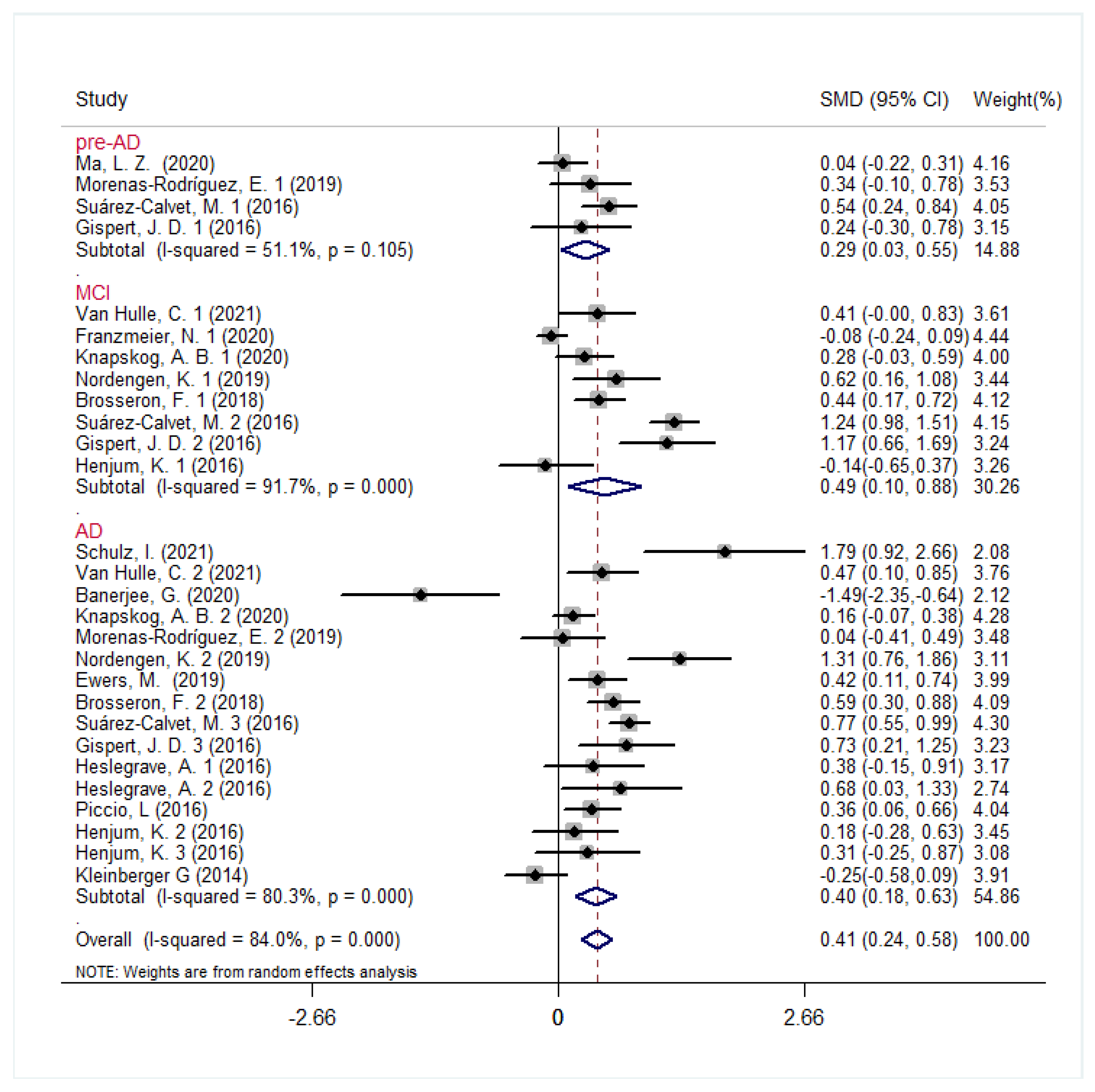
3.2. CSF sTREM2 Levels in AD Continuum Cohorts
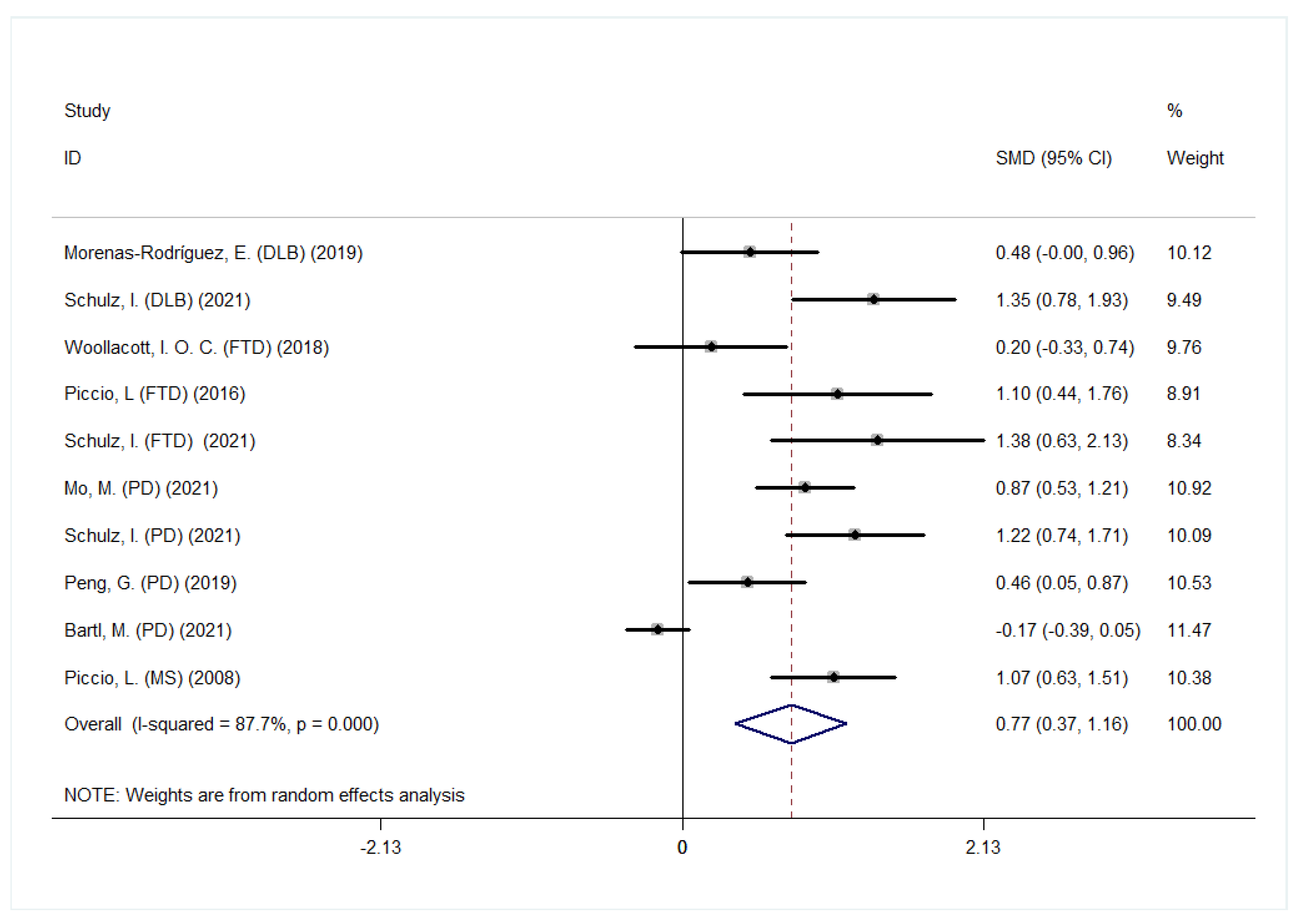
3.3. CSF sTREM2 Levels in Other NDDs
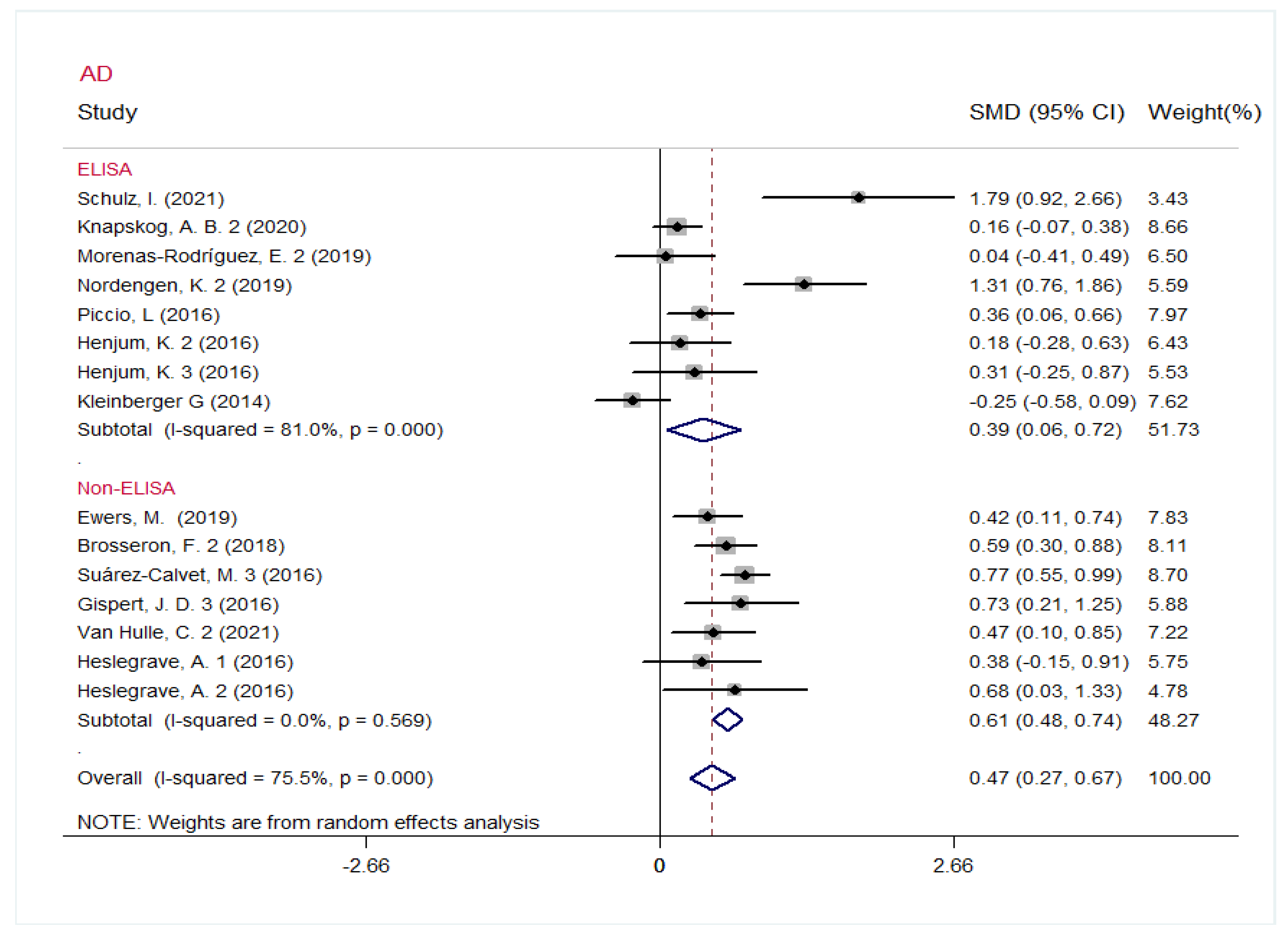
3.4. Heterogeneity Analysis and Subgroup Analysis
3.5. Meta-Regression and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ennerfelt, H.E.; Lukens, J.R. The role of innate immunity in Alzheimer’s disease. Immunol. Rev. 2020, 297, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in Neurodegenerative Diseases. Mol. Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Lewcock, J.W.; Schlepckow, K.; Di Paolo, G.; Tahirovic, S.; Monroe, K.M.; Haass, C. Emerging Microglia Biology Defines Novel Therapeutic Approaches for Alzheimer’s Disease. Neuron 2020, 108, 801–821. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, E.N.; Branda, C.S.; Branda, S.S.; Nevarez, L.; Colonna, M.; Lowell, C.; Hamerman, J.A.; Seaman, W.E. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocytic receptor for bacteria. J. Cell Biol. 2009, 184, 215–223. [Google Scholar] [CrossRef]
- Otero, K.; Shinohara, M.; Zhao, H.; Cella, M.; Gilfillan, S.; Colucci, A.; Faccio, R.; Ross, F.P.; Teitelbaum, S.L.; Takayanagi, H.; et al. TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J. Immunol. 2012, 188, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Rochford, C.D.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J. Exp. Med. 2005, 201, 647–657. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Paloneva, J.; Manninen, T.; Christman, G.; Hovanes, K.; Mandelin, J.; Adolfsson, R.; Bianchin, M.; Bird, T.; Miranda, R.; Salmaggi, A.; et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 2002, 71, 656–662. [Google Scholar] [CrossRef]
- Borroni, B.; Ferrari, F.; Galimberti, D.; Nacmias, B.; Barone, C.; Bagnoli, S.; Fenoglio, C.; Piaceri, I.; Archetti, S.; Bonvicini, C.; et al. Heterozygous TREM2 mutations in frontotemporal dementia. Neurobiol. Aging 2014, 35, 934.e7–934.e10. [Google Scholar] [CrossRef]
