Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Study Population
2.3. Definition of Atrial Fibrillation
2.4. Study Procedures
2.4.1. Implantable Loop Recorder
2.4.2. Rhythm Monitoring
2.5. Statistical Analysis
3. Results
3.1. Clinical Associations with Prevalent Atrial Fibrillation
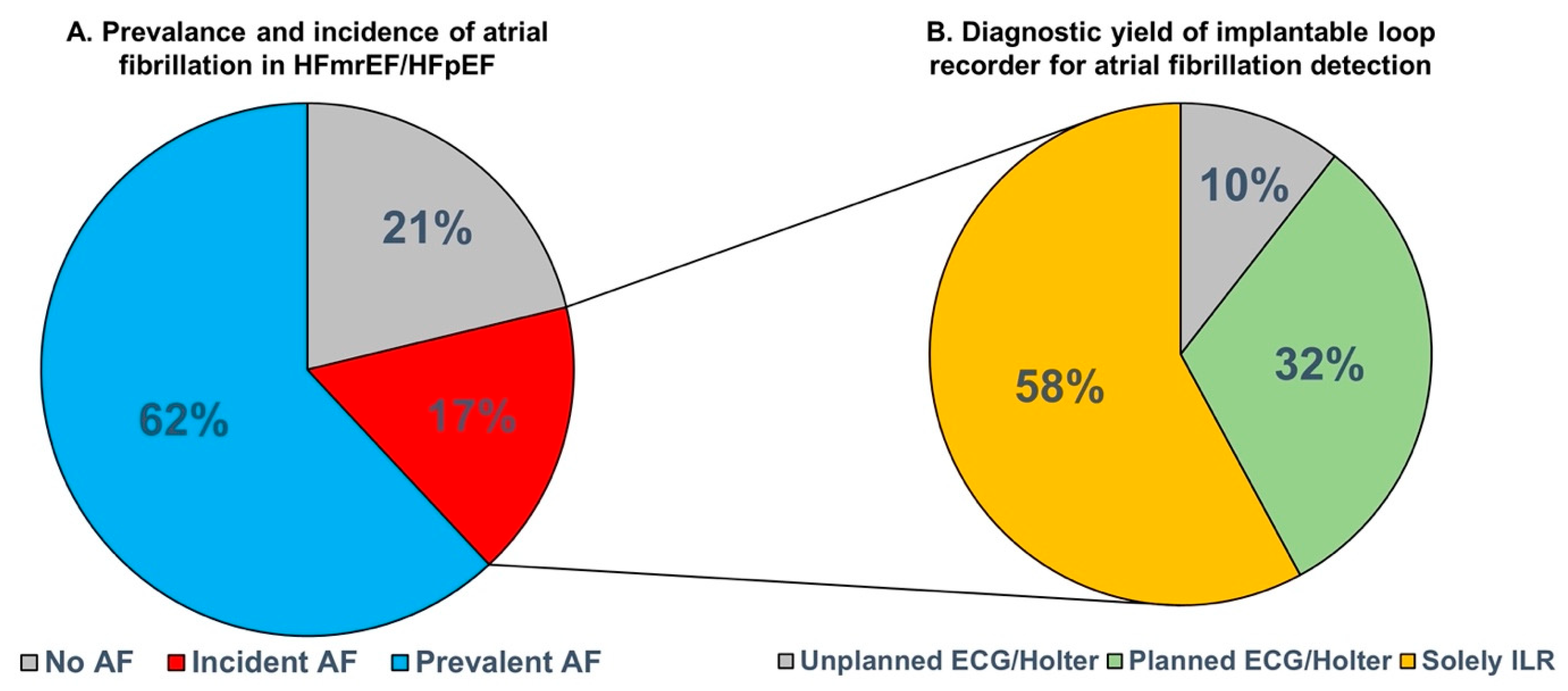
3.2. Incident Atrial Fibrillation and Diagnostic Yield for Atrial Fibrillation Detection
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotecha, D.; Lam, C.S.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Junbo Ge, D.P.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.-V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: A report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlstrom, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef]
- Lam, C.; Rienstra, M.; Tay, W.T.; Liu, L.; Hummel, Y.; Van der Meer, P.; de Boer, R.; Van Gelder, I.; van Veldhuisen, D.; Voors, A.; et al. Atrial Fibrillation in Heart Failure With Preserved Ejection Fraction: Association with Exercise Capacity, Left Ventricular Filling Pressures, Natriuretic Peptides, and Left Atrial Volume. JACC Heart Fail. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.G.; Swedberg, K.; Ducharme, A.; Granger, C.B.; Michelson, E.L.; McMurray, J.J.; Puu, M.; Yusuf, S.; Pfeffer, M.A. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J. Am. Coll. Cardiol. 2006, 47, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Linssen, G.C.; Rienstra, M.; Jaarsma, T.; Voors, A.A.; van Gelder, I.C.; Hillege, H.L.; van Veldhuisen, D.J. Clinical and prognostic effects of atrial fibrillation in heart failure patients with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2011, 13, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, R.; Chamberlain, A.M.; Roger, V.L.; Redfield, M.M. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: A community-based study. Circulation 2013, 128, 1085–1093. [Google Scholar] [CrossRef]
- Van Veldhuisen, D.J.; Van Woerden, G.; Gorter, T.M.; Van Empel, V.P.; Manintveld, O.C.; Tieleman, R.G.; Maass, A.H.; Vernooy, K.; Westenbrink, B.D.; Van Gelder, I.C.; et al. Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: The VIP-HF study. Eur. J. Heart Fail. 2020, 22, 1923–1929. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Ulm, K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am. J. Epidemiol. 