Effects of Deep Neuromuscular Block during Robot-Assisted Transaxillary Thyroidectomy: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
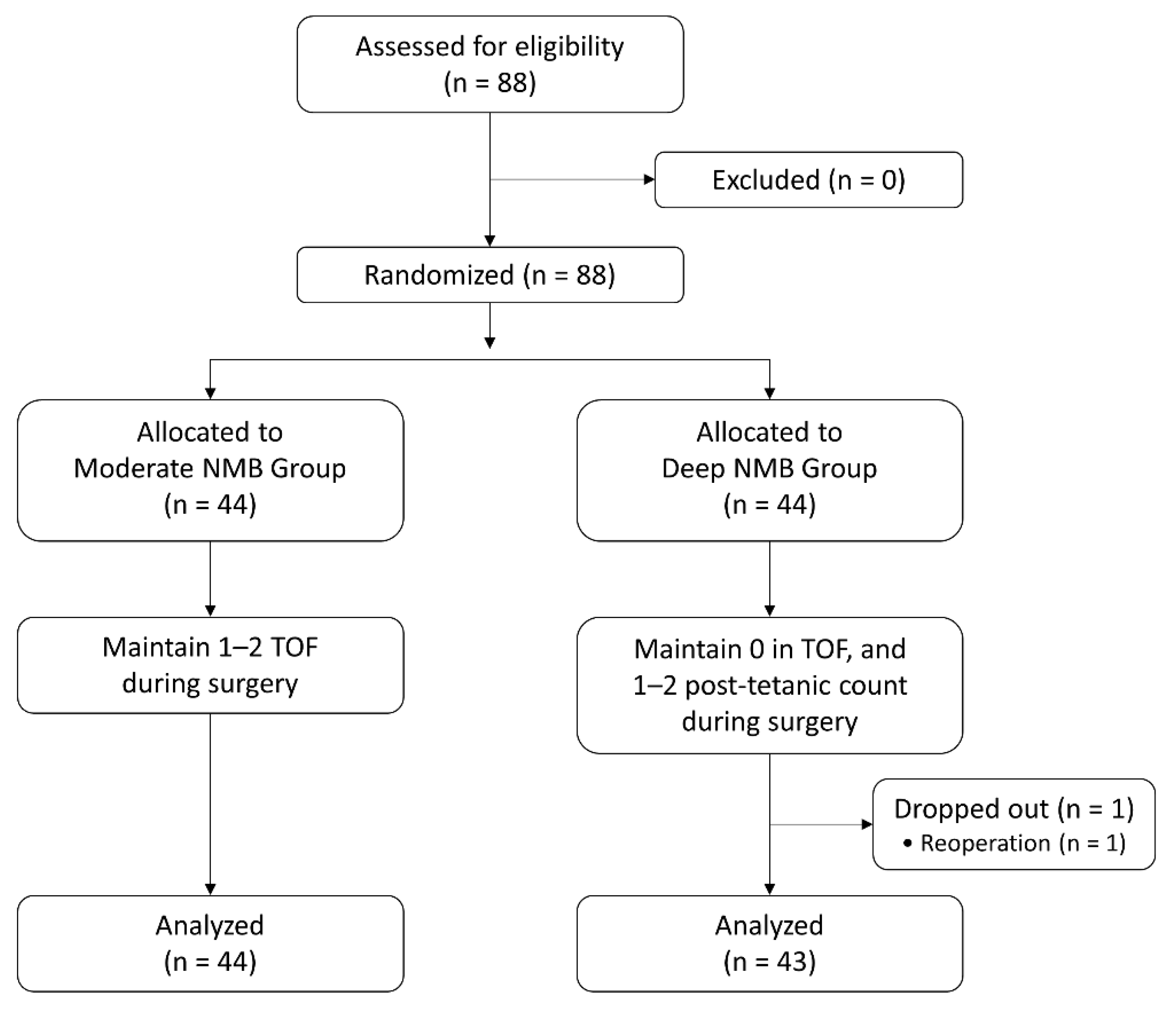
2.1. Patient Enrollment and Randomization
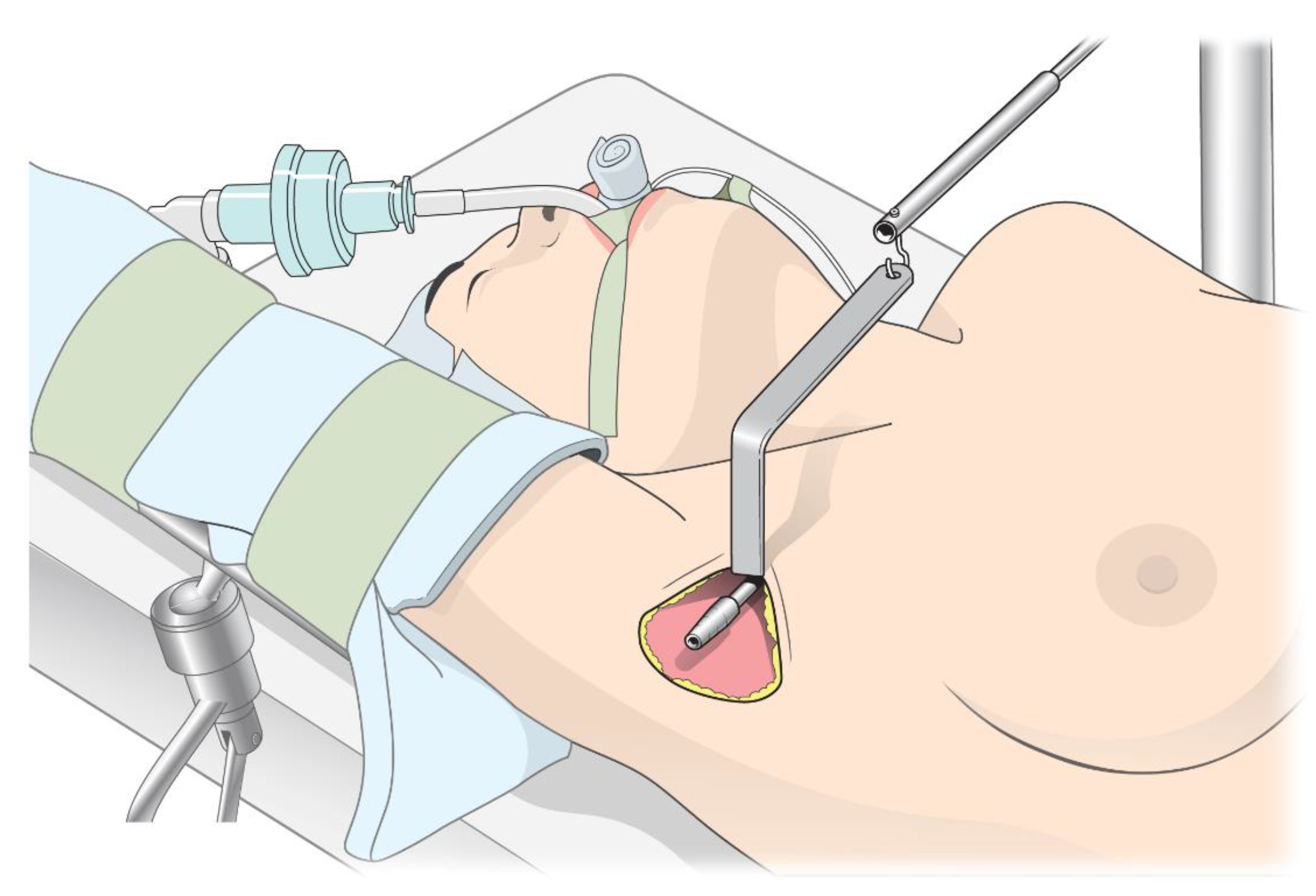
2.2. Perioperative Management and Study Protocol
2.3. Study Endpoints
2.4. Sample Size and Statistical Analysis
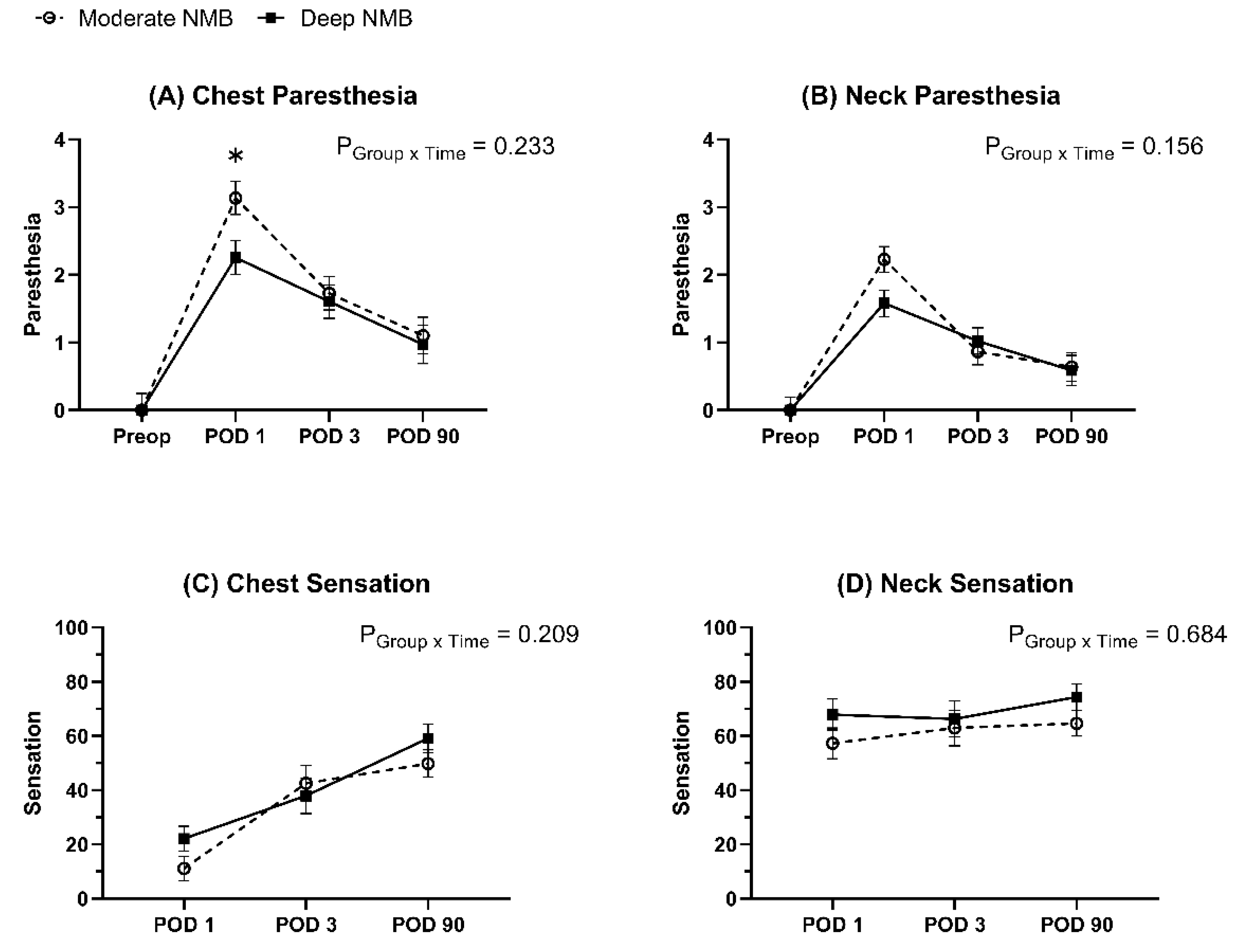
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, E.H.E.; Kim, H.Y.; Koh, Y.W.; Chung, W.Y. Overview of robotic thyroidectomy. Gland Surg. 2017, 6, 218. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.R.; Yao, L.; Tufano, R.P.; Kandil, E.H. Safety of robotic thyroidectomy approaches: Meta-analysis and systematic review. Head Neck. 2014, 36, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.H.; Peress, L.; Pynnonen, M.A. Systematic review and meta-analysis of robotic vs conventional thyroidectomy approaches for thyroid disease. Otolaryngol. Head Neck Surg. 2014, 150, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Nah, K.Y.; Kim, R.M.; Ahn, Y.H.; Soh, E.-Y.; Chung, W.Y. Differences in postoperative outcomes, function, and cosmesis: Open versus robotic thyroidectomy. Surg. Endosc. 2010, 24, 3186–3194. [Google Scholar] [CrossRef]
- Choi, K.W.; Nam, K.-H.; Lee, J.-R.; Chung, W.Y.; Kang, S.-W.; Joe, Y.E.; Lee, J.H. The effects of intravenous lidocaine infusions on the quality of recovery and chronic pain after robotic thyroidectomy: A randomized, double-blinded, controlled study. World J. Surg. 2017, 41, 1305–1312. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Cho, S.H.; Lee, S.H.; Kim, D.S.; Kim, T.W. Early surgical outcomes of robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach for papillary thyroid carcinoma: 2 years’ experience. Head Neck. 2012, 34, 617–625. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, I.S.; Bae, E.H.; Chung, W.Y. Comparative analysis of oncological outcomes and quality of life after robotic versus conventional open thyroidectomy with modified radical neck dissection in patients with papillary thyroid carcinoma and lateral neck node metastases. J. Clin. Endocrinol. Metab. 2013, 98, 2701–2708. [Google Scholar] [CrossRef]
