Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Study Population and SARS-CoV-2 Vaccines Administered
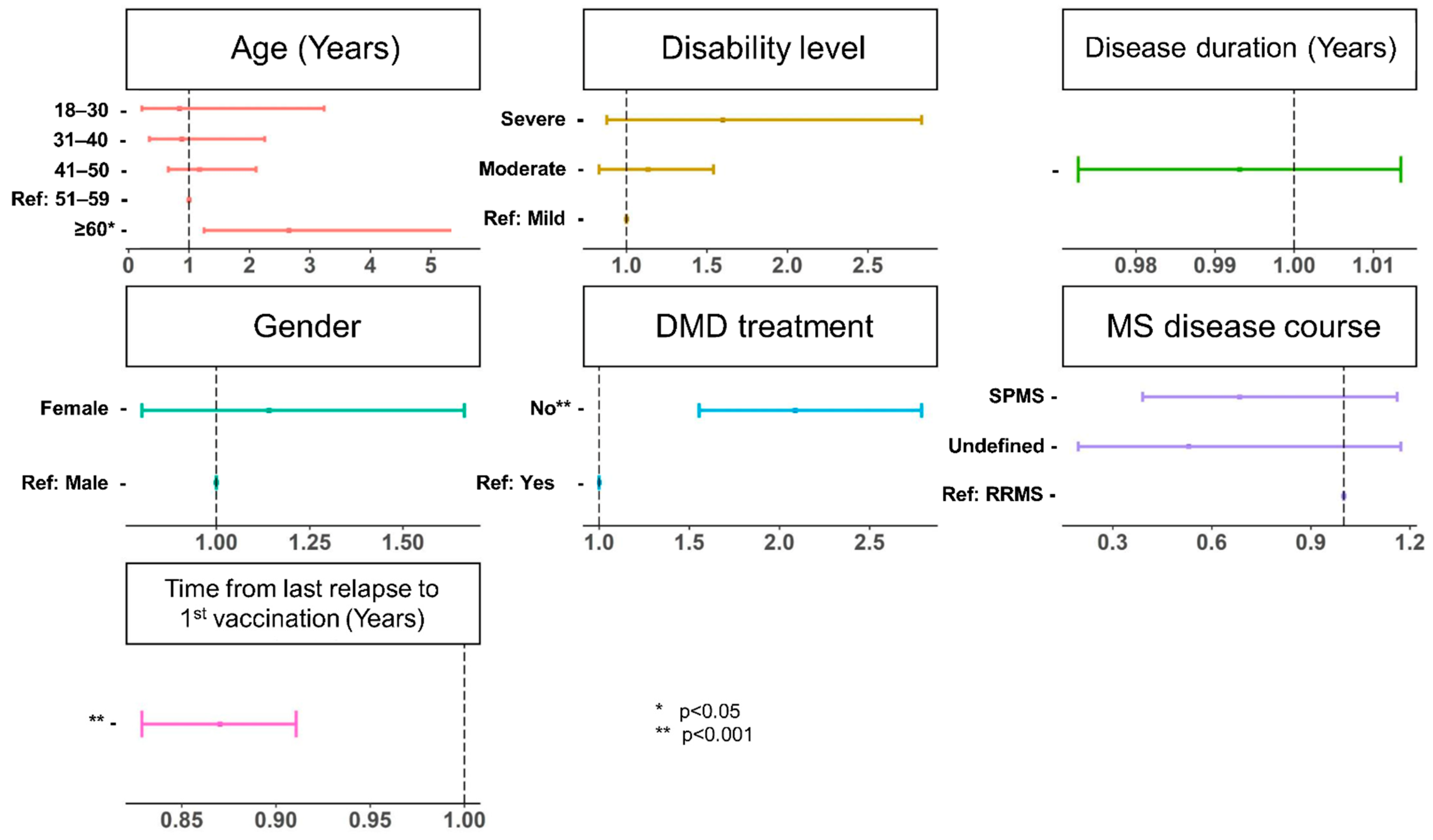
3.2. Relapses before and after SARS-CoV-2 Vaccination in the Study Population
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sormani, M.P.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Filippi, M.; Radaelli, M.; Immovilli, P.; Capobianco, M.; De Rossi, N.; Brichetto, G.; et al. COVID-19 Severity in Multiple Sclerosis: Putting Data Into Context. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1105. [Google Scholar] [CrossRef] [PubMed]
- Arrambide, G.; Llaneza-González, M.Á.; Costa-Frossard França, L.; Meca-Lallana, V.; Díaz, E.F.-; Moreno-Torres, I.; García-Domínguez, J.M.; Ortega-Suero, G.; Ayuso-Peralta, L.; Gómez-Moreno, M.; et al. SARS-CoV-2 Infection in Multiple Sclerosis: Results of the Spanish Neurology Society Registry. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1024. [Google Scholar] [CrossRef] [PubMed]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies with COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef] [PubMed]
- Money, K.M.; Mahatoo, A.; Samaan, S.; Anand, P.; Baber, U.; Bailey, M.; Bakshi, R.; Bouley, A.; Bower, A.; Cahill, J.; et al. A New England COVID-19 Registry of Patients with CNS Demyelinating Disease: A Pilot Analysis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1046. [Google Scholar] [CrossRef]
- Sepúlveda, M.; Llufriu, S.; Martínez-Hernández, E.; Català, M.; Artola, M.; Hernando, A.; Montejo, C.; Pulido-Valdeolivas, I.; Martínez-Heras, E.; Guasp, M.; et al. Incidence and Impact of COVID-19 in MS: A Survey from a Barcelona MS Unit. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e954. [Google Scholar] [CrossRef]
- König, M.; Lorentzen, Å.R.; Torgauten, H.M.; Tran, T.T.; Schikora-Rustad, S.; Vaage, E.B.; Mygland, Å.; Wergeland, S.; Aarseth, J.; Aaberge, I.A.S.; et al. Humoral Immunity to SARS-CoV-2 MRNA Vaccination in Multiple Sclerosis: The Relevance of Time since Last Rituximab Infusion and First Experience from Sporadic Revaccinations. J. Neurol. Neurosurg. Psychiatry 2021, 94, 19–22. [Google Scholar] [CrossRef]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef]
- Reyes, S.; Cunningham, A.L.; Kalincik, T.; Havrdová, E.K.; Isobe, N.; Pakpoor, J.; Airas, L.; Bunyan, R.F.; van der Walt, A.; Oh, J.; et al. Update on the Management of Multiple Sclerosis during the COVID-19 Pandemic and Post Pandemic: An International Consensus Statement. J. Neuroimmunol. 2021, 357, 577627. [Google Scholar] [CrossRef]