1990, 131, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Hwang, S.J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; van Melle, J.P.; Rienstra, M.; Borlaug, B.A.; Hummel, Y.M.; van Gelder, I.C.; Hoendermis, E.S.; Voors, A.A.; van Veldhuisen, D.J.; Lam, C.S. Right Heart Dysfunction in Heart Failure with Preserved Ejection Fraction: The Impact of Atrial Fibrillation. J. Card. Fail. 2018, 24, 177–185. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, S.Z.; Haugan, K.J.; Kronborg, C.; Graff, C.; Højberg, S.; Køber, L.; Krieger, D.; Holst, A.G.; Nielsen, J.B.; Brandes, A.; et al. Comprehensive Evaluation of Rhythm Monitoring Strategies in Screening for Atrial Fibrillation: Insights from Patients at Risk Monitored Long Term with an Implantable Loop Recorder. Circulation 2020, 141, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Kort, R.S.S.; Tuininga, Y.S.; Bosker, H.A.; Janssen, M.; Tukkie, R. Telemonitoring with an implantable loop recorder in outpatient heart failure care: One year follow-up report from a prospective observational Dutch multicentre study. Neth. Heart J. 2019, 27, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.; Wang, J.; Hohnloser, S.H.; Gold, M.R.; Capucci, A.; Lau, C.-P.; Morillo, C.A.; Hobbelt, A.H.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur. Heart J. 2017, 38, 1339–1344. [Google Scholar] [CrossRef]
- Wong, J.A.; Conen, D.; Van Gelder, I.C.; McIntyre, W.F.; Crijns, H.J.; Wang, J.; Gold, M.R.; Hohnloser, S.H.; Lau, C.; Capucci, A.; et al. Progression of Device-Detected Subclinical Atrial Fibrillation and the Risk of Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 2603–2611. [Google Scholar] [CrossRef]
| Total n = 113 | No AF History n = 43 | AF History n = 70 | p-Value | |
|---|---|---|---|---|
| Age | 73 ± 8 | 70 ± 8 | 74 ± 8 | 0.01 |
| Sex, female | 58 (51%) | 18 (42%) | 40 (57%) | 0.1 |
| Body mass index, kg/m2 | 29.8 ± 5.7 | 28.7 ± 5.3 | 30.4 ± 5.9 | 0.1 |
| Comorbidities | ||||
| Hypertension | 88 (78%) | 31 (72%) | 57 (81%) | 0.2 |
| Coronary artery disease | 39 (35%) | 19 (44%) | 20 (29%) | 0.09 |
| Diabetes mellitus | 45 (40%) | 13 (30%) | 32 (46%) | 0.1 |
| Renal dysfunction | 54 (48%) | 19 (44%) | 35 (50%) | 0.5 |
| Obesity | 45 (40%) | 16 (43%) | 29 (45%) | 0.8 |
| Chronic obstructive pulmonary disease | 21 (19%) | 9 (21%) | 12 (17%) | 0.6 |
| CHA2DS2-VASc | 5 [4–6] | 4 [3–6] | 5 [4–6] | 0.3 |
| Echocardiography (n = 113) | ||||
| LV ejection fraction, % | 54 ± 6 | 53 ± 7 | 54 ± 6 | 0.4 |