- Cady, J.; Koval, E.D.; Benitez, B.A.; Zaidman, C.; Jockel-Balsarotti, J.; Allred, P.; Baloh, R.H.; Ravits, J.; Simpson, E.; Appel, S.H.; et al. TREM2 variant p.R47H as a risk factor for sporadic amyotrophic lateral sclerosis. JAMA Neurol. 2014, 71, 449–453. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, S.; Mullen, B.; Baker, M.; Lynch, T.; Finger, E.; Seeley, W.W.; Hatanpaa, K.J.; Lomen-Hoerth, C.; Kertesz, A.; Bigio, E.H.; et al. TREM2 in neurodegeneration: Evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol. Neurodegener. 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kacimi, R.; Kauppinen, T.; Calosing, C.; Kim, J.Y.; Hsieh, C.L.; Nakamura, M.C.; Yenari, M.A. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 2015, 35, 3384–3396. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Song, W.M.; Huang, S.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663.e613. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Jin, S.C.; Benitez, B.A.; Karch, C.M.; Cooper, B.; Skorupa, T.; Carrell, D.; Norton, J.B.; Hsu, S.; Harari, O.; Cai, Y.; et al. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum. Mol. Genet. 2014, 23, 5838–5846. [Google Scholar] [CrossRef]
- Kiianitsa, K.; Kurtz, I.; Beeman, N.; Matsushita, M.; Chien, W.M.; Raskind, W.H.; Korvatska, O. Novel TREM2 splicing isoform that lacks the V-set immunoglobulin domain is abundant in the human brain. J. Leukoc. Biol. 2021, 110, 829–837. [Google Scholar] [CrossRef]
- Del-Aguila, J.L.; Benitez, B.A.; Li, Z.; Dube, U.; Mihindukulasuriya, K.A.; Budde, J.P.; Farias, F.H.G.; Fernández, M.V.; Ibanez, L.; Jiang, S.; et al. TREM2 brain transcript-specific studies in AD and TREM2 mutation carriers. Mol. Neurodegener. 2019, 14, 18. [Google Scholar] [CrossRef]
- Banerjee, G.; Ambler, G.; Keshavan, A.; Paterson, R.W.; Foiani, M.S.; Toombs, J.; Heslegrave, A.; Dickson, J.C.; Fraioli, F.; Groves, A.M.; et al. Cerebrospinal Fluid Biomarkers in Cerebral Amyloid Angiopathy. J. Alzheimers Dis. 2020, 74, 1189–1201. [Google Scholar] [CrossRef]
- Bartl, M.; Dakna, M.; Galasko, D.; Hutten, S.J.; Foroud, T.; Quan, M.; Marek, K.; Siderowf, A.; Franz, J.; Trenkwalder, C.; et al. Biomarkers of neurodegeneration and glial activation validated in Alzheimer’s disease assessed in longitudinal cerebrospinal fluid samples of Parkinson’s disease. PLoS ONE 2021, 16, e0257372. [Google Scholar] [CrossRef] [PubMed]
