The Effect of Virtual Reality on the Reduction of Pain in Women with an Indication for Outpatient Diagnostic Hysteroscopy: A Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
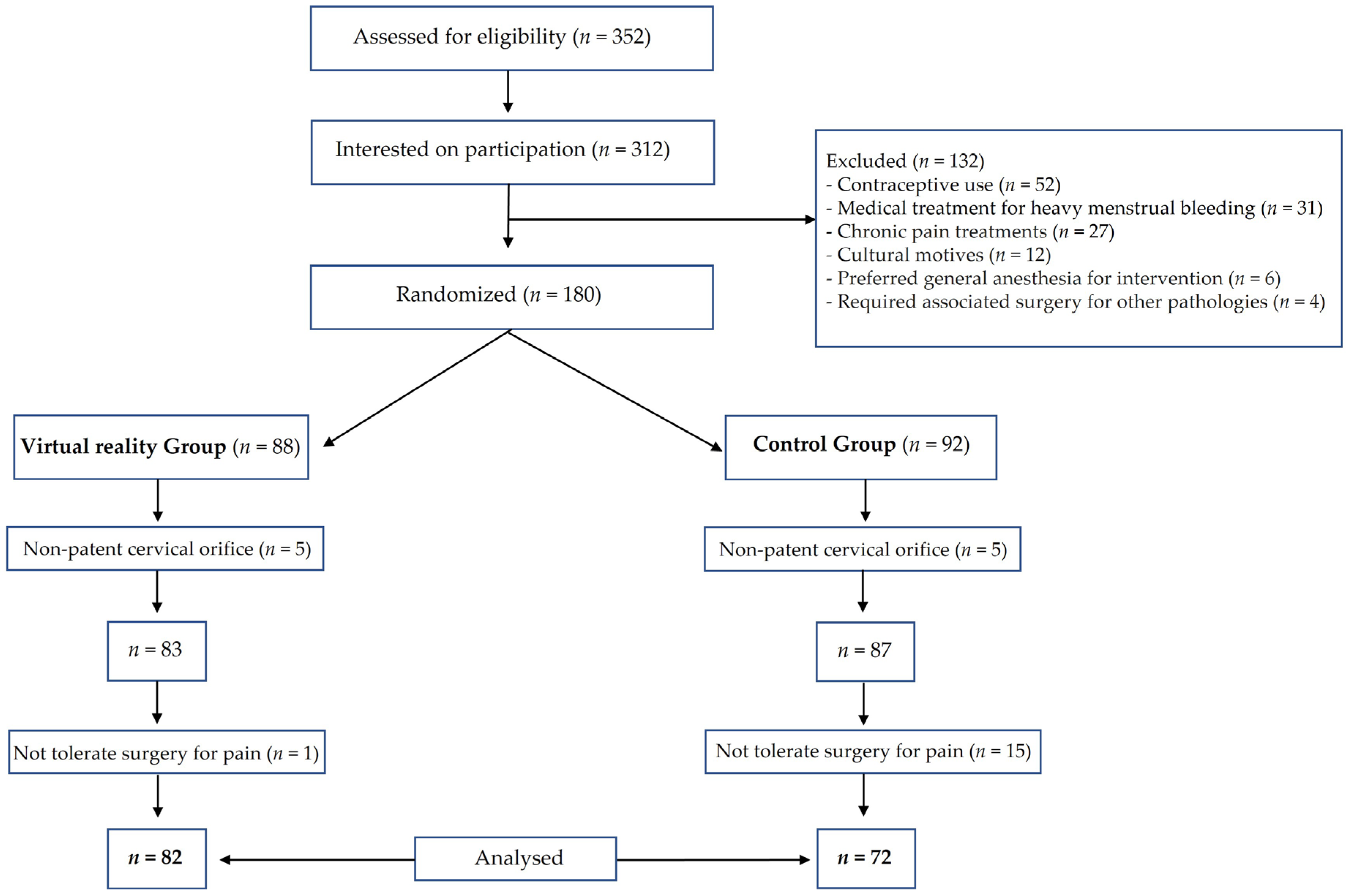
2.1. Patients
2.2. Intervention
2.3. Measurement Variables
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Main Outcomes
3.3. Physiological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valle, R.F. Office hysteroscopy. Clin. Obstet. Gynecol. 1999, 42, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.F.; Chou, H.H.; Wu, H.M.; Lee, C.L.; Chang, T.C. Effectiveness and appropriateness in the application of office hysteroscopy. J. Formos. Med. Assoc. 2019, 118, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Haimovich, S.; Riemma, G.; Ludwin, A.; Zizolfi, B.; De Angelis, M.C.; Carugno, J. Innovations in hysteroscopic surgery: Expanding the meaning of “in-office”. Minim. Invasive Ther. Allied. Technol. 2020, 30, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Loffer, F.D. Complications of hysteroscopy-their cause, prevention, and correction. J. Am. Assoc. Gynecol. Laparosc. 1995, 3, 11–26. [Google Scholar] [CrossRef]
- Paulo, A.A.S.; Solheiro, M.H.R.; Paulo, C.O.S.; Afreixo, V.M.A. What proportion of women refers moderate to severe pain during office hysteroscopy with a mini-hysteroscope? A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 37–46. [Google Scholar] [CrossRef]
- Tasma, M.L.; Louwerse, M.D.; Hehenkamp, W.J.; Geomini, P.M.; Bongers, M.Y.; Veersema, S.; van Kesteren, P.J.; Tromp, E.; Huirne, J.A.; Graziosi, G.C. Misoprostol for cervical priming prior to hysteroscopy in postmenopausal and premenopausal nulliparous women; a multicentre randomised placebo controlled trial. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 81–89. [Google Scholar] [CrossRef]
- Hassan, L.; Gannon, M.J. Anaesthesia and analgesia for ambulatory hysteroscopic surgery. Best Pract. Res. Clin. Obstet. Gynaecol. 2005, 19, 681–691. [Google Scholar] [CrossRef]
- Campo, V.; Campo, S. Hysteroscopy requirements and complications. Minerva Ginecol. 2007, 59, 451–457. [Google Scholar]
- Ahmad, G.; O’Flynn, H.; Attarbashi, S.; Duffy, J.M.; Watson, A. Pain relief for outpatient hysteroscopy. Cochrane Database Syst. Rev. 2010, 10, Cd007710. [Google Scholar] [CrossRef]
- Deo, N.; Khan, K.S.; Mak, J.; Allotey, J.; Gonzalez Carreras, F.J.; Fusari, G.; Benn, J. Virtual reality for acute pain in outpatient hysteroscopy: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2021, 128, 87–95. [Google Scholar] [CrossRef]