- Tae, K.; Ji, Y.B.; Jeong, J.H.; Lee, S.H.; Jeong, M.; Park, C.W. Robotic thyroidectomy by a gasless unilateral axillo-breast or axillary approach: Our early experiences. Surg. Endosc. 2011, 25, 221–228. [Google Scholar] [CrossRef]
- Kang, S.-W.; Jeong, J.J.; Nam, K.-H.; Chang, H.S.; Chung, W.Y.; Park, C.S. Robot-assisted endoscopic thyroidectomy for thyroid malignancies using a gasless transaxillary approach. J. Am. Coll. Surg. 2009, 209, e1–e7. [Google Scholar] [CrossRef]
- Song, C.M.; Ji, Y.B.; Bang, H.S.; Park, C.W.; Kim, H.; Tae, K. Long-term sensory disturbance and discomfort after robotic thyroidectomy. World J. Surg. 2014, 38, 1743–1748. [Google Scholar] [CrossRef]
- Bruintjes, M.; Van Helden, E.; Braat, A.; Dahan, A.; Scheffer, G.; Van Laarhoven, C.; Warlé, M. Deep neuromuscular block to optimize surgical space conditions during laparoscopic surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2017, 118, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Son, Y.G.; Yoo, S.; Lim, T.; Kim, W.H.; Kim, J.-T. Deep vs. Moderate neuromuscular blockade during laparoscopic surgery: A systematic review and meta-analysis. Eur. J. Anaesthesiol. 2018, 35, 867–875. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, K.Y.; Lee, K.Y.; Min, B.S.; Yoo, Y.C. Maintaining optimal surgical conditions with low insufflation pressures is possible with deep neuromuscular blockade during laparoscopic colorectal surgery: A prospective, randomized, double-blind, parallel-group clinical trial. Medicine 2016, 95, e2920. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; Boon, M.; Bevers, R.; Aarts, L.; Dahan, A. Evaluation of surgical conditions during laparoscopic surgery in patients with moderate vs deep neuromuscular block. Br. J. Anaesth. 