- Brosseron, F.; Traschütz, A.; Widmann, C.N.; Kummer, M.P.; Tacik, P.; Santarelli, F.; Jessen, F.; Heneka, M.T. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res. Ther. 2018, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Deming, Y.; Filipello, F.; Cignarella, F.; Cantoni, C.; Hsu, S.; Mikesell, R.; Li, Z.; Del-Aguila, J.L.; Dube, U.; Farias, F.G.; et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 2019, 11, 2291. [Google Scholar] [CrossRef]
- Ewers, M.; Franzmeier, N.; Suárez-Calvet, M.; Morenas-Rodriguez, E.; Caballero, M.A.A.; Kleinberger, G.; Piccio, L.; Cruchaga, C.; Deming, Y.; Dichgans, M.; et al. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019, 11, 6221. [Google Scholar] [CrossRef] [PubMed]
- Franzmeier, N.; Suárez-Calvet, M.; Frontzkowski, L.; Moore, A.; Hohman, T.J.; Morenas-Rodriguez, E.; Nuscher, B.; Shaw, L.; Trojanowski, J.Q.; Dichgans, M.; et al. Higher CSF sTREM2 attenuates ApoE4-related risk for cognitive decline and neurodegeneration. Mol. Neurodegener. 2020, 15, 57. [Google Scholar] [CrossRef] [PubMed]
- Gispert, J.D.; Suárez-Calvet, M.; Monté, G.C.; Tucholka, A.; Falcon, C.; Rojas, S.; Rami, L.; Sánchez-Valle, R.; Lladó, A.; Kleinberger, G.; et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement. 2016, 12, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Henjum, K.; Almdahl, I.S.; Årskog, V.; Minthon, L.; Hansson, O.; Fladby, T.; Nilsson, L.N. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res. Ther. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Heslegrave, A.; Heywood, W.; Paterson, R.; Magdalinou, N.; Svensson, J.; Johansson, P.; Öhrfelt, A.; Blennow, K.; Hardy, J.; Schott, J.; et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol. Neurodegener. 2016, 11, 3. [Google Scholar] [CrossRef]
- Kleinberger, G.; Yamanishi, Y.; Suárez-Calvet, M.; Czirr, E.; Lohmann, E.; Cuyvers, E.; Struyfs, H.; Pettkus, N.; Wenninger-Weinzierl, A.; Mazaheri, F.; et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014, 6, 243ra286. [Google Scholar] [CrossRef]
- Knapskog, A.B.; Henjum, K.; Idland, A.V.; Eldholm, R.S.; Persson, K.; Saltvedt, I.; Watne, L.O.; Engedal, K.; Nilsson, L.N.G. Cerebrospinal fluid sTREM2 in Alzheimer’s disease: Comparisons between clinical presentation and AT classification. Sci. Rep. 2020, 10, 15886. [Google Scholar] [CrossRef]
- Ma, L.Z.; Tan, L.; Bi, Y.L.; Shen, X.N.; Xu, W.; Ma, Y.H.; Li, H.Q.; Dong, Q.; Yu, J.T. Dynamic changes of CSF sTREM2 in preclinical Alzheimer’s disease: The CABLE study. Mol. Neurodegener. 2020, 15, 25. [Google Scholar] [CrossRef]