- Loreto-Quijada, D.; Gutiérrez-Maldonado, J.; Nieto, R.; Gutiérrez-Martínez, O.; Ferrer-García, M.; Saldaña, C.; Fusté-Escolano, A.; Liutsko, L. Differential effects of two virtual reality interventions: Distraction versus pain control. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Dascal, J.; Reid, M.; IsHak, W.W.; Spiegel, B.; Recacho, J.; Rosen, B.; Danovitch, I. Virtual Reality and Medical Inpatients: A Systematic Review of Randomized, Controlled Trials. Innov. Clin. Neurosci. 2017, 14, 14–21. [Google Scholar]
- Raghav, K.; Van Wijk, A.J.; Abdullah, F.; Islam, M.N.; Bernatchez, M.; De Jongh, A. Efficacy of virtual reality exposure therapy for treatment of dental phobia: A randomized control trial. BMC Oral Health 2016, 16, 25. [Google Scholar] [CrossRef]
- Freeman, D.; Haselton, P.; Freeman, J.; Spanlang, B.; Kishore, S.; Albery, E.; Denne, M.; Brown, P.; Slater, M.; Nickless, A. Automated psychological therapy using immersive virtual reality for treatment of fear of heights: A single-blind, parallel-group, randomised controlled trial. Lancet Psychiatry 2018, 5, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.B.; Emmelkamp, P.M. Virtual reality exposure therapy for anxiety disorders: A meta-analysis. J. Anxiety Disord. 2008, 22, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Foster, S.; Sambell, R.; Leong, P. Clinical efficacy of virtual reality for acute procedural pain management: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0200987. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.P.; Bauer, M.E.; Bell, C.L.; Low, L.K.; Hassett, A.L.; Cassidy, R.B.; Boyer, K.D.; Sharar, S.R. Virtual Reality Analgesia in Labor: The VRAIL Pilot Study-A Preliminary Randomized Controlled Trial Suggesting Benefit of Immersive Virtual Reality Analgesia in Unmedicated Laboring Women. Anesth. Analg. 2019, 128, e93–e96. [Google Scholar] [CrossRef]
- Wong, C.L.; Li, C.K.; Chan, C.W.H.; Choi, K.C.; Chen, J.; Yeung, M.T.; Chan, O.N. Virtual Reality Intervention Targeting Pain and Anxiety Among Pediatric Cancer Patients Undergoing Peripheral Intravenous Cannulation: A Randomized Controlled Trial. Cancer Nurs. 2020, 44, 435–442. [Google Scholar] [CrossRef]
- Chuan, A.; Zhou, J.J.; Hou, R.M.; Stevens, C.J.; Bogdanovych, A. Virtual reality for acute and chronic pain management in adult patients: A narrative review. Anaesthesia 2020, 76, 695–704. [Google Scholar] [CrossRef]
