Improvement in Fertility and Pain after Endometriosis Resection and Adhesion Prevention with 4DryField® PH: Follow-up of a Randomized Controlled Clinical Trial
Abstract
1. Introduction
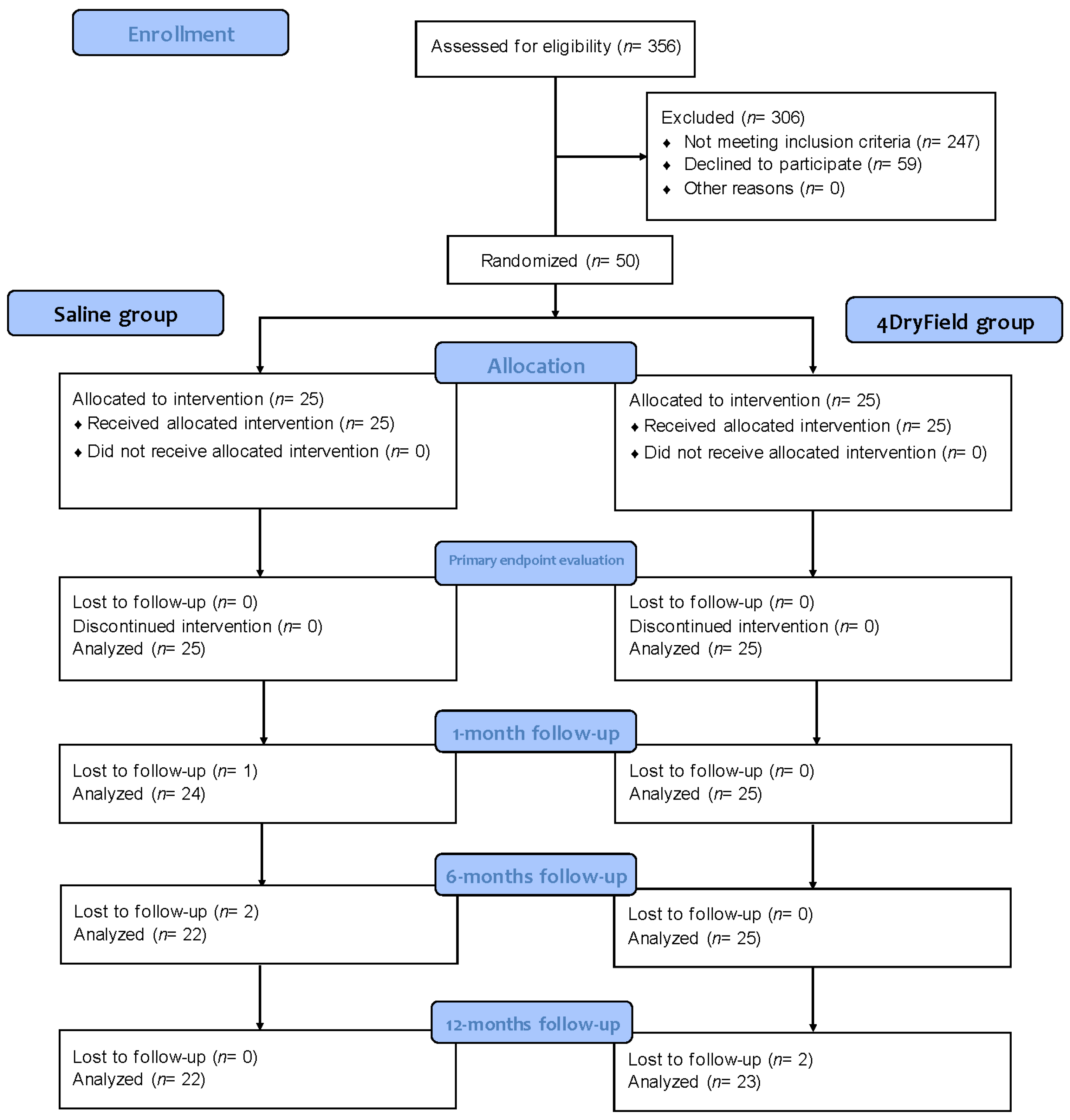
2. Materials and Methods
2.1. Study Design
2.2. Collection of Follow-up Data
2.3. Statistical Evaluation
3. Results
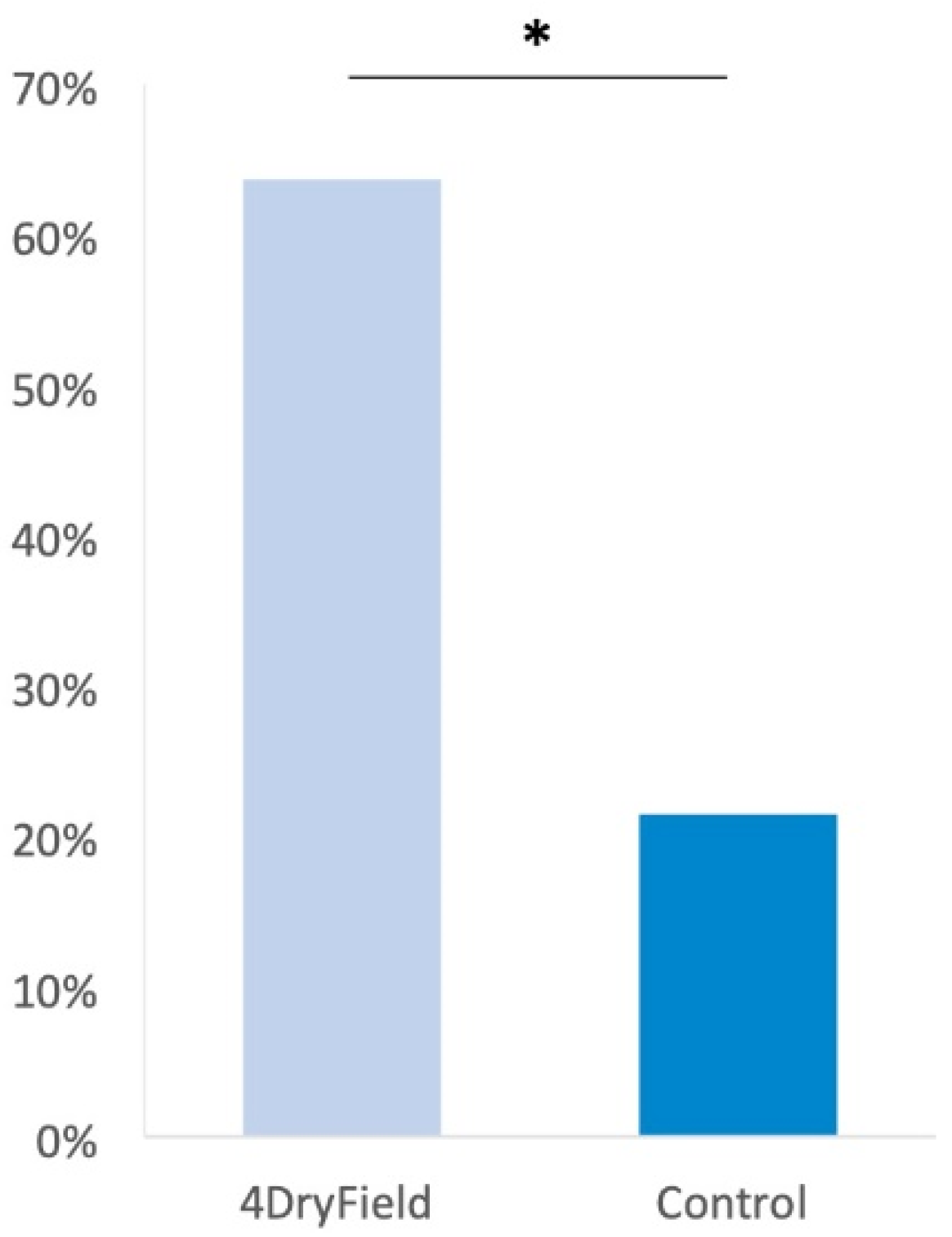
3.1. Reproductive Outcome
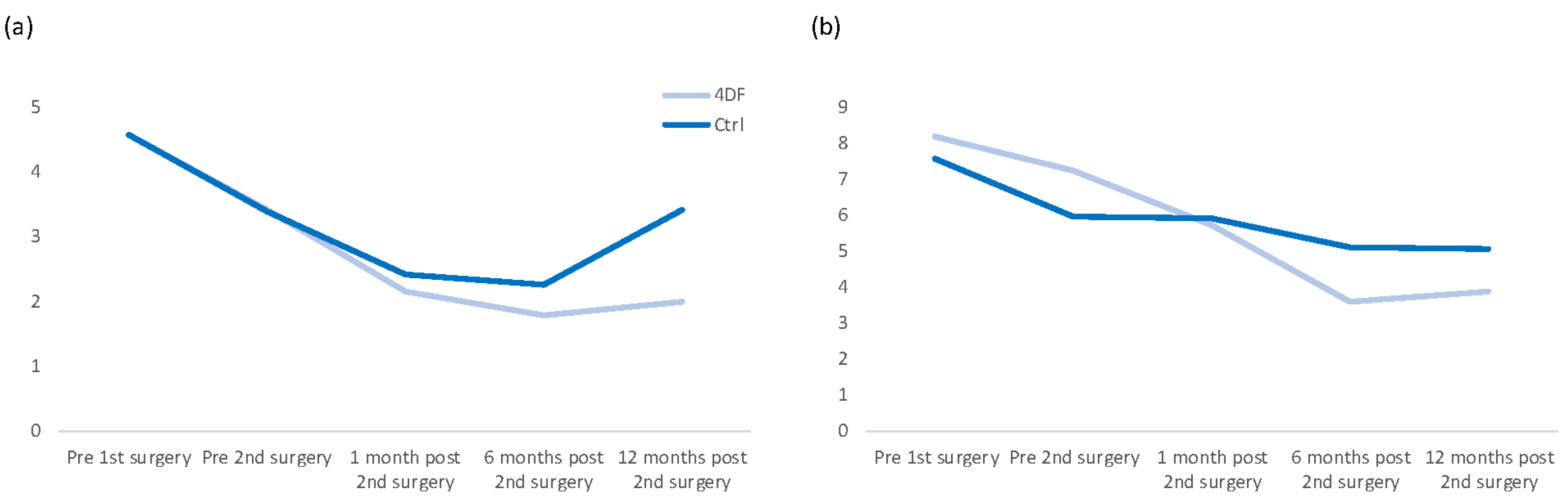
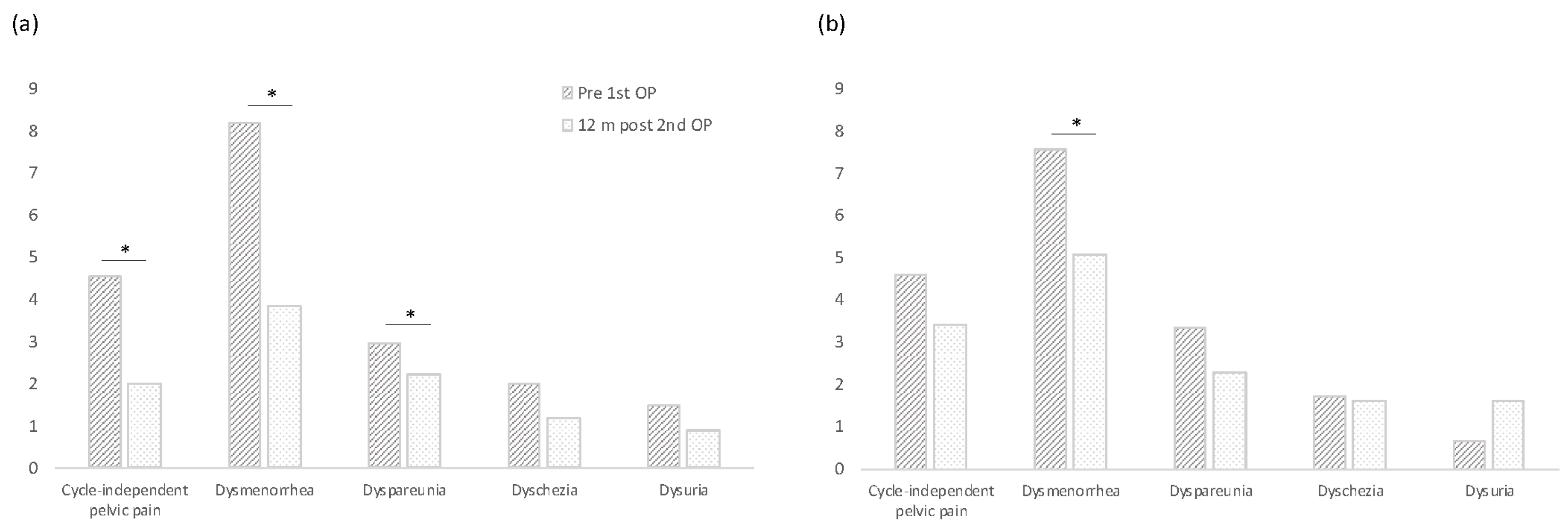
3.2. Pain Development
3.3. Complications
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Comparison with Other Studies
4.3.1. Relationship between Pain and Endometriosis
4.3.2. Relationship between Pain and Adhesions
4.3.3. Relationship between Fertility and Endometriosis/Adhesions
4.4. Clinical Implications
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of endometriosis on quality of life and mental health: Pelvic pain makes the difference. J. Psychosom. Obstet. Gynaecol. 2015, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Benaglia, L.; Busnelli, A.; Vercellini, P.; Fedele, L. Adhesion prevention in endometriosis: A neglected critical challenge. J. Minim. Invasive Gynecol. 2012, 19, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mais, V.; Ajossa, S.; Marongiu, D.; Peiretti, R.F.; Guerriero, S.; Melis, G.B. Reduction of adhesion reformation after laparoscopic endometriosis surgery: A randomized trial with an oxidized regenerated cellulose absorbable barrier. Obstet. Gynecol. 1995, 86, 512–515. [Google Scholar] [CrossRef]
- Howard, F.M. Endometriosis and mechanisms of pelvic pain. J. Minim. Invasive Gynecol. 2009, 16, 540–550. [Google Scholar] [CrossRef]
- Gibson, D.A.; Collins, F.; De Leo, B.; Horne, A.W.; Saunders, P.T.K. Pelvic pain correlates with peritoneal macrophage abundance not endometriosis. Reprod. Fertil. 2021, 2, 47–57. [Google Scholar] [CrossRef]
- Chacko, S.A.; Song, Y.; Nathan, L.; Tinker, L.; de Boer, I.H.; Tylavsky, F.; Wallace, R.; Liu, S. Relations of dietary magnesium intake to biomarkers of inflammation and endothelial dysfunction in an ethnically diverse cohort of postmenopausal women. Diabetes Care 2010, 33, 304–310. [Google Scholar] [CrossRef]