- Mo, M.; Tang, Y.; Wei, L.; Qiu, J.; Peng, G.; Lin, Y.; Zhou, M.; Dai, W.; Zhang, Z.; Chen, X.; et al. Soluble Triggering Receptor Expressed on Myeloid Cells 2 From Cerebrospinal Fluid in Sleep Disorders Related to Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 753210. [Google Scholar] [CrossRef] [PubMed]
- Morenas-Rodríguez, E.; Alcolea, D.; Suárez-Calvet, M.; Muñoz-Llahuna, L.; Vilaplana, E.; Sala, I.; Subirana, A.; Querol-Vilaseca, M.; Carmona-Iragui, M.; Illán-Gala, I.; et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci. Rep. 2019, 9, 7803. [Google Scholar] [CrossRef] [PubMed]
- Nordengen, K.; Kirsebom, B.E.; Henjum, K.; Selnes, P.; Gísladóttir, B.; Wettergreen, M.; Torsetnes, S.B.; Grøntvedt, G.R.; Waterloo, K.K.; Aarsland, D.; et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J. Neuroinflamm. 2019, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Qiu, J.; Liu, H.; Zhou, M.; Huang, S.; Guo, W.; Lin, Y.; Chen, X.; Li, Z.; Li, G.; et al. Analysis of Cerebrospinal Fluid Soluble TREM2 and Polymorphisms in Sporadic Parkinson’s Disease in a Chinese Population. J. Mol. Neurosci. 2020, 70, 294–301. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J., 2nd; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Piccio, L.; Deming, Y.; Del-Águila, J.L.; Ghezzi, L.; Holtzman, D.M.; Fagan, A.M.; Fenoglio, C.; Galimberti, D.; Borroni, B.; Cruchaga, C. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016, 131, 925–933. [Google Scholar] [CrossRef]
- Schulz, I.; Kruse, N.; Gera, R.G.; Kremer, T.; Cedarbaum, J.; Barbour, R.; Zago, W.; Schade, S.; Otte, B.; Bartl, M.; et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov. Disord. 2021, 36, 2874–2887. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol. Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Van Hulle, C.; Jonaitis, E.M.; Betthauser, T.J.; Batrla, R.; Wild, N.; Kollmorgen, G.; Andreasson, U.; Okonkwo, O.; Bendlin, B.B.; Asthana, S.; et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimers Dement. 2021, 17, 431–445. [Google Scholar] [CrossRef]
