Office-Visit Heart Rate and Long-Term Cardiovascular Events in Patients with Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Data Collection
2.2. PCI Procedure and Medical Treatment
2.3. Study Endpoints and Definitions
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
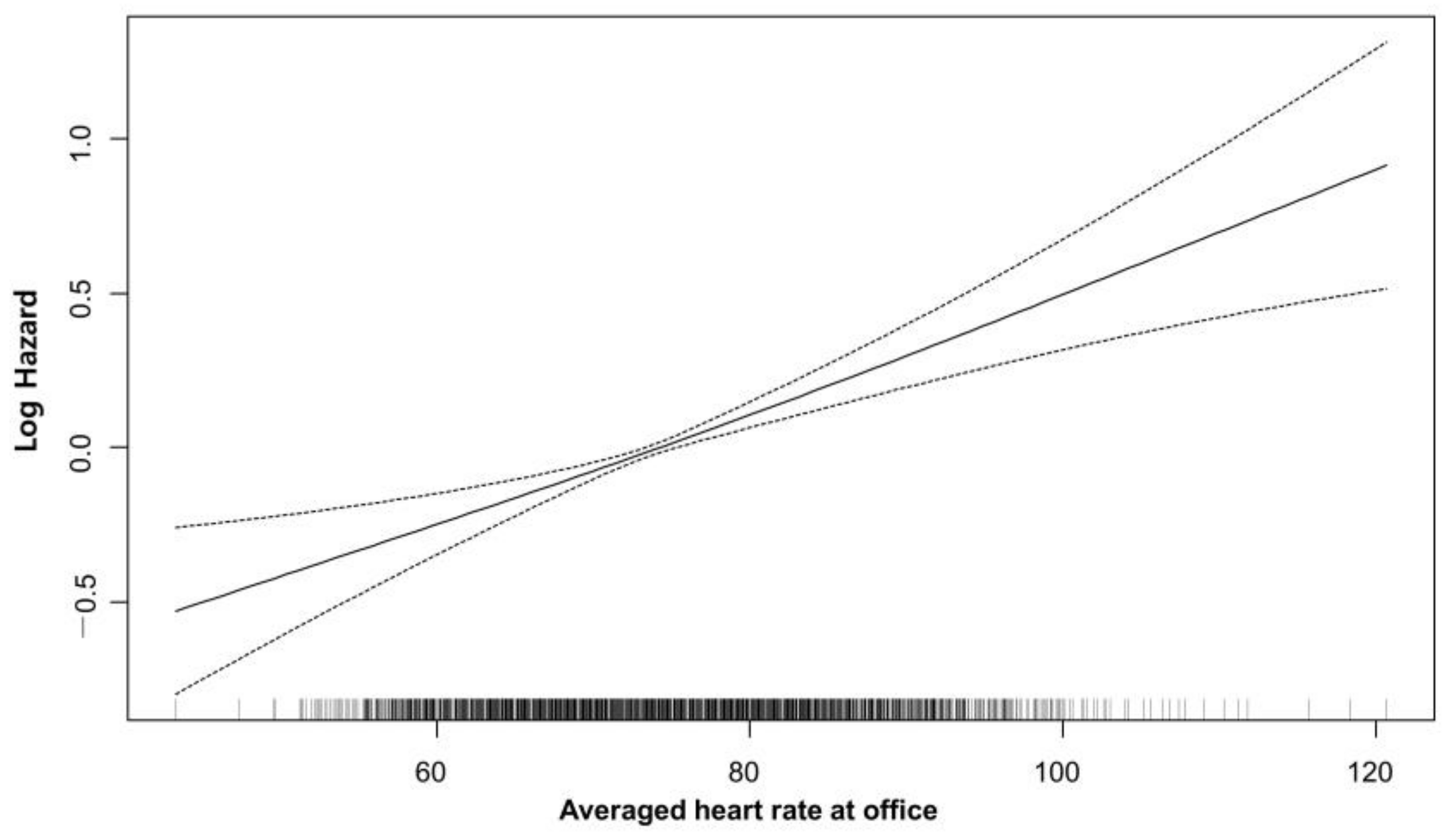
3.2. Average Heart Rate and Cardiovascular Outcome
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolloch, R.; Legler, U.F.; Champion, A.; Cooper-Dehoff, R.M.; Handberg, E.; Zhou, Q.; Pepine, C.J. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: Findings from the INternational VErapamil-SR/trandolapril STudy (INVEST). Eur. Heart J. 2008, 29, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Lechat, P.; Hulot, J.S.; Escolano, S.; Mallet, A.; Leizorovicz, A.; Werhlen-Grandjean, M.; Pochmalicki, G.; Dargie, H. Heart rate and cardiac rhythm relationships with bisoprolol benefit in chronic heart failure in CIBIS II Trial. Circulation 2001, 103, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Bourassa, M.G.; Guertin, M.C.; Tardif, J.C. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur. Heart J. 2005, 26, 967–974. [Google Scholar] [CrossRef]
- Morrow, D.A.; Antman, E.M.; Charlesworth, A.; Cairns, R.; Murphy, S.A.; de Lemos, J.A.; Giugliano, R.P.; McCabe, C.H.; Braunwald, E. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000, 102, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Seronde, M.F.; Geha, R.; Puymirat, E.; Chaib, A.; Simon, T.; Berard, L.; Drouet, E.; Bataille, V.; Danchin, N.; Schiele, F. Discharge heart rate and mortality after acute myocardial infarction. Am. J. Med. 2014, 127, 954–962. [Google Scholar] [CrossRef]
- Antoni, M.L.; Boden, H.; Delgado, V.; Boersma, E.; Fox, K.; Schalij, M.J.; Bax, J.J. Relationship between discharge heart rate and mortality in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Eur. Heart J. 2012, 33, 96–102. [Google Scholar] [CrossRef]