- Garalejić, E.; Bojović-Jović, D.; Damjanović, A.; Arsić, B.; Pantić, I.; Turjacanin-Pantelić, D.; Perović, M. Hamilton anxiety scale (HAMA) in infertile women with endometriosis and its correlation with magnesium levels in peritoneal fluid. Psychiatr. Danub. 2010, 22, 64–67. [Google Scholar]
- Johnson, N.P.; Hummelshoj, L. Consensus on current management of endometriosis. Hum. Reprod. 2013, 28, 1552–1568. [Google Scholar] [CrossRef]
- Abbott, J.; Hawe, J.; Hunter, D.; Holmes, M.; Finn, P.; Garry, R. Laparoscopic excision of endometriosis: A randomized, placebo-controlled trial. Fertil. Steril. 2004, 82, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.A.; Hawe, J.; Clayton, R.D.; Garry, R. The effects and effectiveness of laparoscopic excision of endometriosis: A prospective study with 2–5 year follow-up. Hum. Reprod. 2003, 18, 1922–1927. [Google Scholar] [CrossRef]
- Sutton, C.J.G.; Ewen, S.P.; Whitelaw, N.; Haines, P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil. Steril. 1994, 62, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Lotfallah, H.; Jones, K.; Lovell, D. A randomized trial of excision versus ablation for mild endometriosis. Fertil. Steril. 2005, 83, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Healey, M.; Ang, W.C.; Cheng, C. Surgical treatment of endometriosis: A prospective randomized double-blinded trial comparing excision and ablation. Fertil. Steril. 2010, 94, 2536–2540. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, H.; Gabella, G.; Davis, C.; Mutsaers, S.E.; Boulos, P.; Laurent, G.J.; Herrick, S.E. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann. Surg. 2001, 234, 256–261. [Google Scholar] [CrossRef]
- Peters, A.A.; Trimbos-Kemper, G.C.M.; Admiraal, C.; Trimbos, J.B.M.Z.; Hermans, J. A randomized clinical trial on the benefit of ahesiolysis in patients with intraperitoneal adhesions and chronic pelvic pain. Br. J. Obstet. Gynaecol. 1992, 99, 59–62. [Google Scholar] [CrossRef]
- Diamond, M.P.; Daniell, J.F.; Feste, J.; Surrey, M.W.; McLaughlin, D.S.; Friedman, S.; Vaughn, W.K.; Martin, D.C. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil. Steril. 1987, 47, 864–866. [Google Scholar] [CrossRef]