- Woollacott, I.O.C.; Nicholas, J.M.; Heslegrave, A.; Heller, C.; Foiani, M.S.; Dick, K.M.; Russell, L.L.; Paterson, R.W.; Keshavan, A.; Fox, N.C.; et al. Cerebrospinal fluid soluble TREM2 levels in frontotemporal dementia differ by genetic and pathological subgroup. Alzheimers Res. Ther. 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Calvet, M.; Araque Caballero, M.; Kleinberger, G.; Bateman, R.J.; Fagan, A.M.; Morris, J.C.; Levin, J.; Danek, A.; Ewers, M.; Haass, C. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci. Transl. Med. 2016, 8, 369ra178. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef]
- Korvatska, O.; Leverenz, J.B.; Jayadev, S.; McMillan, P.; Kurtz, I.; Guo, X.; Rumbaugh, M.; Matsushita, M.; Girirajan, S.; Dorschner, M.O.; et al. R47H Variant of TREM2 Associated With Alzheimer Disease in a Large Late-Onset Family: Clinical, Genetic, and Neuropathological Study. JAMA Neurol. 2015, 72, 920–927. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Morenas-Rodríguez, E.; Kleinberger, G.; Schlepckow, K.; Araque Caballero, M.; Franzmeier, N.; Capell, A.; Fellerer, K.; Nuscher, B.; Eren, E.; et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol. Neurodegener. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Schlepckow, K.; Kleinberger, G.; Fukumori, A.; Feederle, R.; Lichtenthaler, S.F.; Steiner, H.; Haass, C. An Alzheimer-associated TREM2 variant occurs at the ADAM cleavage site and affects shedding and phagocytic function. EMBO Mol. Med. 2017, 9, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Sevalle, J.; Deery, M.J.; Fraser, G.; Zhou, Y.; Ståhl, S.; Franssen, E.H.; Dodd, R.B.; Qamar, S.; Gomez Perez-Nievas, B.; et al. TREM2 shedding by cleavage at the H157-S158 bond is accelerated for the Alzheimer’s disease-associated H157Y variant. EMBO Mol. Med. 2017, 9, 1366–1378. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Pan, N.; Liu, C.; Wang, Y.; Zhang, T. Age Matching Is Essential for the Study of Cerebrospinal Fluid sTREM2 Levels and Alzheimer’s Disease Risk: A Meta-Analysis. Front. Aging Neurosci. 2021, 13, 775432. [Google Scholar] [CrossRef]
- Liu, D.; Cao, B.; Zhao, Y.; Huang, H.; McIntyre, R.S.; Rosenblat, J.D.; Zhou, H. Soluble TREM2 changes during the clinical course of Alzheimer’s disease: A meta-analysis. Neurosci. Lett. 2018, 686, 10–16. [Google Scholar] [CrossRef]