| LV ejection fraction ≥50% | 85 (75%) | 28 (65%) | 57 (81%) | 0.051 |
| LV mass index, g/m2 | 102 ± 37 | 116 ± 48 | 94 ± 26 | 0.01 |
| E/e′ | 13.1 ± 5.0 | 12.3 ± 4.6 | 13.9 ± 5.2 | 0.2 |
| Mean e′ septal/lateral wall, cm/s | 7.5 ± 2.1 | 6.6 ± 1.7 | 8.3 ± 2.1 | <0.001 |
| LA volume index, mL/m2 | 47 ± 16 | 40 ± 16 | 50 ± 15 | 0.002 |
| TAPSE. mm | 20.4 ± 4.8 | 21.5 ± 5.6 | 19.7 ± 4.1 | 0.06 |
| RV s′, cm/s | 11.5 ± 2.7 | 12.3 ± 2.7 | 10.8 ± 2.5 | 0.01 |
| TR peak gradient, mmHg | 34 ± 11 | 34 ± 10 | 34 ± 11 | 0.9 |
| Laboratory test (n = 113) | ||||
| Creatinin, µmol/L | 122 ± 54 | 125 ± 59 | 120 ± 51 | 0.6 |
| eGFR, mL/min/1.73 m2 | 52 ± 21 | 53 ± 21 | 52 ± 22 | 0.7 |
| NT-proBNP, ng/L | 1367 [729–2430] | 800 [513–1787] | 1665 [1065–2553] | 0.003 |
| 24-Hour Holter (n = 112) | ||||
| Mean heart rate | 72 ± 13 | 67 ± 8 | 74 ± 15 | 0.001 |
| Cardiac MRI (n = 105) | ||||
| Left ventricle | ||||
| LV ejection fraction, % | 53 ± 8 | 53 ± 9 | 52 ± 8 | 0.8 |
| LV end-diastolic volume index, mL/m2 | 89 ± 25 | 98 ± 27 | 84 ± 22 | 0.004 |
| LV end-systolic volume index, mL/m2 | 43 ± 17 | 48 ± 20 | 41 ± 15 | 0.06 |
| LV mass index, g/m2 | 57 ± 23 | 66 ± 28 | 52 ± 18 | 0.005 |
| LV global longitudinal strain, % | −16.8 ± 5.0 | −17.4 ± 4.6 | −16.4 ± 5.3 | 0.3 |
| LV circumferential strain, % | −22.0 ± 6.1 | −23.4 ± 6.5 | −21.1 ± 5.8 | 0.07 |
| Right ventricle | ||||
| RV ejection fraction, % | 53 ± 10 | 58 ± 11 | 49 ± 8 | <0.001 |
| RV end-diastolic volume index, mL/m2 | 81 ± 20 | 78 ± 16 | 83 ± 22 | 0.2 |
| RV end-systolic volume index, mL/m2 | 39 ± 15 | 33 ± 12 | 43 ± 15 | 0.001 |
| RV global longitudinal strain (%) | −19.4 ± 6.2 | −21.3 ± 6.8 | −18.2 ± 5.5 | 0.01 |
| Stroke volume/end-systolic volume | 1.2 ± 0.6 | 1.5 ± 0.7 | 1.0 ± 0.3 | <0.001 |
| Atria | ||||
| LA volume index, mL/m2 | 62 ± 22 | 54 ± 20 | 68 ± 21 | 0.002 |
| LA emptying fraction, % | 29 ± 16 | 38 ± 15 | 23 ± 14 | <0.001 |
| LA reservoir strain, % | 13.6 ± 9.2 | 18.1 ± 10.5 | 10.8 ± 6.8 | <0.001 |
| LA conduit strain, % (n = 61) | 9.9 ± 5.4 | 10.0 ± 6.1 | 9.9 ± 3.7 | 0.9 |
| LA booster strain, % (n = 61) | 8.0 ± 5.5 | 8.3 ± 6.2 | 7.6 ± 4.1 | 0.7 |
| RA volume index, mL/m2 | 46 ± 22 | 38 ± 18 | 51 ± 24 | 0.002 |
| RA emptying fraction, % | 28.4 ± 15.9 | 39 ± 13 | 21 ± 13 | <0.001 |
| RA reservoir strain, % | 18.5 ± 13.3 | 24.9 ± 13.2 | 14.4 ± 11.7 | <0.001 |
