Glucose Levels of the Oral Glucose Tolerance Test (oGTT) Can Predict Adverse Pregnancy Outcomes in Women with Gestational Diabetes (GDM)
Abstract
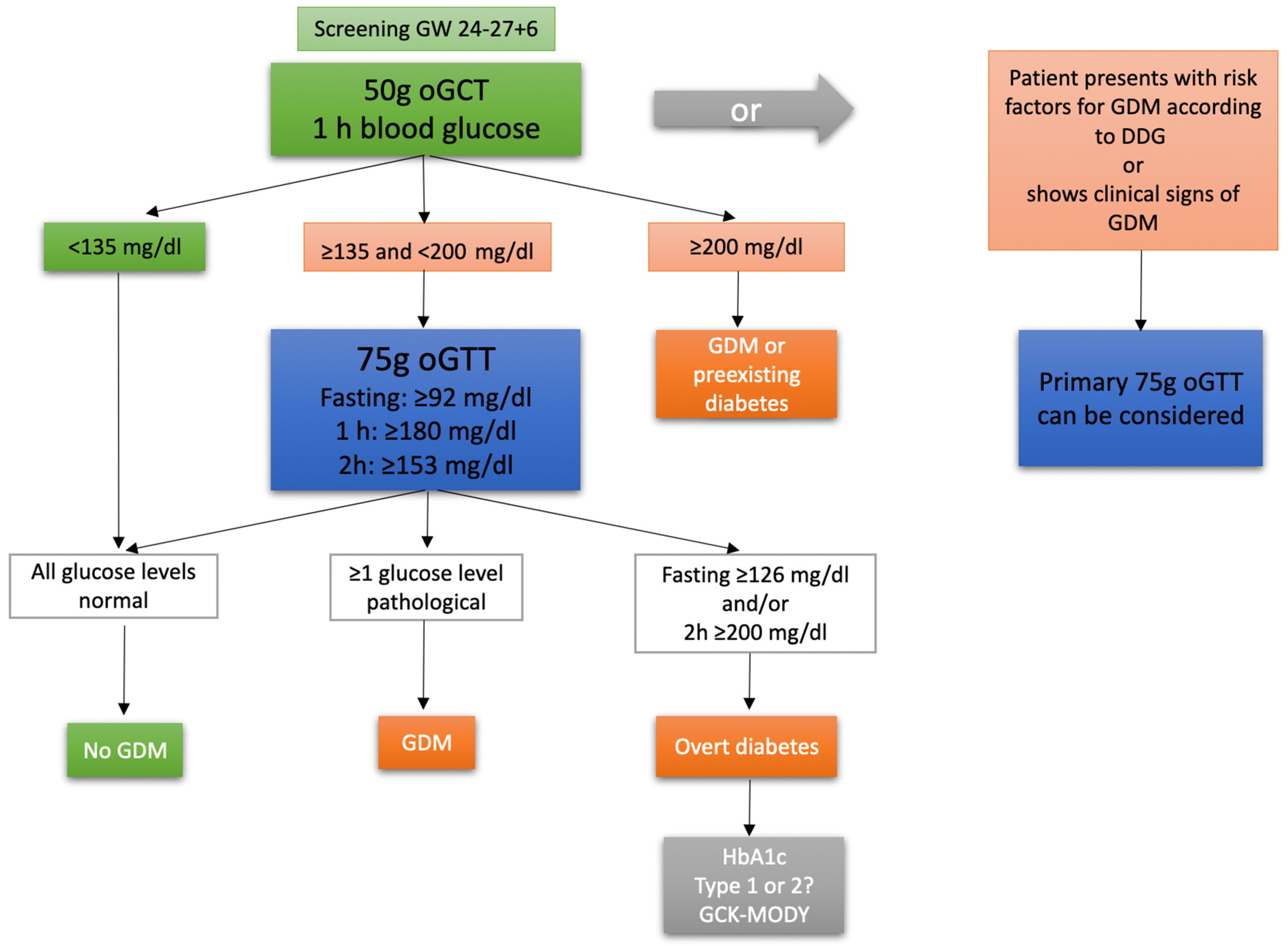
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Characteristics
3.2. Maternal Outcome
3.3. Fetal Outcome
3.4. Effects of Covariates on Fetomaternal Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | gestational diabetes mellitus |
| oGTT | oral glucose tolerance test |
| oGCT | oral glucose challenge test |
| CS | cesarean section |
| GDM-IFH | gestational diabetes with isolated fasting hyperglycemia |
| GDM-IPH | gestational diabetes with isolated postprandial hyperglycemia |
| GDM-CH | gestational diabetes with combined hyperglycemia |
| LGA | large for gestational age |
| IUGR | intrauterine growth retardation |
| SGA | small for gestational age |
| GW | gestational week |
| IUFD | intrauterine fetal death |
References
- Reitzle, L.; Schmidt, C.; Heidemann, C.; Icks, A.; Kaltheuner, M.; Ziese, T.; Scheidt-Nave, C. Gestational diabetes in Germany: Development of screening participation and prevalence. J. Health Monit. 2021, 6, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Graf, U.M.; Gembruch, U.; Kainer, F.; Groten, T.; Hummel, S.; Hösli, I.; Grieshop, M.; Kaltheuner, M.; Bührer, C.; Kautzky-Willer, A.; et al. Gestational Diabetes Mellitus (GDM)—Diagnosis, Treatment and Follow-Up. Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe Frauenheilkd 2018, 78, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Shand, A.W.; Bell, J.C.; McElduff, A.; Morris, J.; Roberts, C.L. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus; a population-based study in New South Wales, Australia, 1998–2002. Diabet. Med. 2008, 25, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Fadl, H.E.; Ostlund, I.K.; Magnuson, A.F.; Hanson, U.S. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet. Med. 2010, 27, 436–441. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. BMJ 2016, 354, i4694. [Google Scholar] [CrossRef]
- Waters, T.P.; Dyer, A.R.; Scholtens, D.M.; Dooley, S.L.; Herer, E.; Lowe, L.P.; Oats, J.J.; Persson, B.; Sacks, D.A.; Metzger, B.E.; et al. Maternal and Neonatal Morbidity for Women Who Would Be Added to the Diagnosis of GDM Using IADPSG Criteria: A Secondary Analysis of the Hyperglycemia and Adverse Pregnancy Outcome Study. Diabetes Care 2016, 39, 2204–2210. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational diabetes mellitus and adverse pregnancy outcomes: Systematic review and meta-analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Tripathy, D.; DeFronzo, R.A. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006, 29, 1130–1139. [Google Scholar] [CrossRef]