- Kosmidou, I.; McAndrew, T.; Redfors, B.; Embacher, M.; Dizon, J.M.; Mehran, R.; Ben-Yehuda, O.; Mintz, G.S.; Stone, G.W. Correlation of Admission Heart Rate With Angiographic and Clinical Outcomes in Patients With Right Coronary Artery ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: HORIZONS-AMI (The Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) Trial. J. Am. Heart Assoc. 2017, 6, e006181. [Google Scholar]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Dargie, H.J. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001, 357, 1385–1390. [Google Scholar]
- Packer, M.; Bristow, M.R.; Cohn, J.N.; Colucci, W.S.; Fowler, M.B.; Gilbert, E.M.; Shusterman, N.H. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N. Engl. J. Med. 1996, 334, 1349–1355. [Google Scholar] [CrossRef]
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999, 353, 9–13. [CrossRef]
- Goldberger, J.J.; Bonow, R.O.; Cuffe, M.; Liu, L.; Rosenberg, Y.; Shah, P.K.; Smith, S.C., Jr.; Subačius, H. Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2015, 66, 1431–1441. [Google Scholar] [CrossRef]
- Hwang, D.; Lee, J.M.; Kim, H.K.; Choi, K.H.; Rhee, T.M.; Park, J.; Park, T.K.; Yang, J.H.; Song, Y.B.; Choi, J.H.; et al. Prognostic Impact of β-Blocker Dose After Acute Myocardial Infarction. Circ. J. 2019, 83, 410–417. [Google Scholar] [CrossRef]
- Perne, A.; Schmidt, F.P.; Hochadel, M.; Giannitsis, E.; Darius, H.; Maier, L.S.; Schmitt, C.; Heusch, G.; Voigtländer, T.; Mudra, H.; et al. Admission heart rate in relation to presentation and prognosis in patients with acute myocardial infarction. Treatment regimens in German chest pain units. Herz 2016, 41, 233–240. [Google Scholar] [CrossRef]
- Alapati, V.; Tang, F.; Charlap, E.; Chan, P.S.; Heidenreich, P.A.; Jones, P.G.; Spertus, J.A.; Srinivas, V.; Kizer, J.R. Discharge Heart Rate After Hospitalization for Myocardial Infarction and Long-Term Mortality in 2 US Registries. J. Am. Heart Assoc. 2019, 8, e010855. [Google Scholar] [CrossRef]