- Tam, W.H.; Yuen, P.M. Use of diclofenac as an analgesic in outpatient hysteroscopy: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2001, 76, 1070–1072. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hwang, J.L.; Huang, L.W.; Chen, H.J. Use of sublingual buprenorphine for pain relief in office hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Spiegel, B.M.R.; Gregory, K.D. Virtual Reality Reduces Pain in Laboring Women: A Randomized Controlled Trial. Am. J. Perinatol. 2020, 38, e167–e172. [Google Scholar] [CrossRef] [PubMed]
- Easterlin, M.C.; Berdahl, C.T.; Rabizadeh, S.; Spiegel, B.; Agoratus, L.; Hoover, C.; Dudovitz, R. Child and Parent Perspectives on the Acceptability of Virtual Reality to Mitigate Medical Trauma in an Infusion Center. Matern. Child Health J. 2020, 24, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Esin, S.; Baser, E.; Okuyan, E.; Kucukozkan, T. Comparison of sublingual misoprostol with lidocaine spray for pain relief in office hysteroscopy: A randomized, double-blind, placebo-controlled trial. J. Minim. Invasive Gynecol. 2013, 20, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Vilos, G.A.; Abu-Rafea, B. New developments in ambulatory hysteroscopic surgery. Best Pract. Res. Clin. Obstet. Gynaecol. 2005, 19, 727–742. [Google Scholar] [CrossRef]
- Cooper, N.A.; Smith, P.; Khan, K.S.; Clark, T.J. Vaginoscopic approach to outpatient hysteroscopy: A systematic review of the effect on pain. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Di Spiezio Sardo, A.; Guida, M.; Pellicano, M.; Nappi, C.; Bettocchi, S. New technique to perform hysteroscopy in ‘women with an intact hymen’ is really just the vaginoscopic approach (no-touch technique). J. Minim. Invasive Gynecol. 2006, 13, 489–490. [Google Scholar] [CrossRef]
- Almeida, Z.M.; Pontes, R.; Costa Hde, L. Evaluation of pain in diagnostic hysteroscopy by vaginoscopy using normal saline at body temperature as distension medium: A randomized controlled trial. Rev. Bras. Gynecol. Obstet. 2008, 30, 25–30. [Google Scholar] [CrossRef]
- Price, D.D.; McGrath, P.A.; Rafii, A.; Buckingham, B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983, 17, 45–56. [Google Scholar] [CrossRef]
- Crossley, K.M.; Bennell, K.L.; Cowan, S.M.; Green, S. Analysis of outcome measures for persons with patellofemoral pain: Which are reliable and valid? Arch. Phys. Med. Rehabil. 2004, 85, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Heart rate monitoring: Applications and limitations. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef] [PubMed]
- Thrane, S.; Cohen, S.M. Effect of Reiki therapy on pain and anxiety in adults: An in-depth literature review of randomized trials with effect size calculations. Pain. Manag. Nurs. 2014, 15, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.J. Virtual pain relief for outpatient hysteroscopy. BJOG Int. J. Obstet. Gynaecol. 2020, 128, 96. [Google Scholar] [CrossRef] [PubMed]