- Sormani, M.P.; Inglese, M.; Schiavetti, I.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Da Rin, G.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Effect of SARS-CoV-2 MRNA Vaccination in MS Patients Treated with Disease Modifying Therapies. EBioMedicine 2021, 72, 103581. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Flechter, S.; Falb, R.; et al. Humoral Immune Response to COVID-19 MRNA Vaccine in Patients with Multiple Sclerosis Treated with High-Efficacy Disease-Modifying Therapies. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211012836. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Dreyer-Alster, S.; Harari, G.; Dolev, M.; Menascu, S.; Magalashvili, D.; Flechter, S.; Givon, U.; Guber, D.; et al. Humoral Immune Response in Multiple Sclerosis Patients Following PfizerBNT162b2 COVID19 Vaccination: Up to 6 Months Cross-Sectional Study. J. Neuroimmunol. 2021, 361, 577746. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Braun, J.; Fauchere, F.; Vanshylla, K.; Loyal, L.; Henze, L.; Kruse, B.; Dingeldey, M.; Jürchott, K.; Mangold, M.; et al. SARS-CoV-2 MRNA Vaccinations Fail to Elicit Humoral and Cellular Immune Responses in Patients with Multiple Sclerosis Receiving Fingolimod. J. Neurol. Neurosurg. Psychiatry 2022, 93, 960–971. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and Humoral Immune Responses Following SARS-CoV-2 MRNA Vaccination in Patients with Multiple Sclerosis on Anti-CD20 Therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-Reactive CD4+ T Cells Enhance SARS-CoV-2 Immune Responses upon Infection and Vaccination. Science 2021, 374, eabh1823. [Google Scholar] [CrossRef]
- Zrzavy, T.; Kollaritsch, H.; Rommer, P.S.; Boxberger, N.; Loebermann, M.; Wimmer, I.; Winkelmann, A.; Zettl, U.K. Vaccination in Multiple Sclerosis: Friend or Foe? Front. Immunol. 2019, 10, 1883. [Google Scholar] [CrossRef]
- Heidler, F.; Baldt, J.; Frahm, N.; Langhorst, S.E.; Mashhadiakbar, P.; Streckenbach, B.; Zettl, U.K.; Richter, J. Vaccination Setting of Patients with Autoimmune Diseases in Times of Severe Acute Respiratory Syndrome Coronavirus Type 2 Pandemic Using the Example of Multiple Sclerosis Patients: A Longitudinal Multicenter Study. ENE 2022, 85, 104–111. [Google Scholar] [CrossRef]
- Heidler, F.; Baldt, J.; Frahm, N.; Langhorst, S.E.; Mashhadiakbar, P.; Streckenbach, B.; Burian, K.; Zettl, U.K.; Richter, J. Vaccination Willingness in Association with Personality Traits in Patients with Multiple Sclerosis in the Course of SARS-CoV-2 Pandemic. Sci. Rep. 2022, 12, 15147. [Google Scholar] [CrossRef]
- Diem, L.; Friedli, C.; Chan, A.; Salmen, A.; Hoepner, R. Vaccine Hesitancy in Patients with Multiple Sclerosis: Preparing for the SARS-CoV-2 Vaccination Challenge. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e991. [Google Scholar] [CrossRef]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.-N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 Vaccination in Patients with Multiple Sclerosis: What We Have Learnt by February 2021. Mult. Scler. 2021, 27, 864–870. [Google Scholar] [CrossRef]