| First Author | Year | Location | Design | Method | Diagnostic Criteria | Disease | Case | Control | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, y, Mean (SD) | No. of Female (%) | sTREM2 (ng/mL) | n | Age, y, Mean (SD) | No. of Female (%) | sTREM2 (ng/mL) | |||||||
| Schulz, I. | 2021 | Germany | CS | ELISA | NINDS-ADRDA | AD | 11 | 74.27 (4.64) | 5 (45) | 7.750 (3.815) | 20 | 68.75 (6.38) | 6 (30) | 3.218 (1.463) |
| Van Hulle, C. | 2021 | US | CS | NTK | NIA-AA | MCI | 33 | 74.1 (7.6) | 13 (39) | 9.75 (3.68) | 70 | 69.1 (6.6) | 45 (64) | 8.51 (2.63) |
| AD | 46 | 72.3 (8.0) | 18 (39) | 9.95 (3.57) | ||||||||||
| Franzmeier, N. | 2020 | ADNI | CS | ELISA | NINCDS-ADRDA | MCI | 414 | 71.82 (7.45) | 170 (41) | 4.095 (2.105) | 221 | 74.25 (6.08) | 106 (48) | 4.256 (2.184) |
| AD | 73 | 74.17 (8.37) | 35 (48) | 4.371 (2.194) | ||||||||||
| Banerjee, G. | 2020 | UK | CC | MSD | NIA-AA | AD | 20 | 62.5 (4.1) | 11 (55) | 6.669 (0.664) | 10 | 62.2 (5.4) | 5 (50) | 7.960 (1.183) |
| Knapskog, A. B. | 2020 | Norway | CS | ELISA | NIA-AA | MCI | 62 | 71.0 (5.4) | 36 (58.1) | 9.9 (4.5) | 113 | 72.3 (6.0) | 54 (47.8) | 8.8 (3.6) |
| AD | 237 | 70.1 (6.8) | 135 (57) | 9.5 (4.8) | ||||||||||
| Ma, L. Z. | 2020 | China | CS | ELISA | NIA-AA | pre-AD stage 1 | 148 | 60.39 (10.41) | 63 (42.6) | 15.12 (6.40) | 242 | 60.81 (9.95) | 96 (39.7) | 18 (6.34) |
| pre-AD stage 2 | 70 | 64.19 (11.09) | 31 (42.3) | 18.28 (7.54) | ||||||||||
| Morenas-Rodríguez, E. | 2019 | Spain | CC | ELISA | NIA-AA | pre-AD | 53 | 72.3 (6.3) | 32 (60.4) | 5 (2.4) (No. = 41) | 44 | 67.4 (5.1) | 25 (56.8) | 4.2 (2.3) (No. = 40) |
| AD | 50 | 74.6 (5.6) | 31 (62) | 4.3 (2.2) (No. = 36) | ||||||||||
| Nordengen, K. | 2019 | Norway | CC | ELISA | NIA-AA | MCI | 40 | 66.6 (7.4) | 23 (57) | 4.0 (1.8) | 36 | 61.1 (9.2) | 19 (53) | 3.1 (0.9) |
| AD | 27 | 67.6 (5.2) | 13 (48) | 4.8 (1.7) | ||||||||||
| Deming, Y. | 2019 | ADNI | CS | ELISA | NINCDS-ADRDA | EMCI | 183 | 71.23 (7.39) | 77 (42.1) | 3.74 (2.07) | 169 | 74.47 (5.85) | 80 (47.3) | 3.99 (1.92) |
| LMCI | 221 | 73.06 (7.41) | 91 (41.2) | 3.92 (1.83) | ||||||||||
| AD | 172 | 74.39 (8.56) | 74 (43.0) | 4.02 (1.95) | ||||||||||
| Ewers, M. | 2019 | ADNI | CS | MSD | NIA-AA | MCI | 184 | 72.9 (7.11) | 77 (41.8) | 4.452 (2.518) | 100 | 72.8 (5.36) | 45 (45) | 3.762 (1.841) |
| AD | 66 | 73.6 (8.51) | 32 (48.5) | 4.608 (2.201) | ||||||||||