- Powe, C.E.; Allard, C.; Battista, M.C.; Doyon, M.; Bouchard, L.; Ecker, J.L.; Perron, P.; Florez, J.C.; Thadhani, R.; Hivert, M.F. Heterogeneous Contribution of Insulin Sensitivity and Secretion Defects to Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1052–1055. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Meng, X.; Zhao, W.; Pan, J.; Tang, J.; Huang, Y.; Tao, M.; Liu, F. Heterogeneity of insulin resistance and beta cell dysfunction in gestational diabetes mellitus: A prospective cohort study of perinatal outcomes. J. Transl. Med. 2018, 16, 289. [Google Scholar] [CrossRef]
- Benhalima, K.; Van Crombrugge, P.; Moyson, C.; Verhaeghe, J.; Vandeginste, S.; Verlaenen, H.; Vercammen, C.; Maes, T.; Dufraimont, E.; De Block, C.; et al. Characteristics and pregnancy outcomes across gestational diabetes mellitus subtypes based on insulin resistance. Diabetologia 2019, 62, 2118–2128. [Google Scholar] [CrossRef]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 2010, 33, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Zawiejska, A.; Wender-Ozegowska, E.; Radzicka, S.; Brazert, J. Maternal hyperglycemia according to IADPSG criteria as a predictor of perinatal complications in women with gestational diabetes: A retrospective observational study. J. Matern. Fetal Neonatal Med. 2014, 27, 1526–1530. [Google Scholar] [CrossRef]
- Kotzaeridi, G.; Blätter, J.; Eppel, D.; Rosicky, I.; Linder, T.; Geissler, F.; Huhn, E.A.; Hösli, I.; Tura, A.; Göbl, C.S. Characteristics of gestational diabetes subtypes classified by oral glucose tolerance test values. Eur. J. Clin. Investig. 2021, 51, e13628. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Harrison, C.L.; Teh, W.T.; Paul, E.; Allan, C.A. Gestational diabetes: Development of an early risk prediction tool to facilitate opportunities for prevention. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 499–504. [Google Scholar] [CrossRef]
- Torloni, M.R.; Betrán, A.P.; Horta, B.L.; Nakamura, M.U.; Atallah, A.N.; Moron, A.F.; Valente, O. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes. Rev. 2009, 10, 194–203. [Google Scholar] [CrossRef]
- Syngelaki, A.; Pastides, A.; Kotecha, R.; Wright, A.; Akolekar, R.; Nicolaides, K.H. First-Trimester Screening for Gestational Diabetes Mellitus Based on Maternal Characteristics and History. Fetal. Diagn. Ther. 2015, 38, 14–21. [Google Scholar] [CrossRef]
- González-Quintero, V.H.; Istwan, N.B.; Rhea, D.J.; Tudela, C.M.; Flick, A.A.; de la Torre, L.; Stanziano, G.J. Antenatal factors predicting subsequent need for insulin treatment in women with gestational diabetes. J. Women’s Health 2008, 17, 1183–1187. [Google Scholar] [CrossRef]