- Lotan, I.; Wilf-Yarkoni, A.; Friedman, Y.; Stiebel-Kalish, H.; Steiner, I.; Hellmann, M.A. Safety of the BNT162b2 COVID-19 Vaccine in Multiple Sclerosis (MS): Early Experience from a Tertiary MS Center in Israel. Eur. J. Neurol. 2021, 28, 3742–3748. [Google Scholar] [CrossRef]
- Lotan, I.; Romanow, G.; Levy, M. Patient-Reported Safety and Tolerability of the COVID-19 Vaccines in Persons with Rare Neuroimmunological Diseases. Mult. Scler. Relat. Disord. 2021, 55, 103189. [Google Scholar] [CrossRef] [PubMed]
- Frahm, N.; Fneish, F.; Ellenberger, D.; Haas, J.; Loebermann, M.; Parciak, T.; Peters, M.; Pöhlau, D.; Rodgers, J.; Röper, A.-L.; et al. SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis in Germany and the United Kingdom: Gender-Specific Results from a Longitudinal Observational Study. Lancet Reg. Health-Eur. 2022, 22, 100502. [Google Scholar] [CrossRef] [PubMed]
- Diagnose und Therapie der Multiplen Sklerose, Neuromyelitis Optica Spektrum und MOG-IgG-assoziierte Erkrankungen. Available online: https://www.kompetenznetz-multiplesklerose.de/wp-content/uploads/2020/09/MS-LL_Hauptteil_Konsultationsfassung_KKNMS_202008_final.pdf (accessed on 9 May 2023).
- Hansen, B.B.; Klopfer, S.O. Optimal Full Matching and Related Designs via Network Flows. J. Comput. Graph. Stat. 2006, 15, 609–627. [Google Scholar] [CrossRef]
- Ohle, L.-M.; Ellenberger, D.; Flachenecker, P.; Friede, T.; Haas, J.; Hellwig, K.; Parciak, T.; Warnke, C.; Paul, F.; Zettl, U.K.; et al. Chances and Challenges of a Long-Term Data Repository in Multiple Sclerosis: 20th Birthday of the German MS Registry. Sci. Rep. 2021, 11, 13340. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Ura, T.; Yamashita, A.; Mizuki, N.; Okuda, K.; Shimada, M. New Vaccine Production Platforms Used in Developing SARS-CoV-2 Vaccine Candidates. Vaccine 2021, 39, 197–201. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Xu, S.; Yang, K.; Li, R.; Zhang, L. MRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020, 21, E6582. [Google Scholar] [CrossRef]
- Monschein, T.; Hartung, H.-P.; Zrzavy, T.; Barnett, M.; Boxberger, N.; Berger, T.; Chataway, J.; Bar-Or, A.; Rommer, P.S.; Zettl, U.K. Vaccination and Multiple Sclerosis in the Era of the COVID-19 Pandemic. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1033–1043. [Google Scholar] [CrossRef]
- Pawlitzki, M.; Zettl, U.K.; Ruck, T.; Rolfes, L.; Hartung, H.-P.; Meuth, S.G. Merits and Culprits of Immunotherapies for Neurological Diseases in Times of COVID-19. EBioMedicine 2020, 56, 102822. [Google Scholar] [CrossRef]
- German Federal Ministry of Health. Das offizielle Dashboard zur Impfkampagne der Bundesrepublik Deutschland. Available online: https://impfdashboard.de/ (accessed on 9 May 2023).