2014, 112, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Buder, T.; Claudius, C.; Skovgaard, L.; Eriksson, L.; Mirakhur, R.; Viby-Mogensen, J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents ii: The stockholm revision. Acta Anaesthesiol. Scand. 2007, 51, 789–808. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tiner, B.; Clark, G.; Bays, R.; Keeling, S.; Rugh, J. A comparison of questionnaire versus monofilament assessment of neurosensory deficit. J. Oral. Maxillofac. Surg. 1996, 54, 454–459. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, E.M.; Nam, K.H.; Chang, D.J.; Nam, S.H.; Kim, K.J. Postoperative intravenous patient-controlled analgesia in thyroid surgery: Comparison of fentanyl and ondansetron regimens with and without the nonsteriodal anti-inflammatory drug ketorolac. Thyroid 2008, 18, 1285–1290. [Google Scholar] [CrossRef]
- Ryu, H.R.; Lee, J.; Park, J.-H.; Kang, S.-W.; Jeong, J.J.; Hong, J.-Y.; Chung, W.Y. A comparison of postoperative pain after conventional open thyroidectomy and transaxillary single-incision robotic thyroidectomy: A prospective study. Ann. Surg. Oncol. 2013, 20, 2279–2284. [Google Scholar] [CrossRef]
- Besson, J. The neurobiology of pain. Lancet 1999, 353, 1610–1615. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Kinins and their receptors in hyperalgesia. Can. J. Physiol. Pharmacol. 1997, 75, 704–712. [Google Scholar] [CrossRef]
- Cohen, S.P.; Mao, J. Neuropathic pain: Mechanisms and their clinical implications. BMJ 2014, 348, f7656. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Kwon, W.-K.; Park, S.; Ji, S.G.; Kim, J.H.; Park, Y.-K.; Lee, S.Y.; Lim, B.G. Comparison of operating conditions, postoperative pain and recovery, and overall satisfaction of surgeons with deep vs. No neuromuscular blockade for spinal surgery under general anesthesia: A prospective randomized controlled trial. J. Clin. Med. 2019, 8, 498. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Kim, N.Y.; Shin, S.; Choi, Y.D.; Hong, J.H.; Kim, C.Y.; Park, H.; Bai, S.J. The intraocular pressure under deep versus moderate neuromuscular blockade during low-pressure robot assisted laparoscopic radical prostatectomy in a randomized trial. PLoS ONE 2015, 10, e0135412. [Google Scholar] [CrossRef] [PubMed]