- Pertot, T.; Molyneaux, L.; Tan, K.; Ross, G.P.; Yue, D.K.; Wong, J. Can common clinical parameters be used to identify patients who will need insulin treatment in gestational diabetes mellitus? Diabetes Care 2011, 34, 2214–2216. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Jalaludin, B. Gestational diabetes mellitus: Who requires insulin therapy? Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.A.; Wong, T.; Ross, G.P.; Jalaludin, B.B.; Wong, V.W.; Smart, C.E.; Collins, C.E.; MacDonald-Wicks, L.; Flack, J.R. A novel validated model for the prediction of insulin therapy initiation and adverse perinatal outcomes in women with gestational diabetes mellitus. Diabetologia 2016, 59, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Uvena-Celebrezze, J.; Fung, C.; Thomas, A.J.; Hoty, A.; Huston-Presley, L.; Amini, S.B.; Catalano, P.M. Relationship of neonatal body composition to maternal glucose control in women with gestational diabetes mellitus. J. Matern. Fetal Neonatal Med. 2002, 12, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Schreiber, H.; Shalev Ram, H.; Ovadia, M.; Shechter-Maor, G.; Biron-Shental, T. Can We Predict Feto-Maternal Adverse Outcomes of Vacuum Extraction? Geburtshilfe Frauenheilkd 2022, 82, 1274–1282. [Google Scholar] [CrossRef]
- Ramos, S.Z.; Waring, M.E.; Leung, K.; Amir, N.S.; Bannon, A.L.; Moore Simas, T.A. Attempted and Successful Vacuum-Assisted Vaginal Delivery by Prepregnancy Body Mass Index. Obstet. Gynecol. 2017, 129, 311–320. [Google Scholar] [CrossRef]
- Athukorala, C.; Crowther, C.A.; Willson, K. Women with gestational diabetes mellitus in the ACHOIS trial: Risk factors for shoulder dystocia. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 37–41. [Google Scholar] [CrossRef]



| Subtype | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM-IFH (n = 553) | GDM-IPH (n = 418) | GDM-CH (n = 693) | |||||||||||
| Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | ||
| Maternal age | 31.89a | 5.78 | 32.62b | 5.37 | 32.70b | 5.40 | |||||||
| Gravida | 2.88a | 1.81 | 2.92a | 2.06 | 3.32b | 1.99 | |||||||
| Para | 1.31a | 1.44 | 1.26a | 1.53 | 1.69b | 1.52 | |||||||
| Parity | Nullipara | 187a | 33.8% | 166a | 39.7% | 173b | 25.0% | ||||||
| ≥Primipara | 366a | 66.2% | 252a | 60.3% | 518b | 75.0% | |||||||
| Preconceptional BMI | 28, 63a | 6,46 | 26, 10b | 5,33 | 29, 69c | 6, 33 | |||||||
| 50 g oGCT | yes | 243a | 43.9% | 252b | 60.3% | 410b | 59.2% | ||||||
| no | 310a | 56.1% | 166b | 39.7% | 283b | 40.8% | |||||||
| GW at 50 g oGCT | 25, 62a | 2.18 | 25, 89a | 2.11 | 25, 85a | 2.38 | |||||||
| Glucose 50 g oGCT in mg/dL | 144, 75a | 18.67 | 157, 69b | 21.71 | 166, 37c | 30.95 | |||||||
| 50 g oGCT ≥ 135 mg/dL | yes | 198a | 85.7% | 225b | 93.4% | 368b | 93.9% | ||||||
| no | 33a | 14.3% | 16b | 6.6% | 24b | 6.1% | |||||||
| GW at 75 g oGTT | 25, 78a | 4.67 | 27, 23b | 3.76 | 26, 53c | 4.27 | |||||||
| 75 g oGTT | before GW 24+0 | 73a | 13.2% | 30b | 7.2% | 82a | 11.8% | ||||||
| after GW 24+0 | 480a | 86.8% | 388b | 92.8% | 611a | 88.2% | |||||||
| Fasting glucose in mg/dL | 99, 02a | 8.35 | 83, 77b | 5.97 | 105, 84c | 14.95 | |||||||