- Gillman, M.W.; Kannel, W.B.; Belanger, A.; D’Agostino, R.B. Influence of heart rate on mortality among persons with hypertension: The Framingham Study. Am. Heart J. 1993, 125, 1148–1154. [Google Scholar] [CrossRef]
- Lonn, E.M.; Rambihar, S.; Gao, P.; Custodis, F.F.; Sliwa, K.; Teo, K.K.; Yusuf, S.; Böhm, M. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: An analysis of ONTARGET/TRANSCEND. Clin. Res. Cardiol. 2014, 103, 149–159. [Google Scholar] [CrossRef]
- Vazir, A.; Claggett, B.; Jhund, P.; Castagno, D.; Skali, H.; Yusuf, S.; Swedberg, K.; Granger, C.B.; McMurray, J.J.; Pfeffer, M.A.; et al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: An analysis of the CHARM program. Eur. Heart J. 2015, 36, 669–675. [Google Scholar] [CrossRef]
- Granger, C.B.; Goldberg, R.J.; Dabbous, O.; Pieper, K.S.; Eagle, K.A.; Cannon, C.P.; Van De Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch. Intern. Med. 2003, 163, 2345–2353. [Google Scholar] [CrossRef]
- Choo, E.H.; Chang, K.; Ahn, Y.; Jeon, D.S.; Lee, J.M.; Kim, D.B.; Her, S.H.; Park, C.S.; Kim, H.Y.; Yoo, K.D.; et al. Benefit of β-blocker treatment for patients with acute myocardial infarction and preserved systolic function after percutaneous coronary intervention. Heart 2014, 100, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA 1982, 247, 1707–1714. [CrossRef] [PubMed]
- Cucherat, M. Quantitative relationship between resting heart rate reduction and magnitude of clinical benefits in post-myocardial infarction: A meta-regression of randomized clinical trials. Eur. Heart J. 2007, 28, 3012–3019. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Feng, D.; Wang, L.; Yang, X.; Su, P.; Li, K.; Chen, M. First post-discharge heart rate and long-term prognosis in patients with acute myocardial infarction. Rev. Cardiovasc. Med. 2022, 23, 24. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Subačius, H.; Marroquin, O.C.; Beau, S.L.; Simonson, J. One-Year Landmark Analysis of the Effect of Beta-Blocker Dose on Survival After Acute Myocardial Infarction. J. Am. Heart Assoc. 2021, 10, e019017. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Böhm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Fox, K.; Ford, I.; Steg, P.G.; Tendera, M.; Ferrari, R. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 807–816. [Google Scholar] [CrossRef]