- Moawad, N.S.; Santamaria, E.; Johnson, M.; Shuster, J. Cost-effectiveness of office hysteroscopy for abnormal uterine bleeding. JSLS J. Soc. Laparoendosc. Surg. 2014, 18, e2014.00393. [Google Scholar] [CrossRef] [PubMed]
- Saridogan, E.; Tilden, D.; Sykes, D.; Davis, N.; Subramanian, D. Cost-analysis comparison of outpatient see-and-treat hysteroscopy service with other hysteroscopy service models. J. Minim. Invasive Gynecol. 2010, 17, 518–525. [Google Scholar] [CrossRef]
- Fouks, Y.; Kern, G.; Cohen, A.; Reicher, L.; Shapira, Z.; Many, A.; Yogev, Y.; Rattan, G. A virtual reality system for pain and anxiety management during outpatient hysteroscopy—A randomized control trial. Eur. J. Pain 2022, 26, 600–609. [Google Scholar] [CrossRef]
| Study Group (n = 82) | Control Group (n = 72) | p-Value | ||
|---|---|---|---|---|
| Age, mean (SD) | 47.19 (8.716) | 49.20 (11.789) | 0.440 | |
| SatO2 initial, mean (SD) | 97.68 (1.873) | 97.00 (2.093) | 0.139 | |
| SAP initial, mean (SD) | 132.03 (23.037) | 139.49 (29.347) | 0.213 | |
| DAP initial, mean (SD) | 79.48 (14.744) | 78.71 (11.668) | 0.807 | |
| Heart Rate, mean (SD) | 83.73 (13.568) | 81.49 (16.823) | 0.526 | |
| VAS, mean (SD) | 3.75 (1.134) | 4.54 (1.253) | 0.174 | |
| Menopause | No, n (%) | 52 (63.4) | 41 (56.9) | 0.144 |
| Yes, n (%) | 30 (36.6) | 31 (43.1) | ||
| Parity | Nulliparous, n (%) | 14 (17.1) | 9 (12.5) | 0.409 |
| Multiparous, n (%) | 68 (82.9) | 63 (87.5) | ||
| Smokers | No, n (%) | 44 (53.6) | 35 (48.6) | 0.415 |
| Yes, n (%) | 38 (46.4) | 37 (51.4) | ||
| Previous Conization | No, n (%) | 78 (95.1) | 66 (91.7) | 0.585 |
| Yes, n (%) | 4 (4.9) | 6 (8.3) | ||
| Biopsy | No, n (%) | 82 (100.0) | 70 (97.2) | 0.892 |
| Yes, n (%) | 0 (0.0) | 2 (2.8) | ||
| Myomectomy | No, n (%) | 80 (97.6) | 68 (94.4) | 0.742 |
| Yes, n (%) | 2 (2.4) | 4 (5.6) | ||
| VAS | |||||
|---|---|---|---|---|---|
| Study Group (n = 82) | Control Group (n = 72) | SMD (95% CI) | ES [95% CI] | p-Value | |
| Final, mean (SD) | 2.451 (1.173) | 3.972 (0.519) | −1.521 (−2.601 to −0.440) | −1.797 [−3.152 to −0.321] | 0.006 |
| 15 min, mean (SD) | 1.769 (0.488) | 3.300 (1.047) | −1.531 (−2.557 to −0.504) | −1.996 [−3.826 to −0.472] | 0.004 |
| 30 min, mean (SD) | 1.621 (0.383) | 2.719 (1.146) | −1.099 (−2.166 to −0.031) | −1.437 [−2.942 to −0.152] | 0.044 |