| Glucose after 1 h in mg/dL | 145, 63a | 22.63 | 188, 11b | 22.31 | 204, 98c | 32.01 | |||||||
| Glucose after 2 h in mg/dL | 116, 16a | 19.93 | 147, 28b | 31.28 | 158, 80c | 36.67 | |||||||
| GW at first presentation | 30, 22a | 4.76 | 30, 72a | 4.42 | 30, 39a | 4.97 | |||||||
| Subtype | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM-IFH (n = 553) | GDM-IPH (n = 418) | GDM-CH (n = 693) | |||||||||||
| Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | ||
| Weight gain | 12.17a | 7.05 | 11.24b | 6.23 | 10.90b | 6.43 | |||||||
| Insulin | long acting insulin only | 72a | 13.0% | 22b | 5.3% | 135c | 19.5% | ||||||
| combined insulin | 21a | 3.8% | 6b | 1.4% | 89c | 12.8% | |||||||
| short acting insulin only | 4a | 0.7% | 9a | 2.2% | 13a | 1.9% | |||||||
| no | 456a | 82.5% | 381b | 91.1% | 456c | 65.8% | |||||||
| Metformin | yes | 1a | 0.2% | 02 | 0.0% | 1a | 0.1% | ||||||
| no | 552a | 99.8% | 4182 | 100.0% | 692a | 99.9% | |||||||
| Preeclampsia | yes | 18a | 3.3% | 7a | 1.7% | 26a | 3.8% | ||||||
| no | 535a | 96.7% | 411a | 98.3% | 667a | 96.2% | |||||||
| HELLP | yes | 2a | 0.4% | 1a | 0.2% | 1a | 0.1% | ||||||
| no | 551a | 99.6% | 417a | 99.8% | 692a | 99.9% | |||||||
| Delivery mode | Vaginal | 279a | 50.5% | 200a | 47.8% | 355a | 51.3% | ||||||
| Vacuum extraction | 45a,b | 8.1% | 41a | 9.8% | 38b | 5.5% | |||||||
| Primary CS | 137a | 24.8% | 76b | 18.2% | 149a,b | 21.5% | |||||||
| Emergent CS | 92a | 16.6% | 100b | 23.9% | 150b | 21.7% | |||||||
| Forceps extraction | 0 | 0.0% | 1 | 0.2% | 0 | 0.0% | |||||||
| Shoulder dystocia | yes | 4a | 1.2% | 0 | 0.0% | 8a | 2.0% | ||||||
| no | 320a | 98.8% | 242 | 100.0% | 385a | 98.0% | |||||||
| Perineal tear | yes | 114a | 35.2% | 95a | 39.3% | 133a | 33.8% | ||||||
| no | 210a | 64.8% | 147a | 60.7% | 260a | 66.2% | |||||||
| Third degree perinealtear | yes | 6a | 1.9% | 3a | 1.2% | 4a | 1.0% | ||||||
| no | 318a | 98.1% | 239a | 98.8% | 389a | 99.0% | |||||||
| Episiotomy | yes | 27a | 8.3% | 28a | 11.6% | 35a | 8.9% | ||||||
| no | 297a | 91.7% | 214a | 88.4% | 358a | 91.1% | |||||||
| Blood loss in mL | 443a | 257 | 450a | 373 | 433a | 257 | |||||||
| Blood loss ≥1000 mL | yes | 26a | 4.8% | 18a | 4.5% | 28a | 4.2% | ||||||
| no | 516a | 95.2% | 385a | 95.5% | 645a | 95.8% | |||||||
| Blood loss ≥1500 mL | yes | 10a | 1.8% | 9a | 2.2% | 8a | 1.2% | ||||||
| no | 532a | 98.2% | 394a | 97.8% | 665a | 98.8% | |||||||
| Postpartum bleeding | yes | 27a | 4.9% | 22a | 5.3% | 38a | 5.5% | ||||||
| no | 526a | 95.1% | 396a | 94.7% | 655a | 94.5% | |||||||
| Subtype | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GDM-IFH (n = 553) | GDM-IPH (n = 418) | GDM-CH (n = 693) | |||||||||||
| n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | ||
| Sex of fetus | male | 305a | 55.2% | 234a | 56.3% | 372a | 54.0% | ||||||