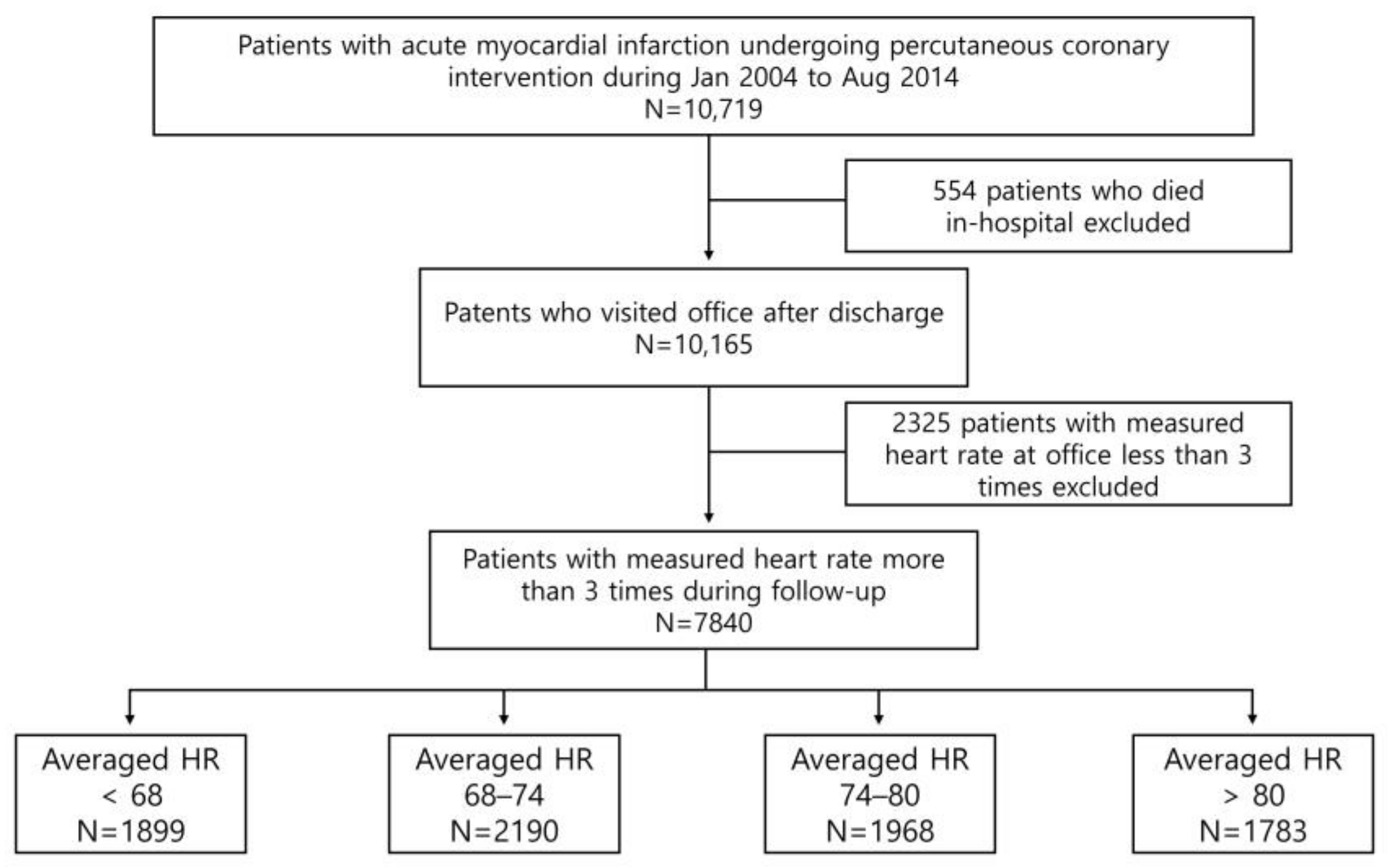


| Total (n = 7840) | <68 bpm (n = 1899) | 68–74 bpm (n = 2190) | 74–80 bpm (n = 1968) | >80 bpm (n = 1783) | p Value | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, y | 61.9 ± 12 | 63 ± 12 | 62 ± 12 | 61 ± 13 | 62 ± 12 | <0.001 |
| Male sex, n (%) | 5825 (74.3%) | 1463 (77.0%) | 1630 (74.4%) | 1445 (73.4%) | 1287 (72.2%) | 0.006 |
| Initial BMI, kg/m2 | 24.33 ± 3.19 | 24.26 ± 3.04 | 24.44 ± 3.17 | 24.38 ± 3.14 | 24.21 ± 3.42 | 0.097 |
| Hypertension, n (%) | 3947 (50.3%) | 969 (51.0%) | 1109 (50.6%) | 972 (49.4%) | 897 (50.3%) | 0.766 |
| Diabetes mellitus, n (%) | 2317 (29.6%) | 397 (20.9%) | 593 (27.1%) | 604 (30.7%) | 723 (40.5%) | <0.001 |
| Hypercholesterolemia, n (%) | 1343 (17.1%) | 341 (18.0%) | 388 (17.7%) | 338 (17.2%) | 276 (15.5%) | 0.182 |
| Currently smoke, n (%) | 3319 (42.3%) | 783 (41.2%) | 890 (40.6%) | 854 (43.4%) | 792 (44.4%) | 0.055 |
| Family history of CAD, n (%) | 240 (3.1%) | 55 (2.9%) | 70 (3.2%) | 64 (3.3%) | 51 (2.9%) | 0.851 |
| Prior MI | 291 (3.7%) | 2 (2.8%) | 84 (3.8%) | 78 (4.0%) | 76 (4.3%) | 0.091 |
| Prior CABG | 36 (0.5%) | 8 (0.4%) | 10 (0.5%) | 8 (0.4%) | 10 (0.6%) | 0.900 |
| Prior PCI | 512 (6.5%) | 113 (6.0%) | 142 (6.5%) | 129 (6.6%) | 128 (7.2%) | 0.516 |
| Prior stroke, n (%) (or CVA) | 496 (6.3%) | 113 (6.0%) | 135 (6.2%) | 120 (6.1%) | 128 (7.2%) | 0.406 |
| Atrial arrhythmia, n (%) | 187 (2.4%) | 40 (2.1%) | 46 (2.1%) | 46 (2.3%) | 55 (3.1%) | 0.16 |
| Peripheral arterial disease, n (%) | 38 (0.5%) | 11 (0.6%) | 9 (0.4%) | 8 (0.4%) | 10 (0.6%) | 0.786 |
| Chronic lung disease, n (%) (or COPD) | 154 (2.0%) | 28 (1.5%) | 34 (1.6%) | 31 (1.6%) | 61 (3.4%) | <0.001 |