| Brosseron, F. | 2018 | Germany | CC | MSD | NIA-AA | MCI | 130 | 71 (8) | 65 (50) | 4.07 (2.54) | 85 | 67 (11) | 65 (76) | 2.99 (2.27) |
| AD | 116 | 74 (8) | 45 (39) | 4.32 (2.24) | ||||||||||
| Suárez-Calvet, M. | 2016 | Multi-center | CC | MSD | NIA-AA | pre-AD | 63 | 70.8 (11) | 38 (60) | 4.09 (2.7) | 150 | 62.4 (11) | 89 (59) | 3.07 (1.4) |
| MCI | 111 | 74.3 (9) | 67 (60) | 5.98 (3.2) | ||||||||||
| AD | 200 | 73.8 (10) | 124 (62) | 5.33 (3.7) | ||||||||||
| Gispert, J. D | 2016 | Spain | CC | MSD | NIA-AA | pre-AD | 19 | 68.53 (7.93) | 13 (68.42) | 2.70 (1.47) | 45 | 60.98 (6.83) | 28 (63.04) | 2.40 (1.14) |
| MCI | 27 | 70.30 (7.35) | 15 (55.56) | 4.16 (1.97) | ||||||||||
| AD | 23 | 66.78 (9.75) | 16 (69.57) | 3.34 (1.53) | ||||||||||
| Heslegrave, A. | 2016 | UK Sweden | CC | UPLC-MS | IWG2 | AD | 37 | 70.51 (7.5) | 19 (53) | 0.231 (0.098) | 22 | 69.2 (8.0) | 10(45) | 0.196 (0.081) |
| AD | 24 | 64.3 (6.8) | 13 (54) | 0.231 (0.097) | 16 | 55.6 (9.7) | 9 (56) | 0.173 (0.065) | ||||||
| Piccio, L | 2016 | Italy/US | CC | ELISA | NINCDS-ADRDA | AD | 73 | 76.6 (5.2) | 36 (49) | 1.028 (0.582) | 107 | 70.2 (8.5) | 57 (53) | 0.832 (0.508) |
| Henjum, K | 2016 | Norway Sweden | CC | ELISA | NIA-AA | MCI | 21 | 67.0 (5.0) | 12 (57) | 4.10 (2.59) | 50 | 66.0 (9.0) | 25 (50) | 4.40 (2.00) |
| AD | 29 | 68.0 (4.8) | 13 (45) | 4.80 (2.67) | ||||||||||
| NINCDS-ADRDA | AD | 25 | 79.0 (6.3) | 18 (72) | 3.80 (2.20) | 25 | 62.0 (9.3) | 17 (68) | 3.20 (1.63) | |||||
| Kleinberger G | 2014 | Germany | CS | ELISA | NINCDS-ADRDA | AD | 56 | 70.4 (8.9) | 38 (68) | 0.3087 (0.191) (RU) | 88 | 60.7 (9.5) | 55 (63) | 0.3834 (0.174) |
| Schulz, I. | 2021 | Germany | CS | ELISA | UKPDSBB | PD | 151 | 69.36 (9.55) | 51 (34) | 6.494 (2.794) | 20 | 68.75 (6.38) | 6 (30) | 3.218 (1.463) |
| FTDC | FTD | 15 | 70.80 (5.58) | 5 (33) | 6.486 (3.210) | |||||||||
| DLB consensus | DLB | 45 | 70.51 (6.51) | 14 (31) | 6.375 (2.620) | |||||||||
| Bartl, M. | 2021 | Multi-center | CC | NTK | UKPDSBB | PD | 252 | 61 (9.8) | 87 (34.5) | 6.9 (2.2) | 115 | 62 (11) | 41 (35.7) | 7.3 (2.7) |
| Mo, M. | 2021 | China | CC | ELISA | UKPDSBB | PD | 80 | 63.59 (8.50) | 32 (40) | 0.419 (0.182) | 65 | 62.49 (6.90) | 26 (40) | 0.290 (0.090) |