- Koo, C.H.; Park, I.; Ahn, S.; Lee, S.; Ryu, J.H. Effect of neuromuscular blockade on intraoperative respiratory mechanics and surgical space conditions during robot-assisted radical prostatectomy: A prospective randomized controlled trial. J. Clin. Med. 2021, 10, 5090. [Google Scholar] [CrossRef]
- Rawal, N. Current issues in postoperative pain management. Eur. J. Anaesthesiol. 2016, 33, 160–171. [Google Scholar] [CrossRef]
- Rahe-Meyer, N.; Fennema, H.; Schulman, S.; Klimscha, W.; Przemeck, M.; Blobner, M.; Wulf, H.; Speek, M.; McCrary Sisk, C.; Williams-Herman, D. Effect of reversal of neuromuscular blockade with sugammadex versus usual care on bleeding risk in a randomized study of surgical patients. Anesthesiology 2014, 121, 969–977. [Google Scholar] [CrossRef]
- Kang, W.-S.; Lim, H.; Kim, B.-S.; Lee, Y.; Hahm, K.-D.; Kim, S.-H. Assessment of the effects of sugammadex on coagulation profiles using thromboelastographic parameters. Sci. Rep. 2020, 10, 11179. [Google Scholar] [CrossRef]
- De Kam, P.-J.; Grobara, P.; Prohn, M.; Höppener, F.; Kluft, C.; Burggraaf, J.; Langdon, R.B.; Peeters, P. Effects of sugammadex on activated partial thromboplastin time and prothrombin time in healthy subjects. Int. J. Clin. Pharmacol. Ther. 2014, 52, 227–236. [Google Scholar] [CrossRef]

| Moderate NMB (n = 44) | Deep NMB (n = 43) | p-Value | |
|---|---|---|---|
| Sex (female) | 41 (93.2) | 40 (93.0) | >0.999 |
| Age (years) | 36.3 ± 9.0 | 35.8 ± 9.1 | 0.795 |
| BMI (kg/m2) | 22.9 ± 2.9 | 22.6 ± 3.4 | 0.663 |
| ASA class | 0.151 | ||
| 1 | 15 (34.1) | 22 (51.2) | |
| 2 | 25 (56.8) | 20 (46.5) | |
| 3 | 4 (9.1) | 1 (2.3) | |
| Hypertension | 0 (0) | 1 (2.3) | 0.494 |
| DM | 0 (0) | 1 (2.3) | 0.494 |
| Old TB | 0 (0) | 1 (2.3) | 0.494 |