| Chronic renal failure, n (%) | 118 (1.5%) | 25 (1.3%) | 30 (1.4%) | 27 (1.4%) | 36 (2.0%) | 0.247 |
| Cancer, n (%) | 254 (3.2%) | 56 (2.9%) | 60 (2.7%) | 68 (3.4%) | 69 (3.8%) | 0.185 |
| Clinical | ||||||
| Initial ECG diagnosis as STEMI, n (%) | 4258 (54.3%) | 988 (52.0%) | 1189 (54.3%) | 1102 (56.0%) | 979 (54.9%) | 0.076 |
| Initial systolic BP, mmHg | 129.2 ± 26.4 | 129.9 ± 26.3 | 129.6 ± 25.8 | 129.2 ± 27.0 | 127.9 ± 26.6 | 0.123 |
| Initial diastolic BP, mmHg | 78.9 ± 16.3 | 78.9 ± 15.9 | 78.9 ± 16.0 | 79.0 ± 16.8 | 78.8 ± 16.8 | 0.966 |
| Heart rate at admission, b.p.m | 78.9 ± 16.3 | 73.1 ± 16.7 | 76.7 ± 17.0 | 79.6 ± 18.2 | 83.3 ± 19.2 | <0.001 |
| Heart rate at discharge, b.p.m | 72 ± 8.6 | 67 ± 7.3 | 70.9 ± 7.2 | 73.2 ± 7.5 | 77.2 ± 9.3 | <0.001 |
| Killip class, n (%) | 0.001 | |||||
| I | 5720 (79.7%) | 1452 (82.0%) | 1628 (80.7%) | 1403 (78.3%) | 1237 (77.2%) | |
| II | 573 (8.0%) | 133 (7.5%) | 166 (8.2%) | 145 (8.1%) | 129 (8.1%) | |
| III | 365 (5.1%) | 68 (3.8%) | 87 (4.3%) | 99 (5.5%) | 111 (6.9%) | |
| IV | 522 (7.3%) | 117 (6.6%) | 136 (6.7%) | 144 (8.0%) | 125 (7.8%) | |
| eGFR, mL/min per 1.73 m2 | 79.1 ± 24.0 | 79.6 ± 23.1 | 80.1 ± 23.3 | 79.8 ± 23.9 | 76.7 ± 25.8 | <0.001 |
| CK-MB, peak | 121.53 ± 225 | 117 ± 181 | 114 ± 165 | 128 ± 321 | 128 ± 204 | 0.098 |
| LV systolic function, EF (%) | 53.8 ± 10.8 | 55.6 ± 9.9 | 54.5 ± 10.9 | 53.2 ± 10.7 | 51.7 ± 11.1 | <0.001 |
| Cardiogenic shock | 232 (3.0%) | 52 (2.7%) | 69 (3.2%) | 61 (3.1%) | 50 (2.8%) | 0.830 |
| Culprit vessel | ||||||
| Left main | 205 (2.6%) | 44 (2.3%) | 56 (2.6%) | 45 (2.3%) | 60 (3.4%) | |
| LAD | 3718 (47.4%) | 888 (46.8%) | 1029 (47.0%) | 940 (47.8%) | 861 (48.3%) | |
| LCX | 1324 (16.9%) | 328 (17.3%) | 356 (16.3%) | 342 (17.4%) | 298 (16.7%) | |
| RCA | 2578 (32.9%) | 634 (33.4%) | 745 (34.0%) | 639 (32.5%) | 560 (31.4%) | |
| Total stent number | 1.56 ± 0.8 | 1.5 ± 0.9 | 1.6 ± 0.9 | 1.5 ± 0.9 | 1.6 ± 1.0 | 0.003 |
| Mean stent diameter | 3.17 ± 0.41 | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 | 0.032 |