- German Standing Committee on Vaccination. Stufenplan der STIKO zur Priorisierung der COVID-19-Impfung. Available online: https://www.rki.de/DE/Content/Infekt/Impfen/ImpfungenAZ/COVID-19/Stufenplan.pdf?__blob=publicationFile (accessed on 9 May 2023).
- Robert Koch Institute COVID-19-Impfungen in Deutschland. Available online: https://github.com/robert-koch-institut/COVID-19-Impfungen_in_Deutschland/blob/4bcef2add8a9df79471f56a74693ed2485391987/Aktuell_Deutschland_Bundeslaender_COVID-19-Impfungen.csv (accessed on 19 January 2022).
- Nabizadeh, F.; Ramezannezhad, E.; Kazemzadeh, K.; Khalili, E.; Ghaffary, E.M.; Mirmosayyeb, O. Multiple Sclerosis Relapse after COVID-19 Vaccination: A Case Report-Based Systematic Review. J. Clin. Neurosci. 2022, 104, 118–125. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Qian, L.; Tartof, S.Y.; Brara, S.M.; Jacobsen, S.J.; Beaber, B.E.; Sy, L.S.; Chao, C.; Hechter, R.; Tseng, H.F. Vaccines and the Risk of Multiple Sclerosis and Other Central Nervous System Demyelinating Diseases. JAMA Neurol. 2014, 71, 1506–1513. [Google Scholar] [CrossRef]
- Amaral, M.P.; Branco, L.M.; Strasser, A.; Dixit, V.M.; Bortoluci, K.R. Paradise Revealed III: Why so Many Ways to Die? Apoptosis, Necroptosis, Pyroptosis, and Beyond. Cell. Death Differ. 2020, 27, 1740–1742. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-ΚB, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Alroughani, R.; Al-Hashel, J.; Abokalawa, F.; AlMojel, M.; Farouk Ahmed, S. COVID-19 Vaccination in People with Multiple Sclerosis, Real-Life Experience. Clin. Neurol. Neurosurg. 2022, 220, 107374. [Google Scholar] [CrossRef]
- Caron, P. Autoimmune and Inflammatory Thyroid Diseases Following Vaccination with SARS-CoV-2 Vaccines: From Etiopathogenesis to Clinical Management. Endocrine 2022, 78, 406–417. [Google Scholar] [CrossRef]
- Chee, Y.J.; Liew, H.; Hoi, W.H.; Lee, Y.; Lim, B.; Chin, H.X.; Lai, R.T.R.; Koh, Y.; Tham, M.; Seow, C.J.; et al. SARS-CoV-2 MRNA Vaccination and Graves’ Disease: A Report of 12 Cases and Review of the Literature. J. Clin. Endocrinol. Metab. 2022, 107, e2324–e2330. