Microsporidial Stromal Keratitis in Post-Keratoplasty Eyes
Abstract
1. Introduction
2. Materials and Methods
3. Results
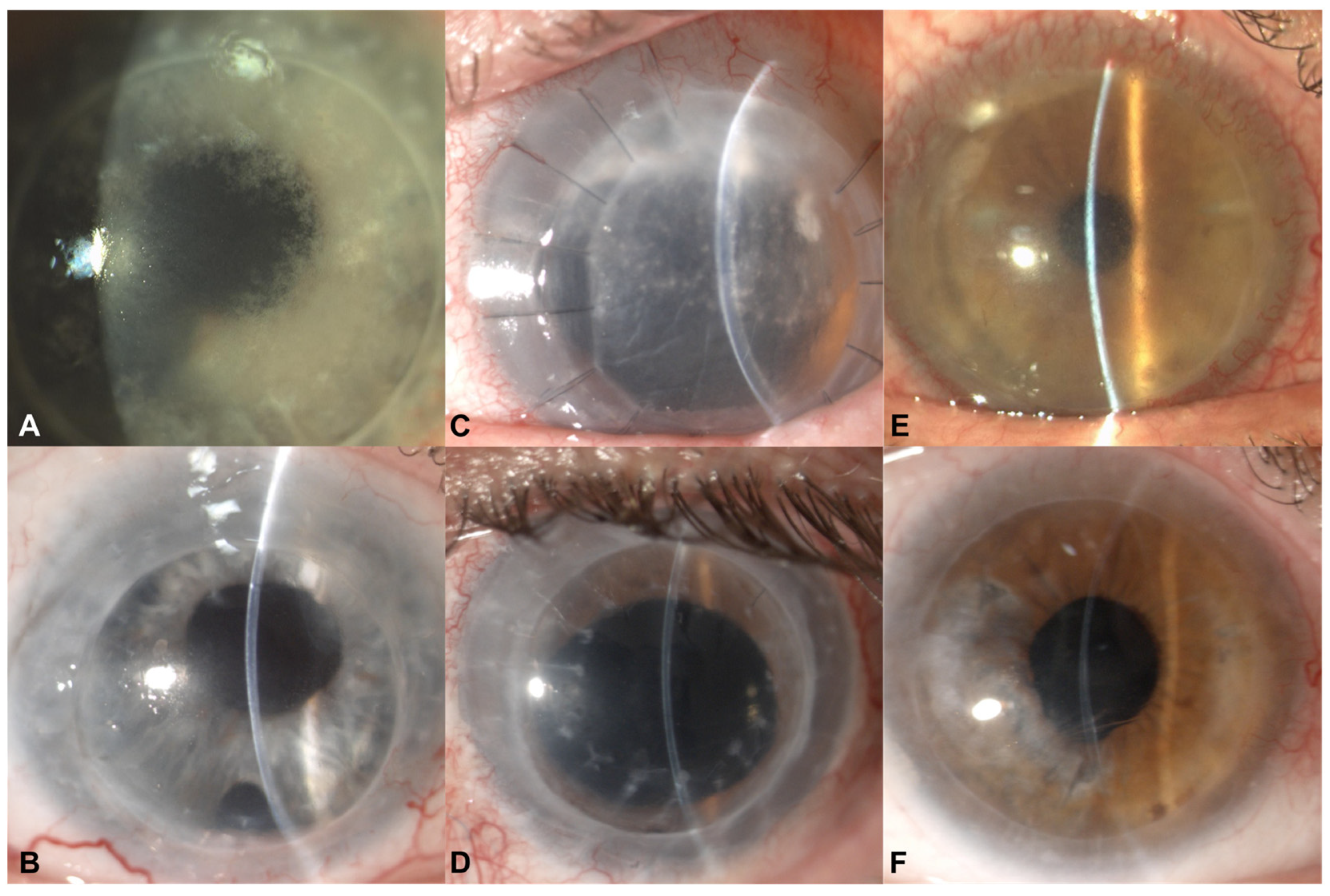
3.1. Case 1
3.2. Case 2
3.3. Case 3
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anane, S.; Attouchi, H. Microsporidiosis: Epidemiology, clinical data and therapy. Gastroenterol. Clin. Biol. 2010, 34, 450–464. [Google Scholar] [CrossRef]
- Han, B.; Weiss, L.M. Microsporidia: Obligate intracellular pathogens within the fungal kingdom. Microbiol. Spectr. 2017, 5, 1–26. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wu, C.L.; Lin, S.H.; Lin, W.C.; Huang, F.C.; Hung, J.H.; Tseng, S.H. Microsporidial stromal keratitis: Characterisation of clinical features, ultrastructural study by electron microscopy and efficacy of different surgical modalities. Br. J. Ophthalmol. 2020, 104, 1613–1620. [Google Scholar] [CrossRef]
- Ang, M.; Mehta, J.S.; Mantoo, S.; Tan, D. Deep anterior lamellar keratoplasty to treat microsporidial stromal keratitis. Cornea 2009, 28, 832–835. [Google Scholar] [CrossRef]
- Sabhapandit, S.; Murthy, S.I.; Garg, P.; Korwar, V.; Vemuganti, G.K.; Sharma, S. Microsporidial stromal keratitis: Clinical features, unique diagnostic criteria, and treatment outcomes in a large case series. Cornea 2016, 35, 1569–1574. [Google Scholar] [CrossRef]
- Matoba, A.; Goosey, J.; Chévez-Barrios, P. Microsporidial Stromal Keratitis: Epidemiological Features, Slit-Lamp Biomicroscopic Characteristics, and Therapy. Cornea 2021, 40, 1532–1540. [Google Scholar] [CrossRef]
- Mittal, R.; Balne, P.K.; Sahu, S.; Das, S.; Sharma, S. Coexistence of herpes simplex virus infection in microsporidial stromal keratitis associated with granulomatous inflammation. Ind. J. Ophthalmol. 2017, 65, 276–281. [Google Scholar] [CrossRef]
- Busin, M.; Spitznas, M. Sustained gentamicin release by presoaked medicated bandage contact lenses. Ophthalmology 1988, 95, 796–798. [Google Scholar] [CrossRef]