| RA conduit strain, % (n = 61) | 10.3 ± 6.5 | 10.7 ± 6.6 | 9.6 ± 6.3 | 0.5 |
| RA booster strain, % (n = 61) | 14.4 ± 9.4 | 14.3 ± 9.6 | 14.6 ± 9.4 | 0.9 |
| Medication | ||||
| Beta blockers | 98 (87%) | 34 (79%) | 64 (91%) | 0.06 |
| ACEi/ARB | 72 (64%) | 31 (72%) | 41 (59%) | 0.1 |
| MRA | 44 (39%) | 18 (42%) | 26 (37%) | 0.6 |
| Loop diuretics | 101 (89%) | 38 (88%) | 63 (90%) | 0.8 |
| Class 1 AAD | 0 | 0 | 0 | |
| Class 3 AAD | 5 (4%) | 0 | 5 (7%) | 0.07 |
| Non-dihydropyridine CCB | 6 (5%) | 3 (7%) | 3 (4%) | 0.5 |
| Digoxin | 23 (20%) | 0 | 23 (33%) | <0.001 |
| DOAC/VKA | 67 (59%) | 0 | 67 (96%) | <0.001 |
| Univariable Cox Regression Analysis | ||
|---|---|---|
| Hazard Ratio [95% CI] | p-Value | |
| Age, per year | 1.45 [0.90–2.34] | 0.1 |
| Male sex | 0.95 [0.38–2.38] | 0.9 |
| Body mass index, per SD increase | 1.35 [0.82–2.22] | 0.2 |
| Heart rate, per SD increase | 0.85 [0.43–1.71] | 0.7 |
| Systolic blood pressure, per SD increase | 1.26 [0.89–1.79] | 0.2 |
| Diastolic blood pressure, per SD increase | 1.04 [0.63–1.71] | 0.9 |
| Comorbidities | ||
| Hypertension | 1.05 [0.37–2.94] | 0.9 |
| Diabetes mellitus | 0.49 [0.16–1.48] | 0.2 |
| Coronary artery disease | 1.02 [0.41–2.52] | 0.97 |
| CHA2DS2-VASc | 1.09 [0.82–1.46] | 0.6 |
| Echocardiography | ||
| LV ejection fraction, per SD increase | 0.93 [0.62–1.41] | 0.7 |
| LV mass index, per SD increase | 1.08 [0.79–1.50] | 0.6 |
| E/e′, per SD increase | 0.85 [0.49–1.50] | 0.6 |
| Mean e′ septal/lateral wall, per SD increase | 0.84 [0.45–1.59] | 0.6 |
| LA volume index, per SD increase | 2.10 [1.30–3.42] | 0.003 |
| TAPSE, per SD increase | 1.21 [0.73–1.72] | 0.6 |
| RV s′, per SD increase | 0.95 [0.59–1.52] | 0.8 |
| >mild mitral regurgitation | 3.46 [0.97–12.33] | 0.056 |
| Laboratory test | ||
| NT-proBNP, per Ln increase | 1.26 [0.80–1.98] | 0.3 |
| eGFR, per Ln increase | 0.98 [0.35–2.77] | 0.98 |
| Medication | ||
| Beta blockers | 2.27 [0.52–9.93] | 0.3 |
| ACEi/ARB | 1.23 [0.45–3.46] | 0.7 |
| MRA | 0.67 [0.25–1.80] | 0.4 |
| Loop diuretics | 1.07 [0.25–4.70] | 0.9 |
| Univariable Cox Regression Analysis | ||
|---|---|---|
| Hazard Ratio [95% CI] | p-Value | |
| LV end-diastolic volume index, per SD increase | 1.10 [0.70–1.72] | 0.7 |
| LV end-systolic volume index, per SD increase | 1.29 [0.85–1.94] | 0.2 |
| LV ejection fraction, per SD increase | 0.70 [0.45–1.10] | 0.1 |
| LV mass index, per SD increase | 0.69 [0.63–1.47] | 0.8 |