| HBV | 1 (2.3) | 1 (2.3) | >0.999 |
| Dyslipidemia | 1 (2.3) | 1 (2.3) | >0.999 |
| Asthma | 3 (6.8) | 1 (2.3) | 0.616 |
| HCD | 3 (6.8) | 2 (4.7) | >0.999 |
| Moderate NMB (n = 44) | Deep NMB (n = 43) | p-Value | |
|---|---|---|---|
| Operation type | 0.730 | ||
| HT | 2 (4.5) | 4 (9.3) | |
| HT with CCND | 36 (81.8) | 33 (76.7) | |
| BTT with CCND | 6 (13.6) | 6 (14.0) | |
| Surgeon | 0.800 | ||
| Dr. Nam | 26 (59.1) | 28 (65.1) | |
| Dr. Kang | 16 (36.4) | 14 (32.6) | |
| Dr. Lee | 2 (4.6) | 1 (2.3) | |
| Tumor size (cm) | 0.9 ± 0.8 | 1.4 ± 1.5 | 0.052 |
| Pathology | 0.069 | ||
| Benign | 4 (9.1) | 5 (11.6) | |
| PTC, conventional | 40 (90.9) | 32 (74.4) | |
| PTC, follicular variant | 0 (0) | 4 (9.3) | |
| PTC, hobnail variant | 0 (0) | 1 (2.3) | |
| FTC | 0 (0) | 1 (2.3) | |
| TNM stage 1 | |||
| T stage | 0.037 | ||
| T1 | 39 (97.5) | 33 (86.8) | |
| T2 | 0 (0) | 4 (10.5) | |
| T3a | 0 (0) | 1 (2.6) | |
| T3b | 1 (2.5) | 0 (0) | |
| N stage | 0.901 | ||
| N0 | 28 (70.0) | 25 (65.8) | |
| N1a | 11 (27.5) | 12 (31.6) | |
| N1b | 1 (2.5) | 1 (2.6) | |
| M stage | >0.999 | ||
| M0 | 40 (100) | 38 (100) |
| Moderate NMB (n = 44) | Deep NMB (n = 43) | p-Value | |
|---|---|---|---|
| Operation time (min) | 99 ± 16 | 102 ± 24 | 0.577 |
| Anesthesia time (min) | 130 ± 17 | 132 ± 23 | 0.651 |
| Fluid input (mL) | 550 ± 117 | 564 ± 175 | 0.664 |
| Propofol (mg) | 101 ± 16 | 104 ± 15 | 0.398 |
| Rocuronium (mg) | 57 ± 9 | 110 ± 28 | <0.001 |
| Remifentanil (μg) | 414 ± 132 | 416 ± 127 | 0.959 |
| Sugammadex (mg) | 121 ± 17 | 244 ± 39 | <0.001 |
| Blood Pressure (mmHg) | |||
| Baseline | 89 ± 13 | 90 ± 12 | 0.731 |
| Before incision | 72 ± 11 | 75 ± 10 | 0.331 |
| Docking robot | 75 ± 9 | 78 ± 9 | 0.201 |
| Undocking robot | 69 ± 9 | 70 ± 7 | 0.364 |
| Heart Rate (beats/min) | |||
| Baseline | 78 ± 13 | 74 ± 13 | 0.167 |
| Before incision | 86 ± 12 | 85 ± 12 | 0.661 |
| Docking robot | 79 ± 12 | 81 ± 10 | 0.342 |
| Undocking robot | 72 ± 11 | 77 ± 9 | 0.021 |
| Peak airway pressure (cm H2O) | |||