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; De Filippo, M.; Licari, A.; Marseglia, A.; Maiocchi, L.; Ricciardi, A.; Corsico, A.; Marseglia, G.; Mondelli, M.U.; Bruno, R. COVID Vaccination and Asthma Exacerbation: Might There Be a Link? Int. J. Infect. Dis. 2021, 112, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Portuguese, A.J.; Sunga, C.; Kruse-Jarres, R.; Gernsheimer, T.; Abkowitz, J. Autoimmune- and Complement-Mediated Hematologic Condition Recrudescence Following SARS-CoV-2 Vaccination. Blood Adv. 2021, 5, 2794–2798. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Rosso, M.; Santoro, J.D. Wilhelm Uhthoff and Uhthoff’s Phenomenon. Mult. Scler. 2020, 26, 1790–1796. [Google Scholar] [CrossRef]
- Winkelmann, A.; Loebermann, M.; Barnett, M.; Hartung, H.-P.; Zettl, U.K. Vaccination and Immunotherapies in Neuroimmunological Diseases. Nat. Rev. Neurol. 2022, 18, 289–306. [Google Scholar] [CrossRef]
- Elser, H.C.; Koch-Henriksen, N.; Magyari, M. Seasonal Patterns of Relapse and Disability in Danish MS Patients: A Population-Based Cohort Study. Mult. Scler. Relat. Disord. 2021, 49, 102739. [Google Scholar] [CrossRef]
- Harding, K.; Tilling, K.; MacIver, C.; Willis, M.; Joseph, F.; Ingram, G.; Hirst, C.; Wardle, M.; Pickersgill, T.; Ben-Shlomo, Y.; et al. Seasonal Variation in Multiple Sclerosis Relapse. J. Neurol. 2017, 264, 1059–1067. [Google Scholar] [CrossRef]
- Whitaker, H.J.; Farrington, C.P.; Spiessens, B.; Musonda, P. Tutorial in Biostatistics: The Self-Controlled Case Series Method. Stat. Med. 2006, 25, 1768–1797. [Google Scholar] [CrossRef]
- Weldeselassie, Y.G.; Whitaker, H.J.; Farrington, C.P. Use of the Self-Controlled Case-Series Method in Vaccine Safety Studies: Review and Recommendations for Best Practice. Epidemiol. Infect. 2011, 139, 1805–1817. [Google Scholar] [CrossRef]
- Galeotti, F.; Massari, M.; D’Alessandro, R.; Beghi, E.; Chiò, A.; Logroscino, G.; Filippini, G.; Benedetti, M.D.; Pugliatti, M.; Santuccio, C.; et al. Risk of Guillain-Barré Syndrome after 2010–2011 Influenza Vaccination. Eur. J. Epidemiol. 2013, 28, 433–444. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. MRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity against SARS-CoV-2 Omicron Variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef]
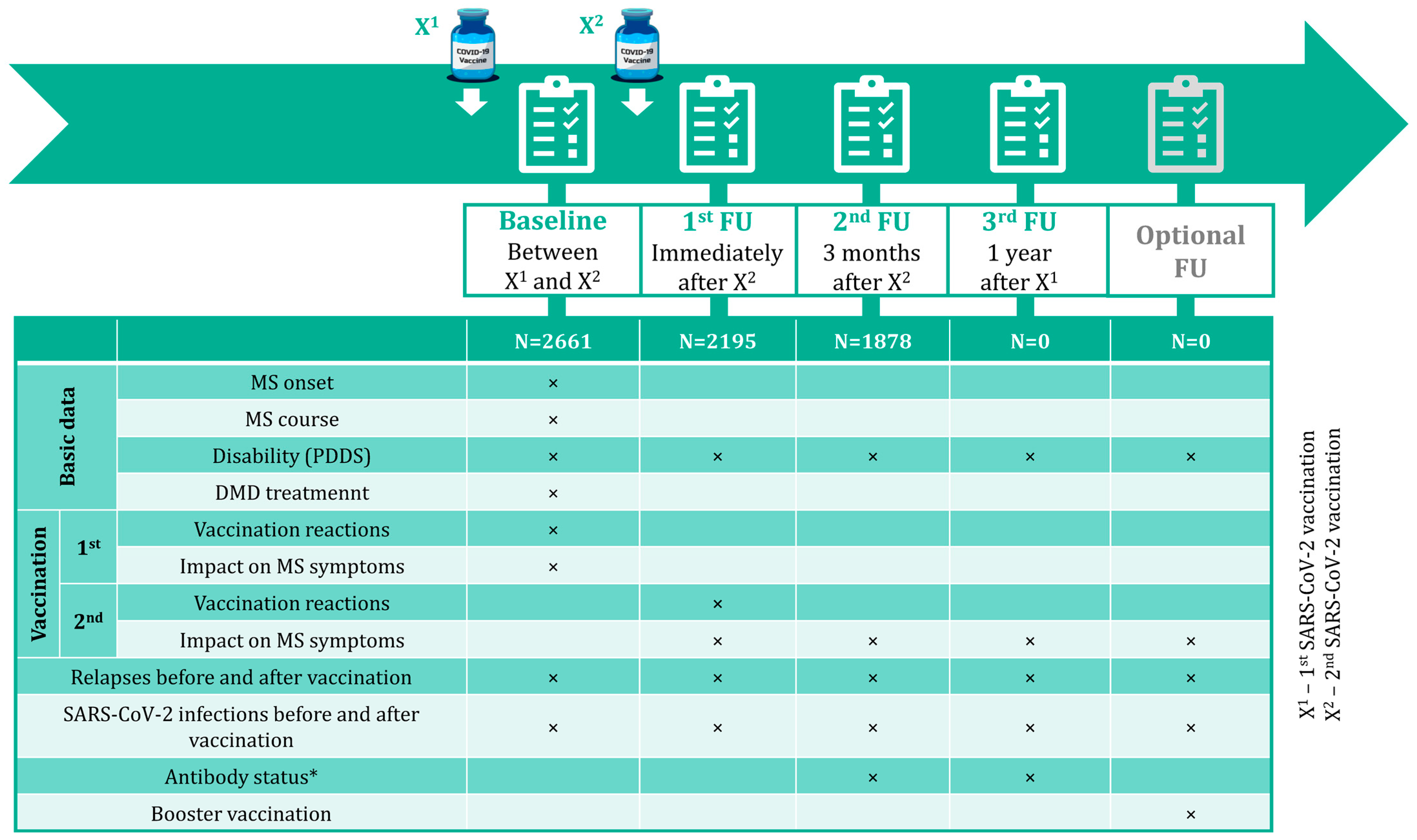

| Baseline (N = 2661) | FU1 (N = 2195) | FU2 (N = 1878) | |