| Total stent length | 34.0 ± 20.7 | 32.4 ± 19.5 | 34.1 ± 20.7 | 34.0 ± 20.5 | 35.7 ± 22.3 | <0.001 |
| Discharge medications | ||||||
| Aspirin | 7737 (98.7%) | 1881 (99.1%) | 2162 (98.7%) | 1940 (98.6%) | 1754 (98.4%) | 0.320 |
| P2Y12 inhibitor | 7015 (89.4%) | 1896 (99.8%) | 2185 (99.7%) | 1964 (99.7%) | 1771 (99.3%) | 0.271 |
| Beta-blocker | 6568 (94.7%) | 1639 (95.9%) | 1884 (95.0%) | 1644 (94.5%) | 1401 (93.2%) | 0.007 |
| RAA blocker | 6168 (78.6%) | 1547 (81.5%) | 1766 (80.6%) | 1560 (79.3% | 1295 (72.6%) | <0.001 |
| Statin | 7411 (94.5%) | 1762 (97.6%) | 2015 (97.0%) | 1811 (97.1%) | 1599 (96.1%) | 0.070 |
| Follow-up | ||||||
| Follow-up mean systolic BP, mm Hg | 123.0 ± 10.7 | 123.7 ± 10.8 | 123.2 ± 10.5 | 123.2 ± 10.6 | 122.1 ± 11.1 | <0.001 |
| Follow-up mean diastolic BP, mm Hg | 73.2 ± 7.1 | 72.1 ± 7.1 | 73.2 ± 6.9 | 74.0 ± 7.1 | 73.7 ± 7.2 | <0.001 |
| Averaged heart rate at office, b.p.m | 74.03 ± 8.42 | 63.8 ± 3.4 | 71.0 ± 1.7 | 76.7 ± 1.7 | 85.7 ± 5.2 | <0.001 |
| Heart rate measurement count | 7.1± 3.0 | 7.2 ± 3.1 | 7.4 ± 3.1 | 7.2 ± 3.0 | 6.7 ± 3.1 | <0.001 |
| Heart rate measurement interval, days | 349.5 ± 268.9 | 350.6 ± 267.8 | 320.7 ± 231.5 | 352.7 ± 270.6 | 373.2 ± 306.0 | <0.001 |
| Total (n = 7840) | <68 bpm (n = 1899) | 68–74 bpm (n = 2190) | 74–80 bpm (n = 1968) | >80 bpm (n = 1783) | p Value | |
|---|---|---|---|---|---|---|
| MACE | 1357 (17.3%) | 261 (13.7%) | 344 (15.7%) | 345 (17.5%) | 407 (22.8%) | <0.0001 |
| Cardiovascular death | 781 (10.0%) | 127 (6.7%) | 197 (9.0%) | 196 (10.0%) | 261 (14.6%) | <0.0001 |
| Myocardial infarction | 474 (6.0%) | 102 (5.4%) | 113 (5.2%) | 126 (6.4%) | 133 (7.5%) | 0.010 |
| Ischemic stroke | 272 (3.5%) | 59 (3.1%) | 72 (3.3%) | 64 (3.3%) | 77 (4.3%) | 0.166 |
| Crude HR (95%CI) | Crude p Value | Adj. HR (95%CI) | Adj. p Value | |
|---|---|---|---|---|
| Averaged heart rate at office (bpm) | ||||
| 68–74 | Reference | |||
| <68 | 0.91 (0.77–1.07) | 0.242 | 0.91 (0.77–1.07) | 0.246 |