- Nahum, Y.; Leon, P.; Ricci-Filipovic, B.A.; Camposampiero, D.; Ponzin, D.; Busin, M. Asymptomatic Infection in Decompensated Full-Thickness Corneal Grafts Referred for Repeat Penetrating Keratoplasty. Cornea 2017, 36, 431–433. [Google Scholar] [CrossRef]
- Joseph, J.; Murthy, S.; Garg, P.; Sharma, S. Use of different stains for microscopic evaluation of corneal scrapings for diagnosis of microsporidial keratitis. J. Clin. Microbiol. 2006, 44, 583–585. [Google Scholar] [CrossRef]
- Sagoo, M.S.; Mehta, J.S.; Hau, S.; Irion, L.D.; Curry, A.; Bonshek, R.E.; Tuft, S.J. Microsporidium stromal keratitis: In vivo confocal findings. Cornea 2007, 26, 870–873. [Google Scholar] [CrossRef]
- Busin, M.; Leon, P.; Nahum, Y.; Scorcia, V. Large (9 mm) Deep Anterior Lamellar Keratoplasty with Clearance of a 6-mm Optical Zone Optimizes Outcomes of Keratoconus Surgery. Ophthalmology 2017, 124, 1072–1080. [Google Scholar] [CrossRef]
- Yu, A.C.; Spena, R.; Pellegrini, M.; Bovone, C.; Busin, M. Deep Anterior Lamellar Keratoplasty: Current Status and Future Directions. Cornea 2022, 41, 539–544. [Google Scholar] [CrossRef]
- Busin, M.; Arffa, R.C.; Zambianchi, L.; Lamberti, G.; Sebastiani, A. Effect of hinged lamellar keratotomy on postkeratoplasty eyes. Ophthalmology 2001, 108, 1845–1851. [Google Scholar] [CrossRef]
- Busin, M.; Halliday, B.L.; Arffa, R.C.; McDonald, M.B.; Kaufman, H.E. Precarved lyophilized tissue for lamellar keratoplasty in recurrent pterygium. Am. J. Ophthalmol. 1986, 102, 222–227. [Google Scholar] [CrossRef]
- Bovone, C.; Nahum, Y.; Scorcia, V.; Giannaccare, G.; Spena, R.; Myerscough, J.; Yu, A.C.; Busin, M. Stromal peeling for deep anterior lamellar keratoplasty in post-penetrating keratoplasty eyes. Br. J. Ophthalmol. 2022, 106, 336–340. [Google Scholar] [CrossRef]
- Scorcia, V.; Giannaccare, G.; Pellegrini, M.; Camposampiero, D.; Ponzin, D.; Yu, A.C.; Busin, M. Stromal peeling for deep anterior lamellar keratoplasty in a post-penetrating keratoplasty eye with hematocornea. Am. J. Ophthalmol. Case Rep. 2023, 29, 101808. [Google Scholar] [CrossRef]