|---|---|---|---|
| Gender, N (%) | |||
| Female | 2058 (77.9) | 1726 (79.3) | 1464 (78.5) |
| Male | 574 (21.7) | 444 (20.4) | 394 (21.1) |
| Divers | 9 (0.3) | 7 (0.3) | 6 (0.3) |
| Age [years], median (range) | 45.2 (18.0–83.8) | 45.5 (18.0–81.0) | 46.7 (18.1–83.8) |
| MS disease course, N (%) | |||
| RRMS | 1987 (74.7) | 1656 (75.4) | 1405 (74.8) |
| SPMS | 456 (17.1) | 368 (16.8) | 326 (17.4) |
| PPMS | 102 (3.8) | 78 (3.6) | 70 (3.7) |
| Undefined | 116 (4.4) | 93 (4.2) | 77 (4.1) |
| Disability level (PDDS), N (%) | |||
| Mild (0–1) | 1376 (51.7) | 1194 (54.6) | 979 (52.4) |
| Moderate (2–4) | 965 (36.3) | 735 (33.6) | 664 (35.6) |
| Severe (≥5) | 320 (12.0) | 256 (11.7) | 224 (12.0) |
| Coincident autoimmune diseases, N (%) | 572 (21.5) | 479 (21.8) | 396 (21.1) |
| DMD treatment, N (%) | 1921 (72.2) | 1603 (73.1) | 1392 (74.1) |
| IFNβ/GLAT | 571 (30.4 a) | 488 (31.1 b) | 418 (30.7 c) |
| CLAD/DMF/TER | 533 (28.4 a) | 451 (28.8 b) | 391 (28.8 c) |
| S1P RM | 333 (17.7 a) | 275 (17.5 b) | 248 (18.2 c) |
| anti-CD20 MAB | 287 (15.3 a) | 222 (14.2 b) | 187 (13.8 c) |
| Natalizumab | 125 (6.6 a) | 104 (6.6 b) | 91 (6.7 c) |
| Other | 31 (1.6 a) | 28 (1.8 b) | 25 (1.8 c) |
| Relapse within the year prior to X1, N (%) | 391 (14.7) | 315 (14.4) | 262 (14.0) |
| Relapse within 6 months prior to X1, N (%) | 213 (8.0) | 169 (7.7) | 139 (7.4) |
| Relapse within 3 months prior to X1, N (%) | 100 (3.8) | 77 (3.5) | 60 (3.2) |
| Time from last relapse (before X1) to X1 [years], median (range) | 3.1 (0.03–40.7) | 3.2 (0.03–40.7) | 3.2 (0.03–40.7) |
| Vaccination Scheme | N (%) | Time between X1 and X2 [Weeks] (25%, 75% Quantiles), Median |
|---|---|---|
| Tozinameran (BNT162b2, Comirnaty® [BioNTech/Pfizer]), 2 doses | 1717 (77.6) | 5.5 (4.2, 5.5) |
| Elasomeran (mRNA-1273, Spikevax® [Moderna]), 2 doses | 218 (9.9) | 5.5 (5.2, 5.5) |
| AZD1222 (Vaxzevria® [AstraZeneca]), 2 doses | 71 (3.2) | 9.7 (8.3, 11.0) |
| Ad26.COV2.S (COVID-19 Vaccine Janssen [Janssen/Johnson & Johnson]), 1 dose | 22 (1.0) | n.a. |
| Heterologous, 2 doses | 184 (8.3) | 10.1 (8.5, 11.0) |
| PwMS | ||
|---|---|---|
| With Relapse after Vaccination (N = 244 *) | Without Relapse after Vaccination (N = 2414) | |
| Gender, N (%) | ||