| Before incision | 13 ± 2 | 14 ± 2 | 0.211 |
| Docking robot | 14 ± 1 | 15 ± 2 | 0.416 |
| Undocking robot | 13 ± 1 | 13 ± 2 | 0.655 |
| TOF 0.9 time (s) | 99 ± 43 | 147 ± 78 | 0.001 |
| PACU time (min) | 39 ± 15 | 39 ± 12 | 0.852 |
| Hospital stay (days) | 4.1 ± 0.5 | 4.0 ± 0.4 | 0.353 |
| ICU admission | 0 (0) | 0 (0) | >0.999 |
| Respiratory failure | 0 (0) | 0 (0) | >0.999 |
| Desaturation | 0 (0) | 0 (0) | >0.999 |
| Postoperative bleeding | 0 (0) | 1 (2.3) | 0.494 |
| Rescue analgesic | |||
| PACU | 21 (47.7) | 19 (44.2) | 0.740 |
| POD 1 | 9 (20.5) | 7 (16.3) | 0.615 |
| POD 3 | 1 (2.3) | 0 (0) | >0.999 |
| PONV (0–3) | |||
| PACU | 0.6 ± 0.9 | 0.4 ± 0.8 | 0.398 |
| POD 1 | 0.0 ± 0.3 | 0.1 ± 0.4 | 0.510 |
| POD 3 | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.323 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, M.I.; Kang, S.-W.; Lee, J.S.; Kim, N.Y.; Lee, B.; Moon, G.; Yoo, Y.C.; Nam, K.-H. Effects of Deep Neuromuscular Block during Robot-Assisted Transaxillary Thyroidectomy: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3633. https://doi.org/10.3390/jcm12113633
Bae MI, Kang S-W, Lee JS, Kim NY, Lee B, Moon G, Yoo YC, Nam K-H. Effects of Deep Neuromuscular Block during Robot-Assisted Transaxillary Thyroidectomy: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(11):3633. https://doi.org/10.3390/jcm12113633
Chicago/Turabian StyleBae, Myung Il, Sang-Wook Kang, Jong Seok Lee, Na Young Kim, Bahn Lee, Gilseong Moon, Young Chul Yoo, and Kee-Hyun Nam. 2023. "Effects of Deep Neuromuscular Block during Robot-Assisted Transaxillary Thyroidectomy: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 11: 3633. https://doi.org/10.3390/jcm12113633
APA StyleBae, M. I., Kang, S.-W., Lee, J. S., Kim, N. Y., Lee, B., Moon, G., Yoo, Y. C., & Nam, K.-H. (2023). Effects of Deep Neuromuscular Block during Robot-Assisted Transaxillary Thyroidectomy: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(11), 3633. https://doi.org/10.3390/jcm12113633