- Ten Broek, R.P.; Kok-Krant, N.; Bakkum, E.A.; Bleichrodt, R.P.; van Goor, H. Different surgical techniques to reduce post-operative adhesion formation: A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 12–25. [Google Scholar] [CrossRef]
- Hamilton, C.J.; Evers, J.L.; Hoogland, H.J. Ovulatory disorders and inflammatory adnexal damage: A neglected cause of the failure of fertility microsurgery. Br. J. Obstet. Gynaecol. 1986, 93, 282–284. [Google Scholar] [CrossRef]
- Franklin, R.R. Reduction of ovarian adhesions by the use of Interceed. Ovarian Adhesion Study Group. Obstet. Gynecol. 1995, 86, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Tulandi, T.; Collins, J.A.; Burrows, E.; Jarrell, J.F.; McInnes, R.A.; Wrixon, W.; Simpson, C.W. Treatment-dependent and treatment-independent pregnancy among women with periadnexal adhesions. Am. J. Obstet. Gynecol. 1990, 162, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Zaschke, S.; Heger, P.; Harnoss, J.C.; Hüttner, F.J.; Mihaljevic, A.L.; Knebel, P.; Diener, M.K. Evidence-based recommendations for blinding in surgical trials. Langenbeck Arch. Surg. 2019, 404, 273–284. [Google Scholar] [CrossRef]
- Schulz, K.F.; Grimes, D.A. Blinding in randomised trials: Hiding who got what. Lancet 2002, 359, 696–700. [Google Scholar] [CrossRef] [PubMed]
- The American Fertility Society. The American Fertility Society classifications of adnexal adhesions, distal tubal occlusion, tubal occlusion secondary to tubal ligation, tubal pregnancies, mullerian anomalies and intrauterine adhesions. Fertil. Steril. 1988, 49, 944–955. [Google Scholar] [CrossRef]
- Krämer, B.; Andress, J.; Neis, F.; Hoffmann, S.; Brucker, S.; Kommoss, S.; Holler, A. Adhesion prevention after endometriosis surgery—Results of a randomized, controlled clinical trial with second-look laparoscopy. Langenbecks Arch. Surg. 2021, 406, 2133–2143. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Korell, M.; Ziegler, N.; De Wilde, R.L. Use of Modified Polysaccharide 4DryField® PH for Adhesion Prevention and Hemostasis in Gynecological Surgery: A Two-Center Observational Study by Second-Look Laparoscopy. Biomed Res. Int. 2016, 2016, 3029264. [Google Scholar] [CrossRef]
- diZerega, G.S.; Coad, J.; Donnez, J. Clinical evaluation of endometriosis and differential response to surgical therapy with and without application of Oxiplex/AP* adhesion barrier gel. Fertil. Steril. 2007, 87, 485–489. [Google Scholar] [CrossRef]