| Female | 203 (83.5) | 1853 (77.4) |
| Male | 39 (16.0) | 534 (22.3) |
| Divers | 1 (0.4) | 8 (0.3) |
| Age [years], median | 41.9 | 45.6 |
| MS disease course, N (%) | ||
| RRMS | 206 (84.4) | 1781 (73.8) |
| SPMS | 28 (11.5) | 428 (17.7) |
| PPMS | 0 (0.0) | 99 (4.1) |
| Undefined | 10 (4.1) | 106 (4.4) |
| Disability level (PDDS), N (%) | ||
| Mild (0–1) | 133 (54.5) | 1243 (51.5) |
| Moderate (2–4) | 90 (36.9) | 873 (36.2) |
| Severe (≥5) | 21 (8.6) | 298 (12.3) |
| Coincident autoimmune disease, N (%) | 60 (24.6) | 510 (21.1) |
| DMD treatment, N (%) | 146 (60.1) | 1774 (73.5) |
| Relapse within the year prior to X1, N (%) | 69 (28.3) | 322 (13.3) |
| Relapse within 6 months prior to X1, N (%) | 43 (17.6) | 170 (7.0) |
| Relapse within 3 months prior to X1, N (%) | 18 (7.4) | 82 (3.4) |
| Time from last relapse (before X1) to X1 [years], median (range) | 1.4 (0.05–24.2) | 3.3 (0.03–40.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frahm, N.; Fneish, F.; Ellenberger, D.; Haas, J.; Löbermann, M.; Peters, M.; Pöhlau, D.; Röper, A.-L.; Schilling, S.; Stahmann, A.; et al. Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study. J. Clin. Med. 2023, 12, 3640. https://doi.org/10.3390/jcm12113640
Frahm N, Fneish F, Ellenberger D, Haas J, Löbermann M, Peters M, Pöhlau D, Röper A-L, Schilling S, Stahmann A, et al. Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study. Journal of Clinical Medicine. 2023; 12(11):3640. https://doi.org/10.3390/jcm12113640
Chicago/Turabian StyleFrahm, Niklas, Firas Fneish, David Ellenberger, Judith Haas, Micha Löbermann, Melanie Peters, Dieter Pöhlau, Anna-Lena Röper, Sarah Schilling, Alexander Stahmann, and et al. 2023. "Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study" Journal of Clinical Medicine 12, no. 11: 3640. https://doi.org/10.3390/jcm12113640
APA StyleFrahm, N., Fneish, F., Ellenberger, D., Haas, J., Löbermann, M., Peters, M., Pöhlau, D., Röper, A. -L., Schilling, S., Stahmann, A., Temmes, H., Paul, F., & Zettl, U. K. (2023). Frequency and Predictors of Relapses following SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis: Interim Results from a Longitudinal Observational Study. Journal of Clinical Medicine, 12(11), 3640. https://doi.org/10.3390/jcm12113640