| 74–80 | 1.13 (0.97–1.31) | 0.116 | 1.12 (0.96–1.3) | 0.135 |
| >80 | 1.51 (1.31–1.74) | <0.001 | 1.39 (1.2–1.61) | <0.001 |
| Age | 1.05 (1.05–1.06) | <0.001 | 1.04 (1.04–1.05) | <0.001 |
| Body mass index | 0.93 (0.91–0.95) | <0.001 | 0.98 (0.96–0.99) | 0.009 |
| Killip class | ||||
| 1 | Reference | |||
| 2 | 1.64 (1.36–1.97) | <0.001 | 1.27 (1.05–1.52) | 0.013 |
| ≥3 | 1.81 (1.56–2.09) | <0.001 | 1.28 (1.1–1.49) | 0.001 |
| Hypertension | 1.67 (1.5–1.87) | <0.001 | 1.2 (1.07–1.35) | 0.002 |
| Diabetes | 1.69 (1.51–1.88) | <0.001 | 1.31 (1.17–1.46) | <0.001 |
| Previous MI | 2.13 (1.72–2.62) | <0.001 | 1.33 (1.04–1.71) | 0.024 |
| Previous PCI | 2.03 (1.7–2.41) | <0.001 | 1.39 (1.13–1.71) | 0.002 |
| Peripheral artery disease | 2.53 (1.5–4.29) | <0.001 | 1.97 (1.16–3.35) | 0.012 |
| End stage renal disease | 5.26 (4.08–6.79) | <0.001 | 3.74 (2.87–4.86) | <0.001 |
| Chronic lung disease | 2.05 (1.53–2.75) | <0.001 | 1.34 (0.99–1.8) | 0.054 |
| LV dysfunction | 2.21 (1.93–2.52) | <0.001 | 1.57 (1.36–1.8) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, J.; Choo, E.H.; Choi, I.J.; Lee, K.Y.; Hwang, B.-H.; Kim, C.J.; Jeon, D.S.; Ahn, Y.; Jeong, M.H.; Chang, K. Office-Visit Heart Rate and Long-Term Cardiovascular Events in Patients with Acute Myocardial Infarction. J. Clin. Med. 2023, 12, 3734. https://doi.org/10.3390/jcm12113734
Byeon J, Choo EH, Choi IJ, Lee KY, Hwang B-H, Kim CJ, Jeon DS, Ahn Y, Jeong MH, Chang K. Office-Visit Heart Rate and Long-Term Cardiovascular Events in Patients with Acute Myocardial Infarction. Journal of Clinical Medicine. 2023; 12(11):3734. https://doi.org/10.3390/jcm12113734
Chicago/Turabian StyleByeon, Jaeho, Eun Ho Choo, Ik Jun Choi, Kwan Yong Lee, Byung-Hee Hwang, Chan Joon Kim, Doo Soo Jeon, Youngkeun Ahn, Myung Ho Jeong, and Kiyuk Chang. 2023. "Office-Visit Heart Rate and Long-Term Cardiovascular Events in Patients with Acute Myocardial Infarction" Journal of Clinical Medicine 12, no. 11: 3734. https://doi.org/10.3390/jcm12113734