- Kucukbas, M.; Kurek Eken, M.; Ilhan, G.; Senol, T.; Herkiloglu, D.; Kapudere, B. Which factors are associated with the recurrence of endometrioma after cystectomy? J. Obstet. Gynaecol. 2018, 38, 372–376. [Google Scholar] [CrossRef]
- Parker, M.C.; Wilson, M.S.; van Goor, H.; Moran, B.J.; Jeekel, J.; Duron, J.J.; Menzies, D.; Wexner, S.D.; Ellis, H. Adhesions and colorectal surgery—Call for action. Colorectal Dis. 2007, 9 (Suppl. 2), 66–72. [Google Scholar] [CrossRef] [PubMed]
- Attard, J.A.; MacLean, A.R. Adhesive small bowel obstruction: Epidemiology, biology and prevention. Can. J. Surg. 2007, 50, 291–300. [Google Scholar] [PubMed]
- Ellis, H.; Moran, B.J.; Thompson, J.N.; Parker, M.C.; Wilson, M.S.; Menzies, D.; McGuire, A.; Lower, A.M.; Hawthorn, R.J.S.; O’Brian, F.; et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: A retrospective cohort study. Lancet 1999, 353, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Vignali, M.; Bianchi, S.; Candiani, M.; Spadaccini, G.; Oggioni, G.; Busacca, M. Surgical treatment of deep endometriosis and risk of recurrence. J. Minim. Invasive Gynecol. 2005, 12, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.D.; Sinaii, N.; Segars, J.H.; Godoy, H.; Winkel, C.; Stratton, P. Adhesion formation after laparoscopic excision of endometriosis and lysis of adhesions. Fertil. Steril. 2005, 84, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Mais, V.; Cipriani, S.; per lo Studio dell’Endometriosi, G.I. Adhesions and pain in women with first diagnosis of endometriosis: Results from a cross-sectional study. J. Minim. Invasive Gynecol. 2006, 13, 49–54. [Google Scholar] [CrossRef]
- Hao, M.; Zhao, W.H.; Wang, Y.H. Correlation between pelvic adhesions and pain symptoms of endometriosis. Zhonghua Fu Chan Ke Za Zhi 2009, 44, 333–336. [Google Scholar]
- Abd El-Kader, A.I.; Gonied, A.S.; Lotfy Mohamed, M.; Lotfy Mohamed, S. Impact of Endometriosis-Related Adhesions on Quality of Life among Infertile Women. Int. J. Fertil. Steril. 2019, 13, 72–76. [Google Scholar] [CrossRef]