| Initial | Procedure | Final | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study Group (n = 82) | Control Group (n = 72) | p-Value | Study Group (n = 82) | Control Group (n = 72) | p-Value | Study Group (n = 82) | Control Group (n = 72) | p-Value | |
| SatO2, mean (SD) | 97.68 (1.873) | 97.00 (2.093) | 0.797 | 98.05 (1.749) | 97.11 (2.246) | 0.732 | 98.15 (1.514) | 97.17 (2.332) | 0.633 |
| SAP, mean (SD) | 132.03 (23.037) | 139.49 (29.347) | 0.213 | 133.13 (20.756) | 137.46 (17.094) | 0.440 | 127.54 (19.316) | 133.94 (24.352) | 0.212 |
| DAP, mean (SD) | 79.48 (14.744) | 78.71 (11.668) | 0.807 | 76.85 (11.142) | 77.23 (12.291) | 0.889 | 73.56 (12.703) | 76.11 (14.338) | 0.420 |
| Blood Pressure, mean (SD) | 96.38 (16.429) | 98.54 (15.257) | 0.558 | 95.05 (13.199) | 96.89 (15.479) | 0.584 | 91.03 (13.523) | 94.66 (15.961) | 0.293 |
| Heart Rate, mean (SD) | 83.73 (13.568) | 81.49 (16.823) | 0.526 | 76.79 (14.577) | 72.89 (13.486) | 0.237 | 74.41 (11.621) | 75.20 (15.262) | 0.802 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelazas-Hernández, J.A.; Varillas-Delgado, D.; González-Casado, T.; Cristóbal-Quevedo, I.; Alonso-Bermejo, A.; Ronchas-Martínez, M.; Cristóbal-García, I. The Effect of Virtual Reality on the Reduction of Pain in Women with an Indication for Outpatient Diagnostic Hysteroscopy: A Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3645. https://doi.org/10.3390/jcm12113645
Pelazas-Hernández JA, Varillas-Delgado D, González-Casado T, Cristóbal-Quevedo I, Alonso-Bermejo A, Ronchas-Martínez M, Cristóbal-García I. The Effect of Virtual Reality on the Reduction of Pain in Women with an Indication for Outpatient Diagnostic Hysteroscopy: A Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(11):3645. https://doi.org/10.3390/jcm12113645
Chicago/Turabian StylePelazas-Hernández, Jesus A., David Varillas-Delgado, Teresa González-Casado, Ignacio Cristóbal-Quevedo, Agustina Alonso-Bermejo, Marina Ronchas-Martínez, and Ignacio Cristóbal-García. 2023. "The Effect of Virtual Reality on the Reduction of Pain in Women with an Indication for Outpatient Diagnostic Hysteroscopy: A Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 11: 3645. https://doi.org/10.3390/jcm12113645
APA StylePelazas-Hernández, J. A., Varillas-Delgado, D., González-Casado, T., Cristóbal-Quevedo, I., Alonso-Bermejo, A., Ronchas-Martínez, M., & Cristóbal-García, I. (2023). The Effect of Virtual Reality on the Reduction of Pain in Women with an Indication for Outpatient Diagnostic Hysteroscopy: A Randomized Controlled Trial. Journal of Clinical Medicine, 12(11), 3645. https://doi.org/10.3390/jcm12113645