| RV end-diastolic volume index, per SD increase | 1.12 [0.62–2.03] | 0.7 |
| RV end-systolic volume index, per SD increase | 1.26 [0.72–2.21] | 0.4 |
| RV ejection fraction, per SD increase | 0.86 [0.56–1.31] | 0.4 |
| RV mass index, per SD increase | 0.82 [0.52–1.30] | 0.4 |
| LA end-systolic volume index, per SD increase | 1.94 [1.12–3.34] | 0.017 |
| LA end-diastolic volume index, per SD increase | 2.14 [1.24–3.69] | 0.006 |
| LA emptying fraction, per SD increase | 0.75 [0.43–1.30] | 0.3 |
| LA reservoir strain, per SD increase | 0.86 [0.54–1.35] | 0.5 |
| LA passive strain, per SD increase | 0.91 [0.61–1.36] | 0.6 |
| LA active strain, per SD increase | 0.89 [0.54–1.45] | 0.6 |
| RA volume index, per SD increase | 1.02 [0.54–1.93] | 0.9 |
| RA emptying fraction, per SD increase | 0.71 [0.39–1.30] | 0.3 |
| RA reservoir strain, per SD increase | 0.83 [0.50–1.36] | 0.4 |
| RA passive strain, per SD increase | 0.95 [0.58–1.55] | 0.8 |
| RA active strain, per SD increase | 0.60 [0.44–1.31] | 0.3 |
| LV global longitudinal strain, per SD increase | 0.90 [0.55–1.46] | 0.7 |
| LV circumferential strain, per SD increase | 0.97 [0.63–1.49] | 0.9 |
| RV global longitudinal strain, per SD increase | 1.12 [0.73–1.72] | 0.6 |
| RV coupling, per SD increase | 0.85 [0.53–1.38] | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorter, T.M.; van Veldhuisen, D.J.; Mulder, B.A.; Artola Arita, V.A.; van Empel, V.P.M.; Manintveld, O.C.; Tieleman, R.G.; Maass, A.H.; Vernooy, K.; van Gelder, I.C.; et al. Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders. J. Clin. Med. 2023, 12, 3682. https://doi.org/10.3390/jcm12113682
Gorter TM, van Veldhuisen DJ, Mulder BA, Artola Arita VA, van Empel VPM, Manintveld OC, Tieleman RG, Maass AH, Vernooy K, van Gelder IC, et al. Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders. Journal of Clinical Medicine. 2023; 12(11):3682. https://doi.org/10.3390/jcm12113682
Chicago/Turabian StyleGorter, Thomas M., Dirk J. van Veldhuisen, Bart A. Mulder, Vicente A. Artola Arita, Vanessa P. M. van Empel, Olivier C. Manintveld, Robert G. Tieleman, Alexander H. Maass, Kevin Vernooy, Isabelle C. van Gelder, and et al. 2023. "Prevalence and Incidence of Atrial Fibrillation in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: (Additive) Value of Implantable Loop Recorders" Journal of Clinical Medicine 12, no. 11: 3682. https://doi.org/10.3390/jcm12113682