- Sekiba, K. Use of Interceed(TC7) absorbable adhesion barrier to reduce postoperative adhesion reformation in infertility and endometriosis surgery. The Obstetrics and Gynecology Adhesion Prevention Committee. Obstet. Gynecol. 1992, 79, 518–522. [Google Scholar]
- Wallwiener, D.; Meyer, A.; Bastert, G. Adhesion formation of the parietal and visceral peritoneum: An explanation for the controversy on the use of autologous and alloplastic barriers? Fertil. Steril. 1998, 69, 132–137. [Google Scholar] [CrossRef]
- Ahmad, G.; Kim, K.; Thompson, M.; Agarwal, P.; O’Flynn, H.; Hindocha, A.; Watson, A. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst. Rev. 2020, 3, Cd000475. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G.; Vercellini, P.; Biffignandi, F.; Costantini, W.; Cortesi, I.; Imparato, E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertil. Steril. 1996, 66, 706–711. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Ma, L.; McKinnon, B.; Mueller, M.D. Recurrence Patterns after Surgery in Patients with Different Endometriosis Subtypes: A Long-Term Hospital-Based Cohort Study. J. Clin. Med. 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- van den Beukel, B.A.W.; de Ree, R.; van Goor, H.; van der Wal, S.E.I.; Ten Broek, R.P.G. Analgesia in patients with adhesions-related chronic abdominal and pelvic pain after surgery: A systematic review. Acta Chir. Belg. 2021, 122, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kresch, A.J.; Seifer, D.B.; Sachs, L.B.; Barrese, I. Laparoscopy in 100 women with chronic pelvic pain. Obstet. Gynecol. 1984, 64, 672–674. [Google Scholar] [PubMed]
- Steege, J.F.; Stout, A.L. Resolution of chronic pelvic pain after laparoscopic lysis of adhesions. Am. J. Obstet. Gynecol. 1991, 165, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.C.; Reading, I.; Bailey, S.; Sadek, K.; Ledger, W.; Li, T.C. Should women with chronic pelvic pain have adhesiolysis? BMC Womens Health 2014, 14, 36. [Google Scholar] [CrossRef]
- Chan, C.L.; Wood, C. Pelvic adhesiolysis—The assessment of symptom relief by 100 patients. Aust. N. Z. J. Obstet. Gynaecol. 1985, 25, 295–298. [Google Scholar] [CrossRef]
- Daniell, J.F. Laparoscopic Enterolysis for Chronic Abdominal Pain. J. Gynecol. Surg. 1989, 5, 61–66. [Google Scholar] [CrossRef]