| female | 248a | 44.8% | 182a | 43.8% | 317a | 46.0% | |||||||
| GW at delivery | 39.70a | 1.50 | 39.51a,b | 2.08 | 39.41b | 1.92 | |||||||
| Prematurity <37 GW | yes | 16a | 2.9% | 25a | 6.0% | 35a | 5.1% | ||||||
| no | 537a | 97.1% | 393a | 94.0% | 657a | 94.9% | |||||||
| Birthweight | 3470.71a | 556.88 | 3327.59b | 609.55 | 3460.21a | 605.72 | |||||||
| Birthweight percentile | 54.68a | 30.16 | 49.43b | 29.52 | 57.51a | 29.65 | |||||||
| IUGR | yes | 9a | 1.6% | 13a | 3.1% | 9a | 1.3% | ||||||
| no | 543a | 98.4% | 404a | 96.9% | 682a | 98.7% | |||||||
| SGA | yes | 42a | 7.6% | 48b | 11.5% | 49a | 7.1% | ||||||
| no | 510a | 92.4% | 369b | 88.5% | 642a | 92.9% | |||||||
| Low fetal weight | yes | 145a,b | 26.3% | 128a | 30.7% | 149b | 21.6% | ||||||
| no | 407a,b | 73.7% | 289a | 69.3% | 542b | 78.4% | |||||||
| LGA | yes | 68a | 12.3% | 27b | 6.5% | 93a | 13.5% | ||||||
| no | 484a | 87.7% | 390b | 93.5% | 598a | 86.5% | |||||||
| APGAR at 1 min | 8.62a | 1.20 | 8.64a | 1.03 | 8.49a | 1.38 | |||||||
| APGAR at 5 min | 9.56a | 0.91 | 9.49a | 0.96 | 9.43a | 1.17 | |||||||
| APGAR at 10 min | 9.81a | 0.66 | 9.75a | 0.77 | 9.70a | 1.01 | |||||||
| Cord pH | 7.24a | 0.07 | 7.24a | 0.07 | 7.24a | .07 | |||||||
| Base excess | −4.44a | 2.96 | −4.38a | 3.25 | −4.36a | 3.24 | |||||||
| NICU | yes | 28a | 5.1% | 36a | 8.6% | 45a | 6.5% | ||||||
| no | 525a | 94.9% | 382a | 91.4% | 648a | 93.5% | |||||||
| IUFD | yes | 1a | 0.2% | 0 | 0.0% | 5a | 0.7% | ||||||
| no | 552a | 99.8% | 418 | 100.0% | 687a | 99.3% | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balke, S.; Weid, P.; Fangmann, L.; Rostin, P.; Henrich, W.; Koenigbauer, J.T. Glucose Levels of the Oral Glucose Tolerance Test (oGTT) Can Predict Adverse Pregnancy Outcomes in Women with Gestational Diabetes (GDM). J. Clin. Med. 2023, 12, 3709. https://doi.org/10.3390/jcm12113709
Balke S, Weid P, Fangmann L, Rostin P, Henrich W, Koenigbauer JT. Glucose Levels of the Oral Glucose Tolerance Test (oGTT) Can Predict Adverse Pregnancy Outcomes in Women with Gestational Diabetes (GDM). Journal of Clinical Medicine. 2023; 12(11):3709. https://doi.org/10.3390/jcm12113709
Chicago/Turabian StyleBalke, Selina, Petra Weid, Laura Fangmann, Paul Rostin, Wolfgang Henrich, and Josefine Theresia Koenigbauer. 2023. "Glucose Levels of the Oral Glucose Tolerance Test (oGTT) Can Predict Adverse Pregnancy Outcomes in Women with Gestational Diabetes (GDM)" Journal of Clinical Medicine 12, no. 11: 3709. https://doi.org/10.3390/jcm12113709
APA StyleBalke, S., Weid, P., Fangmann, L., Rostin, P., Henrich, W., & Koenigbauer, J. T. (2023). Glucose Levels of the Oral Glucose Tolerance Test (oGTT) Can Predict Adverse Pregnancy Outcomes in Women with Gestational Diabetes (GDM). Journal of Clinical Medicine, 12(11), 3709. https://doi.org/10.3390/jcm12113709