- Busin, M.; Bovone, C.; Scorcia, V.; Rimondi, E.; Nahum, Y.; Myerscough, J.; Yu, A.C. Ultrastructural Alterations of Grafted Corneal Buttons: The Anatomic Basis for Stromal Peeling Along a Natural Plane of Separation. Am. J. Ophthalmol. 2021, 231, 144–153. [Google Scholar] [CrossRef]
- Lucisano, A.; Lionetti, G.; Yu, A.C.; Giannaccare, G.; D’Angelo, S.; Busin, M.; Scorcia, V. Outcomes of Conventional 8.0-mm Versus Large 9.0-mm Diameter Deep Anterior Lamellar Keratoplasty for Keratoconus. Cornea, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Didier, E.S.; Maddry, J.A.; Kwong, C.D.; Green, L.C.; Snowden, K.F.; Shadduk, J.A. Screening of compounds for antimicrosporidial activity in vitro. Folia Parasitol. 1998, 45, 129–139. [Google Scholar]
- Moshirfar, M.; Somani, S.N.; Shmunes, K.M.; Espandar, L.; Gokhale, N.S.; Ronquillo, Y.C.; Hoopes, P.C. A Narrative Review of Microsporidial Infections of the Cornea. Ophthalmol. Ther. 2020, 9, 265–278. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spena, R.; Bovone, C.; Ciarmatori, N.; Pellegrini, M.; Yu, A.C.; Zauli, G.; Busin, M. Microsporidial Stromal Keratitis in Post-Keratoplasty Eyes. J. Clin. Med. 2023, 12, 3706. https://doi.org/10.3390/jcm12113706
Spena R, Bovone C, Ciarmatori N, Pellegrini M, Yu AC, Zauli G, Busin M. Microsporidial Stromal Keratitis in Post-Keratoplasty Eyes. Journal of Clinical Medicine. 2023; 12(11):3706. https://doi.org/10.3390/jcm12113706
Chicago/Turabian StyleSpena, Rossella, Cristina Bovone, Nicolò Ciarmatori, Marco Pellegrini, Angeli Christy Yu, Giorgio Zauli, and Massimo Busin. 2023. "Microsporidial Stromal Keratitis in Post-Keratoplasty Eyes" Journal of Clinical Medicine 12, no. 11: 3706. https://doi.org/10.3390/jcm12113706
APA StyleSpena, R., Bovone, C., Ciarmatori, N., Pellegrini, M., Yu, A. C., Zauli, G., & Busin, M. (2023). Microsporidial Stromal Keratitis in Post-Keratoplasty Eyes. Journal of Clinical Medicine, 12(11), 3706. https://doi.org/10.3390/jcm12113706