- Sutton, C.; MacDonald, R. Laser laparoscopic adhesiolysis. J. Gynecol. Surg. 1990, 6, 155–159. [Google Scholar] [CrossRef]
- Saravelos, H.G.; Li, T.C.; Cooke, I.D. An analysis of the outcome of microsurgical and laparoscopic adhesiolysis for chronic pelvic pain. Hum. Reprod. 1995, 10, 2895–2901. [Google Scholar] [CrossRef]
- Heim, L.J. Evaluation and differential diagnosis of dyspareunia. Am. Fam. Physician 2001, 63, 1535–1544. [Google Scholar] [PubMed]
- Mama, S.T. Advances in the management of endometriosis in the adolescent. Curr. Opin. Obstet. Gynecol. 2018, 30, 326–330. [Google Scholar] [CrossRef]
- Muzii, L.; Marana, R.; Pedullà, S.; Catalano, G.F.; Mancuso, S. Correlation between endometriosis-associated dysmenorrhea and the presence of typical or atypical lesions. Fertil. Steril. 1997, 68, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Koninckx, P.R.; Piazze, J.; Natili, M.; Colagrande, S.; Cosmi, E.V. Correlation between endometriosis and pelvic pain. J. Am. Assoc. Gynecol. Laparosc. 1999, 6, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier, A.; Chapron, C.; Dubuisson, J.-B.; Vieira, M.; Dousset, B.; Bréart, G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil. Steril. 2002, 78, 719–726. [Google Scholar] [CrossRef]
- Chapron, C.; Fauconnier, A.; Dubuisson, J.B.; Barakat, H.; Vieira, M.; Bréart, G. Deep infiltrating endometriosis: Relation between severity of dysmenorrhoea and extent of disease. Hum. Reprod. 2003, 18, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.; Turan, C.; Mungan, T.; Aydogdu, T.; Sahin, Y.; Gokmen, O. Laparoscopy for adhesion prevention following myomectomy. Int. J. Gynaecol. Obstet. 1996, 53, 145–149. [Google Scholar] [CrossRef]
- van der Wal, J.B.C.; Halm, J.A.; Jeekel, J. Chronic abdominal pain: The role of adhesions and benefit of laparoscopic adhesiolysis. Gynecol. Surg. 2006, 3, 168–174. [Google Scholar] [CrossRef]
- Haydardedeoglu, B.; Zeyneloglu, H.B. The impact of endometriosis on fertility. Womens Health 2015, 11, 619–623. [Google Scholar] [CrossRef]
- Evans, M.B.; Decherney, A.H. Fertility and Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef] [PubMed]