| Peng, G. | 2019 | China | CC | ELISA | UKPDSBB | PD | 55 | 59.8 (8.9) | 28 (51) | 0.4331 (0.0247) | 40 | 55.6 (13.4) | 19 (47.5) | 0.2752 (0.0179) |
| Morenas-Rodríguez, E. | 2019 | Spain | CC | ELISA | DLB consensus | DLB | 37 | 76.5 (5) | 20 (54.1) | 5.3 (2.3) (No. = 28) | 44 | 67.4 (5.1) | 25 (56.8) | 4.2 (2.3) (No. = 40) |
| Woollacott, I. O. C. | 2018 | UK | CC | MSD | FTDC | FTD | 64 | 64.6 (6.5) | 19 (29.7) | 7.4 (3.2) | 17 | 63.7 (6.4) | 11 (64.7) | 6.8 (1.6) |
| Piccio, L | 2016 | Italy/US | CC | ELISA | FTDC | FTD | 10 | - | - | 1.396 (0.563) | 107 | 70.2 (8.5) | 57 (53) | 0.832 (0.508) |
| Piccio, L. | 2008 | US | CC | ELISA | McDonald criteria | MS | 52 | 54 (9) | 31 (59.6) | 0.9 (0.55) | 41 | 44 (15) | 31 (76) | 0.43 (0.23) |
| Diseases | No. of Trials | Subgroup | Overall Effect | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
| SMD | 95% CI | p Value | p of I2 | I2 | |||
| MCI | 6 | Overall | 0.45 | 0.18~0.73 | 0.001 | 0.014 | 65.0% |
| 3 | ELISA | 0.27 | −0.10~0.64 | 0.157 | 0.096 | 57.3% | |
| 3 | Non-ELISA | 0.63 | 0.22~1.05 | 0.003 | 0.037 | 69.6% | |
| AD | 15 | Overall | 0.47 | 0.27~0.67 | 0.000 | 0.000 | 75.5% |
| 8 | ELISA | 0.39 | 0.06~0.72 | 0.020 | 0.000 | 81.0% | |
| 7 | Non-ELISA | 0.61 | 0.48~0.74 | 0.000 | 0.569 | 0.0% | |
| Other NDDs | 8 | ELISA | 0.94 | 0.70~1.19 | 0.000 | 0.052 | 49.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Zhou, Y.; Li, J. Association between Cerebrospinal Fluid Soluble TREM2, Alzheimer’s Disease and Other Neurodegenerative Diseases. J. Clin. Med. 2023, 12, 3589. https://doi.org/10.3390/jcm12103589
Zhou W, Zhou Y, Li J. Association between Cerebrospinal Fluid Soluble TREM2, Alzheimer’s Disease and Other Neurodegenerative Diseases. Journal of Clinical Medicine. 2023; 12(10):3589. https://doi.org/10.3390/jcm12103589
Chicago/Turabian StyleZhou, Wenchuan, Yutong Zhou, and Jing Li. 2023. "Association between Cerebrospinal Fluid Soluble TREM2, Alzheimer’s Disease and Other Neurodegenerative Diseases" Journal of Clinical Medicine 12, no. 10: 3589. https://doi.org/10.3390/jcm12103589
APA StyleZhou, W., Zhou, Y., & Li, J. (2023). Association between Cerebrospinal Fluid Soluble TREM2, Alzheimer’s Disease and Other Neurodegenerative Diseases. Journal of Clinical Medicine, 12(10), 3589. https://doi.org/10.3390/jcm12103589