- Burghaus, S.; Schäfer, S.D.; Beckmann, M.W.; Brandes, I.; Brünahl, C.; Chvatal, R.; Drahoňovský, J.; Dudek, W.; Ebert, A.D.; Fahlbusch, C.; et al. Diagnosis and Treatment of Endometriosis. Guideline of the DGGG, SGGG and OEGGG (S2k Level, AWMF Registry Number 015/045, August 2020). Geburtshilfe Frauenheilkd. 2021, 81, 422–446. [Google Scholar] [CrossRef]
- Ziegler, N.; De Wilde, R.L. Reduction of adhesion formation after gynaecological adhesiolysis surgery with 4DryField PH—A retrospective, controlled study with second look laparoscopies. J. Obstet. Gynaecol. 2022, 42, 658–664. [Google Scholar] [CrossRef]
- Krämer, B.; Neis, F.; Brucker, S.Y.; Kommoss, S.; Andress, J.; Hoffmann, S. Peritoneal Adhesions and their Prevention—Current Trends. Surg. Technol. Int. 2021, 38, 221–233. [Google Scholar] [CrossRef] [PubMed]
| 4DryField Group | Control | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Cycle-independent pelvic pain | Prior to 1st surgery | 4.5 | 3.3 | 4.6 | 3.5 |
| Prior to 2nd surgery | 3.4 | 2.7 | 3.4 | 3.3 | |
| After 1 month | 2.1 | 2.4 | 2.4 | 2.5 | |
| After 6 months | 1.8 | 2.1 | 2.3 | 2.8 | |
| After 12 months | 2.0 | 2.3 | 3.4 | 3.2 | |
| Dysmenorrhea | Prior to 1st surgery | 8.2 | 2.2 | 7.6 | 2.5 |
| Prior to 2nd surgery | 7.2 | 3.1 | 6.0 | 3.6 | |
| After 1 month | 5.7 | 3.1 | 5.9 | 3.7 | |
| After 6 months | 3.6 | 2.9 | 5.1 | 3.0 | |
| After 12 months | 3.9 | 3.6 | 5.1 | 3.5 | |
| Dyspareunia | Prior to 1st surgery | 3.0 | 3.0 | 3.3 | 3.4 |
| Prior to 2nd surgery | 2.3 | 2.6 | 3.0 | 3.2 | |
| After 1 month | 2.0 | 2.7 | 0.6 | 1.4 | |
| After 6 months | 1.7 | 2.8 | 1.7 | 2.4 | |
| After 12 months | 2.2 | 2.6 | 2.3 | 2.5 | |
| Dyschezia | Prior to 1st surgery | 2.0 | 3.0 | 1.7 | 3.0 |
| Prior to 2nd surgery | 2.2 | 3.2 | 2.0 | 3.3 | |
| After 1 month | 1.2 | 1.8 | 0.8 | 2.2 | |
| After 6 months | 0.8 | 2.0 | 1.2 | 2.5 | |
| After 12 months | 1.2 | 2.0 | 1.6 | 2.8 | |
| Dysuria | Prior to 1st surgery | 1.5 | 2.4 | 0.6 | 1.6 |
| Prior to 2nd surgery | 1.0 | 2.4 | 0.9 | 2.2 | |
| After 1 month | 0.5 | 1.0 | 1.0 | 2.3 | |
| After 6 months | 0.2 | 0.6 | 0.5 | 1.7 | |
| After 12 months | 0.9 | 2.1 | 1.6 | 3.1 | |
| (a) CIPP | 4DryField | Control | p |
| Prior to 1st surgery | 4.5 | 4.6 | 0.9835 |
| After 12 months | 2.0 | 3.4 | 0.0932 |
| p | 0.0024 * | 0.1413 | |
| (b) Dysmenorrhea | 4DryField | Control | p |
| Prior to 1st surgery | 8.2 | 7.6 | 0.32 |
| After 12 months | 3.9 | 5.1 | 0.2525 |
| p | <0.0001 * | 0.0009 * | |
| (c) Dyspareunia | 4DryField | Control | p |
| Prior to 1st surgery | 3.0 | 3.3 | 0.7693 |
| After 12 months | 2.2 | 2.3 | 0.977 |
| p | 0.0239 * | 0.1761 | |
| (d) Dyschezia | 4DryField | Control | p |
| Prior to 1st surgery | 2.0 | 1.7 | 0.7347 |
| After 12 months | 1.2 | 1.6 | 0.5715 |
| p | 0.1816 | 0.4062 | |
| (e) Dysuria | 4DryField | Control | p |
| Prior to 1st surgery | 1.5 | 0.6 | 0.0873 |
| After 12 months | 0.9 | 1.6 | 0.7586 |
| p | 0.2422 | 0.2969 |
| Our Control Group | Healey et al. [15] | |||
|---|---|---|---|---|
| Improvement | SD | Improvement | SD | |
| CIPP | 1.2 | 3.7 | 2.6 | 3.5 |
| Dysmenorrhea | 2.6 | 2.5 | 2.4 | 3.9 |
| Dyschezia | 0.9 | 2.7 | 1.8 | 3.5 |
| Dysuria | 0.4 | 2.7 | 0.4 | 2.3 |
| Dyspareunia | −0.9 | 2.9 | 3.1 | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krämer, B.; Andress, J.; Neis, F.; Hoffmann, S.; Brucker, S.; Kommoss, S.; Höller, A. Improvement in Fertility and Pain after Endometriosis Resection and Adhesion Prevention with 4DryField® PH: Follow-up of a Randomized Controlled Clinical Trial. J. Clin. Med. 2023, 12, 3597. https://doi.org/10.3390/jcm12103597
Krämer B, Andress J, Neis F, Hoffmann S, Brucker S, Kommoss S, Höller A. Improvement in Fertility and Pain after Endometriosis Resection and Adhesion Prevention with 4DryField® PH: Follow-up of a Randomized Controlled Clinical Trial. Journal of Clinical Medicine. 2023; 12(10):3597. https://doi.org/10.3390/jcm12103597
Chicago/Turabian StyleKrämer, Bernhard, Jürgen Andress, Felix Neis, Sascha Hoffmann, Sara Brucker, Stefan Kommoss, and Alice Höller. 2023. "Improvement in Fertility and Pain after Endometriosis Resection and Adhesion Prevention with 4DryField® PH: Follow-up of a Randomized Controlled Clinical Trial" Journal of Clinical Medicine 12, no. 10: 3597. https://doi.org/10.3390/jcm12103597
APA StyleKrämer, B., Andress, J., Neis, F., Hoffmann, S., Brucker, S., Kommoss, S., & Höller, A. (2023). Improvement in Fertility and Pain after Endometriosis Resection and Adhesion Prevention with 4DryField® PH: Follow-up of a Randomized Controlled Clinical Trial. Journal of Clinical Medicine, 12(10), 3597. https://doi.org/10.3390/jcm12103597





